Summary
Aims
Although extensive investigation has revealed that an astrocyte‐specific protein aquaporin‐4 (AQP4) participates in regulating synaptic plasticity and memory, a functional relationship between AQP4 and learning processing has not been clearly established. This study was designed to test our hypothesis that AQP4 modulates the aversive motivation in Morris water maze (MWM).
Methods and Results
Using hidden platform training, we observed that AQP4 KO mice significantly decreased their swimming velocity compared with wild‐type (WT) mice. To test for a relationship between velocities and escape motivation, we removed the platform and subjected a new group of mice similar to the session of hidden platform training. We found that KO mice exhibited a gradual reduction in swimming velocity, while WT mice did not alter their velocity. In the subsequent probe trial, KO mice after no platform training significantly decreased their mean velocity compared with those KO mice after hide platform training. However, all of KO mice were not impaired in their ability to locate a visible, cued escape platform.
Conclusions
Our findings, along with a previous report that AQP4 regulates memory consolidation, implicate a novel role for this glial protein in modulating the aversive motivation in spatial learning paradigm.
Keywords: Aquaporin‐4, Astrocyte, Memory, Motivation, Synaptic plasticity
Introduction
It has been well established that astrocytes play an important role in structural and metabolic support to neurons. However, increasing evidence for the roles of astrocytes in synaptic plasticity has emerged over the past few years 1, 2. Astrocytes express membrane receptors for neurotransmitters and can release their own chemical messengers (gliotransmitters) 3. Thus, they can establish a cross talk with both pre‐ and postsynaptic neurons, forming a currently recognized novel functional unit, the tripartite synapse 4. However, the role of astrocyte‐specific proteins and transporters in synaptic plasticity is only beginning to be elucidated.
Recently, using mice with a deletion of the astrocyte‐specific channel aquaporin‐4 (AQP4), many studies including our own have indicated the potential roles of AQP4 in hippocampal synaptic plasticity and spatial memory function 5, 6, 7, 8, 9. AQP4 is the primary aquaporin as water channels in the central nervous system 10. Generally, it is involved in the regulation of edema 10, 11, 12 and other types of pathological conditions, such as seizure threshold in epilepsy 10, 13. As AQP4 is coupled to a subset of potassium channels and can regulate extracellular space volume dynamics during high‐frequency synaptic stimulation, it could influence synaptic transmission and spatial memory in the hippocampus. Using AQP4 knockout (KO) mice generated in our laboratory, we demonstrated that AQP4 KO mice exhibited impaired cognitive abilities in Morris water maze (MWM) accompanied by defects in long‐term potentiation (LTP) 7. Li et al. 8 also found that AQP4 deficiency impaired LTP in the thalamo‐lateral amygdala (LA) pathway and amygdala‐dependent fear memory of adult mice. Using AQP4 KO mice generated in Alan Verkman's laboratory, Skucas et al. 6 also found that AQP4 KO mice exhibited a selective defect both in LTP and in long‐term depression (LTD) related specifically to brain‐derived neurotropic factor. However, in their experiments, cognitive abilities in AQP4 KO mice were impaired only using location‐specific object memory, but not MWM or contextual fear conditioning. These studies support an unanticipated role for AQP4 in synaptic plasticity and memory function, but a functional relationship between AQP4 and learning processing has not been clearly established.
Morris water maze is an aversively motivated spatial learning paradigm that has been used extensively to study the neurobiology of spatial learning and memory in rodents 14. In general, the MWM is used to study spatial memory and learning 15, 16. Its learning process relies on two major functional systems, motivation (reward related, climbing onto the platform to escape from the water) and information processing (acquisition, consolidation, and retrieval of the platform location). Thus, it can also be used to evaluate aversive motivational aspects of learning 17, 18. We previously observed that AQP4 KO mice appeared to find the platform more easily and more flexibly on the first day of training, but could not reach the same execution levels on escape latency compared with WT mice at the end of training 7. We hypothesized that AQP4 KO mice may exhibit not only impaired information processing but also reduced motivation for reward in learning and memory.
In MWM, information processing is best measured during a probe trial in which the hidden platform is removed from the swimming pool 19. Animals that have learned the location of the hidden platform in relation to spatial cues spent more time in the quadrant where the platform was previously located than in any of the other quadrants, that is, they show spatial bias or spatial memory 20. On the other hand, other behavioral tests measure motivation by using a reward approach 21. As reaching the platform is considered rewarding, motivation to reach this reward is best measured by “mean swimming velocity” during trials 18. In this study, to test our hypothesis in AQP4 KO mice, we evaluated swimming velocity during the learning processing of AQP4 KO and wild‐type (WT) mice using hide platform training (with rewards, acquiring training) and nonplatform training (without rewards, only swimming). We found that AQP4 KO mice showed reduced swimming velocity during training with rewards and without rewards. In subsequent probe trials, AQP4 KO mice without rewards significantly decreased their mean velocity compared with those AQP4 KO mice with rewards. In our study, all AQP4 KO mice were not impaired in their ability to locate a visible, cued escape platform, suggesting that AQP4 does not cause visual impairments. Thus, our findings indicate that AQP4 may regulate aversive motivation of spatial memory.
Materials and Methods
Animals
Generation of the transgenic animals is given in detail by Fan et al. 22. Male mice aged 3–4 months were housed in same‐sex littermate groups (2–3 animals per cage) and were kept under environmentally controlled conditions (ambient temperature, 22 ± 1°C; humidity, 40%) on a 12‐h light–dark cycle with food and water ad libitum. All experiments were approved by the Institutional Animal Care and Use Committee at the Nanjing Medical University. All efforts were made to minimize animal suffering and to reduce the number of animals used for the experiments.
Apparatus
The apparatus and room were as previously described 7. Briefly, a galvanized circular pool (100 cm diameter, 60 cm deep) held opaque water colored with nontoxic white. The water temperature was between 22°C and 24°C during testing. The platform (10 cm2) was approximately 0.7 cm below the surface of the water. Around the test room, multiple cues including mouse cages, TV and VCR equipment, the experimenter, and cardboard shapes on the wall were visible to the swimming mouse. The mice were moved to the testing room 2 days before testing to acclimatize to the new environment and to reduce the stress incurred by moving. Video tracks were analyzed using clever system TopScan software (Clever Sys Inc., Reston, VA, USA). For trials, “latency to reach the platform,” “distance moved,” and “mean swimming velocity” were recorded. The latter parameter was measured only when mice were swimming (velocity > 3.0 cm/seconds). Movements at lower velocity were considered to be floating. Daily scores were averaged (four trials).
Procedure of MWM
Hide Platform Training (Acquisition Training, with Rewards)
On the first day of training, mice were alternated between standing on the platform (reward) and swimming for 10 seconds to familiarize the animals with the test apparatus. The mice were given a block consisting of four trials each for a total of 9 days as illustrated in Figure 1A. On each trial, the starting position was randomized between four possible positions. Each trial lasted 60 seconds or until the animal located the platform. Animals that did not find the platform were guided to the platform and given a latency score of 60 seconds. All animals received a 30‐seconds rest period on the escape platform between trials.
Figure 1.
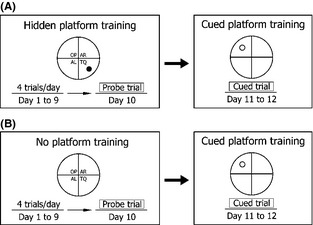
Illustration of the Morris water maze (MWM) training protocol. (A) Wild‐type (WT) and AQP4 knockout (KO) mice were first trained to find the hidden platform (placed in the center of training quadrant) by four trials daily for 9 days and tested by the probe trial on day 10. Starting from day 11, the same animals were subjected to the cued platform training, during which the platform was moved from training quadrant to the opposite quadrant (as illustrated). Animals were trained by four trials daily for 2 days (from day 11 to day 12). (B) WT and AQP4 KO mice were trained to swim without the platform similar to the session of the MWM. TQ, training quadrant; AR, adjacent right quadrant; AL, adjacent left quadrant; OP, opposite quadrant.
Nonplatform Training (Only Swimming, Without Rewards)
The apparatus was the same pool used in the hide platform training experiments, from which the platform was removed (without rewards). The task consisted of the same sessions as that in the hide platform training as illustrated in Figure 1B. During these nine sessions, each mouse was allowed to swim for 60 seconds in the pool. We recorded the swimming distances and speed of each group during the trials.
The Probe Trial
After training on the ninth day, animals (with or without rewards) were given only a 60‐seconds probe trial during which the platform was removed from the pool. The probe trial started from a start position opposite to the quadrant that originally contained the platform.
Cued Platform Task
One day after the probe trial, mice were tested in a cued platform task for two consecutive days. In this task, the platform was made visible by attaching a black cubic landmark to the platform.
Statistics
If not stated otherwise, error bars represent SEM. Comparison of groups and effects was performed with ANOVA using SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, USA). Initial statistical analyses were performed using repeated measures or univariate ANOVA. After significant effects involving genotype, follow‐up comparisons were performed using separate ANOVAs within each genotype or Tukey's post hoc tests. During the cued platform task, one WT mice and three AQP4 KO mice could not reach the cued platform in 60 seconds; thus, these mice were removed from analysis of this task.
Results
AQP4 KO Mice Show Reduced Swimming Velocity During Hidden Platform Training
We trained AQP4 KO and WT mice with the MWM (as illustrated in Figure 1A). We first subjected mice to 9 days of hidden platform training (four trials per day). While AQP4 KO and WT mice all improved gradually in escape latency, there is no significant difference between these two genotypes (trial day: F 8,136 = 27.727, P < 0.001; genotypes: F 1,17 = 0.207, P = 0.655, Figure 2A). However, as previously reported 7, AQP4 KO mice showed a shorter latency to find the platform compared with WT mice on the first training day, whereas they took longer time to escape the water on the last training days (KO: 14.19 ± 2.86, WT: 7.12 ± 1.00, P = 0.040). This was confirmed by statistical analysis as we found a significant interaction term (trial day × genotype: F 8,136 = 7.643, P < 0.001). Thus, AQP4 KO mice did not reach the same execution levels on escape latency compared with WT mice. Interestingly, while both genotypes showed increasingly shorter pathways from the starting point to the hidden platform during the training phase, AQP4 KO mice traveled significantly shorter distances compared with WT mice (trial day: F 8,136 = 39.790, P < 0.001; genotype: F 1,17 = 14.741, P = 0.001, Figure 2B). No genotype‐dependent differences in the distance to the platform were observed between AQP4 KO and WT mice on the last training days.
Figure 2.
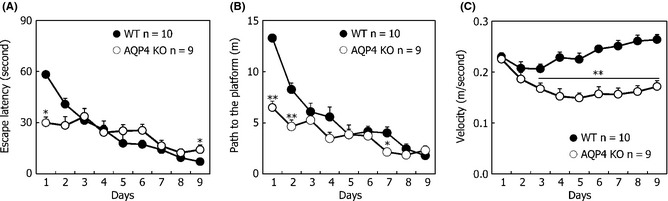
Performance in the Morris water maze using hidden platform training was assessed by escape latency (A), distance to the platform (B), and swim velocity (C) during nine training days. Each point represents the mean (±SEM) for four trials of each group. **P < 0.01 and **P < 0.05 indicated significant post hoc differences (Tukey's test) between wild‐type (WT) mice and knockout (KO) mice.
With respect to motivation, a significant difference in velocity was found between the two genotypes (genotype: F 1,17 =1015.077, P < 0.001, Figure 2C). This was confirmed by statistical analysis as we found a significant interaction term (trial day × genotype: F 8,136 = 7.865, P < 0.001). In addition, repeated measurements per genotype showed that WT mice increased their mean swimming velocity over days (trial day: F 8,72 = 12.328, P < 0.001), while AQP4 KO mice significantly decreased their velocity (trial day: F 8,64 = 8.564, P < 0.001). Post hoc analysis per day revealed that significant differences between genotypes were present on the latter 7 days. These findings suggest that the increased escape latency of AQP4 KO mice was due, at least in part, to a reduced swimming velocity.
These results confirmed our conclusion that AQP4 KO mice exhibit impaired spatial learning in MWM. Furthermore, AQP4 KO mice exhibited lower swimming velocities in the acquisition training compared with wild‐type mice, which indicates that AQP4 KO mice have reduced motivation for reaching the platform.
AQP4 KO Mice Exhibit Reduced Swimming Velocity During Nonplatform Training
To gain further insight into the reduced motivation of AQP4 KO mice in the MWM, we removed the platform and subjected another group of AQP4 KO and WT mice to similar sessions of the MWM (as illustrated in Figure 1B). We analyzed swimming distance and velocity during 60 seconds under nonplatform circumstances. A significant difference in distance (genotype: F 1,16 = 29.018, P < 0.001, Figure 3A) and velocity (genotype: F 1,16 = 29.271, P < 0.001, Figure 3B) was found. This was confirmed by statistical analysis as we found a significant interaction term (distance: trial day × genotype: F 8,128 = 18.388, P < 0.001; velocity: trial day × genotype: F 8,128 = 17.844, P < 0.001). Repeated measurements per genotype showed that AQP4 KO mice significantly decreased their mean swimming distance (trial day: F 8,64 = 37.778, P < 0 .001) and velocity (trial day: F 8,64 = 36.970, P < 0 .001). No genotype‐dependent differences in the length of swimming path and the raw average swimming speeds were observed between AQP4 KO and WT mice on the first day during the 60 seconds of trial. However, during trials on following days, significant differences in swimming distance and velocity were detected between AQP4 KO and WT mice. According to trial‐by‐trial learning curves (Figure 3C), both genotypes of mice showed the same length in the swimming pool during the daily four‐trial sessions. In contrast to WT mice, AQP4 KO mice exhibited gradual reduction in travel length during the 60 seconds following eight consecutive days of training.
Figure 3.
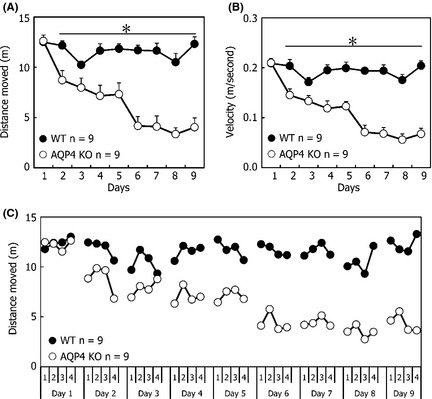
Performance using nonplatform training, as assessed by distance moved (A), swim velocity (B), and trial‐by‐trial learning curves (C) during nine training days. Each point represents the mean (±SEM) for four trials in each group. *P < 0.05 indicated significant post hoc differences (Tukey's test) between wild‐type (WT) mice and knockout (KO) mice.
AQP4 KO Mice Display Impaired Spatial Learning in the Probe Trial
To test whether the mice had indeed learned the spatial location of the hidden platform and were able to remember this information, the probe trials were conducted after the acquisition (for mice with the platform) or only swimming (for mice with no platform).
After the acquisition training of 9 days, only WT mice (%Time: F 3,36 = 5.946, P = 0.002; %Distance: F 3,36 = 6.358, P = 0.001), not AQP4 KO mice (%Time: F 3,36 = 0.294, P = 0.829; % Distance: F 3,36 = 0.185, P = 0.906), showed preference to the target quadrant (Figure 4A,B). WT mice spent more time (P = 0.045) and traveled more distances (P = 0.021) in the target quadrant. There also was a significant difference between WT and AQP4 KO mice in the numbers of platform crossed (P = 0.023, Figure 4C). For mice only swimming for 9 days, both AQP4 KO and WT mice, as expected, showed no significant preference to any quadrant (Figure 4D,E).
Figure 4.
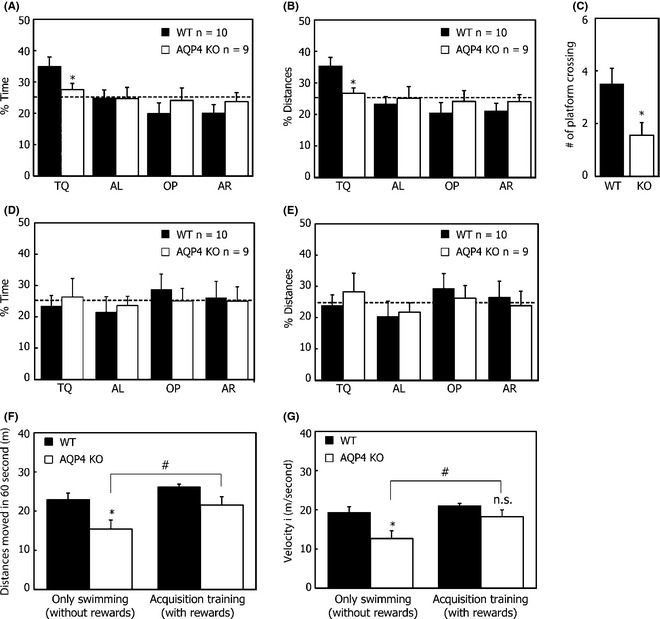
Performance in percentage of time spent in each quadrant (A, D), distance spent in each quadrant (B, E), number of crossings over the target platform location (C), distance moved in 60 seconds (F), and velocity (G) during the probe trial. Each point represents the mean (±SEM) for each group. *P < 0.05 indicated significant post hoc differences (Tukey's test) compared with wild‐type (WT) mice. # P < 0.05 indicated significant post hoc differences (Tukey's test) compared with corresponding knockout (KO) mice. TQ, training quadrant; AR, adjacent right quadrant; AL, adjacent left quadrant; OP, opposite quadrant.
To investigate the contribution of AQP4 on motivation in the MWM, we compared the effect of AQP4 deletion on swimming distance and velocity during 60 seconds under different training conditions. A significant difference in distance (genotype: F 1,33 = 9.900, P = 0.003; training: F 1,33 = 5.533, P = 0.025, Figure 4F) and velocity (genotype: F 1,33 = 9.624, P = 0.004; training: F 1,33 = 5.557, P = 0.024, Figure 4G) was found. WT mice showed no significant differences in swimming distance and velocity between acquisition training and swimming, while AQP4 KO mice (without rewards) showed significant shorter swimming distances and a decrease in their mean swimming velocity compared with AQP4 KO mice with rewards. Interestingly, although AQP4 KO mice with rewards showed lower swimming speed during the acquisition training, there were no significant differences between AQP4 KO and WT mice in the probe trials (P = 0.065). It seems likely that this was attributed to a reduction in swimming speed of WT mice (from 0.26 ± 0.01 m/seconds on the last training day to 0.22 ± 0.02 m/seconds in the probe test) rather than an increase in the swimming speed of AQP4 KO mice (from 0.17 ± 0.01 m/seconds on the last training day to 0.18 ± 0.05 m/seconds in the probe test).
AQP4 KO Mice Show Normal Spatial Learning in the Cued Platform Task
We next subjected these same animals to the cued platform task. As illustrated in Figure 1A,B, we moved the visible platform to the opposite quadrant. In the first trial, AQP4 KO and WT mice after acquisition training spent less time and traveled fewer distances to locate the cued platform compared with mice without acquisition training, indicating that acquisition training can improve the ability to reach the visible platform. Furthermore, the performance of AQP4 KO mice and WT littermates after acquisition training did not differ in the escape latency (genotype: F 1,17 = 0.213, P = 0.650, Figure 5A) and swimming distance (genotype: F 1,17 = 2.139, P = 0.162, Figure 5B). For mice without acquisition training, when excluding the four mice (one of nine WT mice and three of eight KO mice), which were unable to locate a cued platform, AQP4 KO mice and WT littermates showed significant improvements in their escape latency (F 1,11 = 44.907, P < 0.001, Figure 5A) and swimming distance (F 1,11 = 22.440, P = 0.001, Figure 5B). Meanwhile, WT and AQP4 KO mice showed similar escape latency (genotype: F 1,11 = 0.092, P = 0.767), while AQP4 KO mice traveled fewer distances to the cued platform only in the first trial (genotype: F 1,11 = 7.970, P = 0.01, Figure 5B) because of the reduced swimming velocity (Figure 5C). Despite the presence and absence of rewards, there were no significant differences in the swimming velocity between AQP4 KO and WT mice on the second day of cued trial (Figure 5C).
Figure 5.
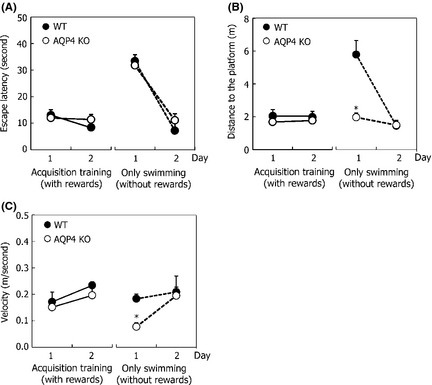
Performance in escape latency (A), distance (B), and velocity (C) during the cued platform trial. Each point represents the mean (±SEM) for each group. *P < 0.05 indicated significant post hoc differences (Tukey's test) between wild‐type (WT) mice and knockout (KO) mice.
Discussion
In the present study, we firstly confirmed the dissociation between acquisition and spatial probe found in AQP4 KO mice as previous report 7. As a continuing effort to understand the role of AQP4 in regulating the learning processing of spatial memory, our study sought to describe the effects of AQP4 deficiency on learning process using MWM. Interestingly, we observed significant differences in the mean swimming velocity between AQP4 KO and WT mice. In particular, the swimming velocity of AQP4 KO mice was significantly decreased from day 3 and 9 during hide platform training. Although AQP4 KO mice appeared more capable of finding the platform on the first day, they could not reach the same execution levels on escape latency compared with WT mice, as a result of a reduction in swimming velocity. Furthermore, when only swimming without platform (without rewards), AQP4 KO mice exhibited significantly less speed compared with WT mice. Our study is the first to provide evidence that AQP4 plays an important role in the regulation of motivation. Our data clearly show that, as a consequence of AQP4 deletion, AQP4 KO mice had reduced aversive motivation, which resulted in impairments in their learning and spatial memory abilities.
We ascribe our results to a deficit in aversive motivation, rather than a motor problem for the following reasons. First, AQP4 KO mice appeared to be capable of finding the platform on the first day of acquiring training. They also showed no significant difference in swimming distance on the first day under nonplatform circumstances compared with WT mice. These data implicate that AQP4 KO mice were able to swim as well as WT mice. On the first training day, no significant differences between genotypes were found in swimming velocity. Therefore, we suggest that, independent of genotype, maximum motivation to reach the platform is present at the beginning of training. Second, as training proceeded, AQP4 KO mice spent more time “floating” (data not show), especially upon removal of the platform (no reward). This represents an inability to switch from a passive strategy to a spatially selective strategy and is typically indicative of decreased motivation 23. Finally, in the presence or absence of rewards, KO mice had normal acquisition when trained in the cued platform task, which involved two sequential trials, and was performed after the hidden platform training or only swimming training. Consequently, if AQP4 KO mice were fatigued, this should be more evident in the cued platform task. In addition, according to trial‐by‐trial learning curves without rewards, AQP4 KO mice showed the same length in the swimming pool during the daily four‐trial sessions. Thus, these factors are consistent with the hypothesis that AQP4 KO mice “give up” when the task is too difficult, suggesting motivational deficits instead of muscular fatigue or weakness.
Both the hippocampus 24 and the meso‐accumbens dopamine system 25 are involved in MWM. Motivation is thought to be dependent on the meso‐accumbens dopamine system 26. AQP4 participates in the regulation of neurotransmission 22, 27, 28, especially dopamine 29. Using in vivo microdialysis and tissue homogenates from KO mice, we found that the basal extracellular levels of dopamine and its metabolites were significantly increased in areas of the brain known to play an important role in reward and motivation, the striatum, and ventromedial prefrontal cortex 22, 28. Studies in both animals and humans have demonstrated that direct manipulation of the neurotransmitter dopamine significantly impacts cost/benefit decision‐making 30. High levels of dopamine can decrease work motivation 31. On the other hand, AQP4 deficiency is able to downregulate glutamate uptake and glutamate transporter‐1 expression in astrocytes and induce prolonged excess of glutamate in the extracellular space 32. Stronger glutamate input to indirect pathway neurons may decrease the motivation of AQP4 KO mice 33. Recently, Yan et al. 34 found that AQP4 deficiency attenuated morphine tolerance and physical dependence through suppressing glutamate transporter‐1 downregulation and maintaining glutamate homeostasis. Furthermore, these findings from AQP4 KO mice showed an abnormal reward–motivational behaviors, such as depression 35, addiction resistance 36, 37, 38, and other psychosis 39. We reported that AQP4 deficiency disrupted behavioral improvement after fluoxetine treatment under both basal condition and chronic mild stress‐induced depression 35. AQP4 deficiency can attenuate locomotor activity in acute and repeated cocaine‐exposed mice 36 and inhibit morphine pharmacological actions 34, 38. However, it is clear that further experiments need to be performed to examine how AQP4 specifically impacts these reward–motivational systems.
In conclusion, using a separate parameter indicative of aversive motivation in MWM, we demonstrated that deletion of AQP4 has a distinct effect not only on information processing but also on the aversive motivational component.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
We thank Mr. Jason Davis (Georgia Regents University) for help with revising the manuscript. This study was supported by the grants from the National Key Basic Research Program of China (No. 2011CB504103), the National Natural Science Foundation of China (No. 81173032), and the key project of Natural Science Foundation of the Higher Education Institutions of Jiangsu Province (No. 11KJA310003).
References
- 1. Bains JS, Oliet SH. Glia: They make your memories stick! Trends Neurosci 2007;30:417–424. [DOI] [PubMed] [Google Scholar]
- 2. Barker AJ, Ullian EM. Astrocytes and synaptic plasticity. Neuroscientist 2010;16:40–50. [DOI] [PubMed] [Google Scholar]
- 3. Parpura V, Zorec R. Gliotransmission: Exocytotic release from astrocytes. Brain Res Rev 2010;63:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Santello M, Cali C, Bezzi P. Gliotransmission and the tripartite synapse. Adv Exp Med Biol 2012;970:307–331. [DOI] [PubMed] [Google Scholar]
- 5. Liu L, Lu Y, Kong H, et al. Aquaporin‐4 deficiency exacerbates brain oxidative damage and memory deficits induced by long‐term ovarian hormone deprivation and D‐galactose injection. Int J Neuropsychopharmacol 2012;15:55–68. [DOI] [PubMed] [Google Scholar]
- 6. Skucas VA, Mathews IB, Yang J, et al. Impairment of select forms of spatial memory and neurotrophin‐dependent synaptic plasticity by deletion of glial aquaporin‐4. J Neurosci 2011;31:6392–6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fan Y, Liu M, Wu X, et al. Aquaporin‐4 promotes memory consolidation in Morris water maze. Brain Struct Funct 2013;218:39–50. [DOI] [PubMed] [Google Scholar]
- 8. Li YK, Wang F, Wang W, et al. Aquaporin‐4 deficiency impairs synaptic plasticity and associative fear memory in the lateral amygdala: Involvement of downregulation of glutamate transporter‐1 expression. Neuropsychopharmacology 2012;37:1867–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scharfman HE, Binder DK. Aquaporin‐4 water channels and synaptic plasticity in the hippocampus. Neurochem Int 2013. doi: 10.1016/j.neuint.2013.05.003 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci 2013;14:265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zeng XN, Xie LL, Liang R, Sun XL, Fan Y, Hu G. AQP4 knockout aggravates ischemia/reperfusion injury in mice. CNS Neurosci Ther 2012;18:388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yan JH, Khatibi NH, Han HB, et al. p53‐induced uncoupling expression of aquaporin‐4 and inwardly rectifying K+ 4.1 channels in cytotoxic edema after subarachnoid hemorrhage. CNS Neurosci Ther 2012;18:334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li W, Hu B, Li GL, et al. Heterozygote genotypes at rs2222823 and rs2811712 SNP loci are associated with cerebral small vessel disease in Han Chinese population. CNS Neurosci Ther 2012;18:558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McNamara RK, Skelton RW. The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain Res Brain Res Rev 1993;18:33–49. [DOI] [PubMed] [Google Scholar]
- 15. Ma J, Xiong JY, Hou WW, et al. Protective effect of carnosine on subcortical ischemic vascular dementia in mice. CNS Neurosci Ther 2012;18:745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang LY, Tang SS, Wang XY, et al. PPARgamma agonist pioglitazone reverses memory impairment and biochemical changes in a mouse model of type 2 diabetes mellitus. CNS Neurosci Ther 2012;18:659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Francis GJ, Martinez JA, Liu WQ, et al. Intranasal insulin prevents cognitive decline, cerebral atrophy and white matter changes in murine type I diabetic encephalopathy. Brain 2008;131:3311–3334. [DOI] [PubMed] [Google Scholar]
- 18. Lubbers ME, van den Bos R, Spruijt BM. Mu opioid receptor knockout mice in the Morris Water Maze: A learning or motivation deficit? Behav Brain Res 2007;180:107–111. [DOI] [PubMed] [Google Scholar]
- 19. Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: Development of a learning index for performance in the Morris water maze. Behav Neurosci 1993;107:618–626. [DOI] [PubMed] [Google Scholar]
- 20. Morris R. Developments of a water‐maze procedure for studying spatial learning in the rat. J Neurosci Methods 1984;11:47–60. [DOI] [PubMed] [Google Scholar]
- 21. Bokkers EA, Koene P. Motivation and ability to walk for a food reward in fast‐ and slow‐growing broilers to 12 weeks of age. Behav Processes 2004;67:121–130. [DOI] [PubMed] [Google Scholar]
- 22. Fan Y, Zhang J, Sun XL, et al. Sex‐ and region‐specific alterations of basal amino acid and monoamine metabolism in the brain of aquaporin‐4 knockout mice. J Neurosci Res 2005;82:458–464. [DOI] [PubMed] [Google Scholar]
- 23. Wolfer DP, Stagljar‐Bozicevic M, Errington ML, Lipp HP. Spatial memory and learning in transgenic mice: Fact or artifact? News Physiol Sci 1998;13:118–123. [DOI] [PubMed] [Google Scholar]
- 24. Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature 1982;297:681–683. [DOI] [PubMed] [Google Scholar]
- 25. Ploeger GE, Spruijt BM, Cools AR. Spatial localization in the Morris water maze in rats: Acquisition is affected by intra‐accumbens injections of the dopaminergic antagonist haloperidol. Behav Neurosci 1994;108:927–934. [DOI] [PubMed] [Google Scholar]
- 26. Salamone JD, Correa M. Motivational views of reinforcement: Implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res 2002;137:3–25. [DOI] [PubMed] [Google Scholar]
- 27. Sun XL, Ding JH, Fan Y, Zhang J, Gao L, Hu G. Aquaporin 4 regulates the effects of ovarian hormones on monoamine neurotransmission. Biochem Biophys Res Commun 2007;353:457–462. [DOI] [PubMed] [Google Scholar]
- 28. Ding JH, Sha LL, Chang J, Zhou XQ, Fan Y, Hu G. Alterations of striatal neurotransmitter release in aquaporin‐4 deficient mice: An in vivo microdialysis study. Neurosci Lett 2007;422:175–180. [DOI] [PubMed] [Google Scholar]
- 29. Fan Y, Kong H, Shi X, et al. Hypersensitivity of aquaporin 4‐deficient mice to 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyrindine and astrocytic modulation. Neurobiol Aging 2008;29:1226–1236. [DOI] [PubMed] [Google Scholar]
- 30. Salamone JD, Correa M, Farrar A, Mingote SM. Effort‐related functions of nucleus accumbens dopamine and associated forebrain circuits (Berl). Psychopharmacology 2007;191:461–482. [DOI] [PubMed] [Google Scholar]
- 31. Treadway MT, Buckholtz JW, Cowan RL, et al. Dopaminergic mechanisms of individual differences in human effort‐based decision‐making. J Neurosci 2012;32:6170–6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zeng XN, Sun XL, Gao L, Fan Y, Ding JH, Hu G. Aquaporin‐4 deficiency down‐regulates glutamate uptake and GLT‐1 expression in astrocytes. Mol Cell Neurosci 2007;34:34–39. [DOI] [PubMed] [Google Scholar]
- 33. Bock R, Shin JH, Kaplan AR, et al. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci 2013;16:632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yan HT, Wu N, Lu XQ, Su RB, Zheng JQ, Li J. Aquaporin‐4 deficiency attenuates opioid dependence through suppressing glutamate transporter‐1 down‐regulation and maintaining glutamate homeostasis. CNS Neurosci Ther 2013;19:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kong H, Sha LL, Fan Y, et al. Requirement of AQP4 for antidepressive efficiency of fluoxetine: Implication in adult hippocampal neurogenesis. Neuropsychopharmacology 2009;34:1263–1276. [DOI] [PubMed] [Google Scholar]
- 36. Li Z, Gao L, Liu Q, et al. Aquaporin‐4 knockout regulated cocaine‐induced behavior and neurochemical changes in mice. Neurosci Lett 2006;403:294–298. [DOI] [PubMed] [Google Scholar]
- 37. Xie LL, Sun XL, Fan Y, Kong H, Ding JH, Hu G. Aquaporin 4 knockout resists negative regulation of neural cell proliferation by cocaine in mouse hippocampus. Int J Neuropsychopharmacol 2009;12:843–850. [DOI] [PubMed] [Google Scholar]
- 38. Wu N, Lu XQ, Yan HT, et al. Aquaporin 4 deficiency modulates morphine pharmacological actions. Neurosci Lett 2008;448:221–225. [DOI] [PubMed] [Google Scholar]
- 39. Su CJ, Xu XQ, Fan Y, Du RH, Hu G. Aquaporin‐4 knockout abolishes apomorphine‐induced tardive dyskinesia following chronic treatment with neuroleptics. CNS Neurosci Ther 2012;18:1024–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]


