Summary
Solid organ transplantations became a clinical option in the 1950s. The hand allograft was the pioneer of composite tissue allotransplantation (CTA), successfully started near the end of the last century despite arguments over the practicality and methods. Since then, CTA such as hand and face has continued to progress from the theoretical to clinical reality. The treatment principles, drug combinations, and mechanisms of the immunosuppression medications on which contemporary transplant surgeries have been based continue to develop as researchers and physicians gain more experience in the CTA field. It could be argued that the ethical issues associated with CTA have prevented evolution of the field rather than surgical or technical skill. This is particularly true for allo‐head and body reconstruction (AHBR). How can leaders in the field of CTA develop a model that would satisfy ethical concerns? Bolstered by recent successes in the field, is it time to traverse the next frontier? Can AHBR ever be a feasible option in the clinical setting? The reader will be provided with a brief history of CTA from theory to research to clinical practice. A concise description of AHBR as it pertains to the critical procedure (i.e., surgery design) will also be discussed.
Keywords: Spinal cord injury, Composite tissue allotransplantation, Head transplantation
Introduction
Head transplantation was attempted earlier by Guthrie in dog model in 1908 1, Demichow in 1954 2, Zhao in 1959 3, and White in 1965 4. White successfully documented this highly challenging operation when he transplanted the head/body of one primate onto anothers 5, 6. In these pioneer publications, the surgical procedures were well described from an anatomic and physiologic perspective, including the experimental techniques of vascular, spinal cord separation from the parent body, and cephalon functional maintenance strategies 7. Neurochemical and neurophysiologic dynamic monitoring were also investigated during the operation 8, 9.
These earlier transplant models were designed to place the head of one animal onto the intact body of another, with the exception of White who transferred the isolated head to a new isolated body (the first real “head or body transplantation”), a true clinical model for the future. More recently, this model was explored in rats 10, 11, 12. However, these previous studies were limited by a lack of concision, long‐term rejection prevention information, or an efficacious spinal functional recovery strategy. Of allo‐head and body reconstruction (AHBR) model in the past, are used cervical third, fourth incision. Such donor transplant body both center of life, breathing and circulation, because the loss of brainstem central control, unable to sustain independent life activities. Artificial respiration machine can temporarily support the heart and lung function, but this machine quit ventilation device will bring damage to the organs, so that the animal cannot be long‐term survival.
Despite these failures in previous studies, surgeons have been given encouragement with the successes of composite tissue allotransplantation (CTA) in hand and face models 13, 14, 15, 16, 17, 18, 19. As such, allo‐head and body transplantation have rapidly captured the interest and imagination of physician scientists. This strategy could provide unique a surgical option to save a life with current available medical technology. But how does one even define this most challenging surgical strategy? The idea itself remains controversial, and it has been called “head transplantation” or “whole body transplantation.” However, in scientific point, this novel operation should be recognized as a body‐to‐head transplantation. Here, we try to coin the term: allo‐head and body reconstruction, or AHBR. This defines AHBR as a unique surgical procedure involving the reconstruction of a master organism's head onto the body of another to save a life.
In this review, we first briefly present the history of CTA from research to clinical application and then outline some of the key arguments against AHBR; finally, we emphasize the requirement of developing a potential mouse model as a primary biological model to build up a fundamental platform to develop a feasible surgical procedure and spinal recovery strategies. We hope that this review will help guide the surgical team that would be considering perform human AHBR in the future.
The History of CTA
The interest in using CTA to reconstruct soft tissue and musculoskeletal defects dates at least as far as back as the fourth century. It was then when the saintly twin brothers Cosmas and Damian were reputed to have replaced a diseased limb 20. Advances in reconstructive microsurgery, an increased experience with organ transplantation, and the recent development of more efficacious immunotherapy have led to the possibility of CTA research 21, 22, 23 and its clinical applicability 13, 14, 15, 16, 17, 19.
Louisville team developed a successful immunosuppressive combination in a series of animal experiments that later had clinical applicability 13, 21, 22, 23, 24 and the large animal model demonstrated rejection‐free long‐term graft survival 21, 23. Based on these findings, during 1998 and 1999, teams in Louisville (USA), Lyon (France), and Guangzhou (China) performed the first four human hand transplantations using FK‐506/MMF/corticosteroid combination therapy 13. These breakthroughs captured the imagination of surgeons and led to a great deal of discussion in the scientific communities. Bolstered by the success of the human hand transplantation, the first human facial allograft was successfully performed in 2005 19.
Expanding the Paradigm of CTA: What is the Next Frontier in CTA?
Definition of the Unique Surgical Strategy
Until now, the term “head transplantation” was used to describe a surgical procedure whereby an isolated head was transplanted from one organism onto another intact body. This approach was similar to organ transplantation whereby the revascularized head would become an affiliate organ, but not master of the body 1, 2, 3. The real head transplantation model in which the head would be master was not employed until the 1970s by White 5, 6. He designed a surgical procedure whereby an isolated head was transplanted to a decapitated body. This truly employed the concept of “whole body transplantation” instead of “head transplantation.” It is from this model that the new term “AHBR” has been developed (see Figure 1). AHBR differs completely from classical organ transplantation and allograft because of the head's function as an organ capable of thought and awareness. Secondary to this, the head in AHBR functions as both recipient and master in the procedure, while the isolated body becomes a donor. This precise definition is necessary to redefine this unique operative procedure to avoid misleading terminology.
Figure 1.
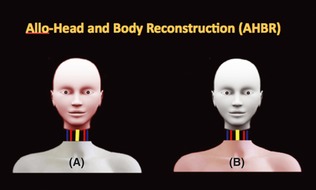
Proposed scheme for AHBR. (A) shows isolated head as receipted of AHBR, and (B) shows decapitated body as donor of AHBR, while pink color presents health part of body individually.
Is AHBR a Clinical Possibility?
Yes. Attempts have been made with limited success over the years, and there are lessons to be gleaned from prior CTA models that have been developed. Advances in technology, microsurgery, immunosuppressive therapy, and central nervous system regenerative therapies allow for serious consideration of AHBR. Surgical techniques, postoperative management, and functional rehabilitation have also advanced markedly in recent years; it is time to consider adapting the AHBR technique to humans. But of course, none of this will occur if a feasible biological model is not first developed.
Gaps in Current Knowledge: What is a Feasible Biological Model for AHBR?
Of course, to make a clinical transition to AHBR, a feasible biological model must be developed. This model should incorporate maximal preservation of the isolated body's own basic functions, which would naturally include the circulatory and respiratory centers before focusing on spinal function and recovery.
Strategies to Make the AHBR Postoperative Body Has Spontaneous Breathing and Blood Circulation
In the final design, White points out an operative incision at the level of the mid‐brain instead of the traditional section through the upper cervical cord 7, but not yet to see the results of experimental study published. This idea makes intuitive sense and would be applicable to AHBR, as preserving the CNS at this level allows the recipient body's basic functions of respiration and circulation to continue uninterrupted without equipment support. In the pilot studies, our outcomes also demonstrated by donor brainstem reserved to instead cervical one is critical and feasible (unpublished).
Multiple Approaches for Spinal Functional Recovery that Includes Stem Cell Therapy, Costimuli, Electric Stimuli, and Neural Interface System (3D)
Stem cell engineering has offered an attractive approach to replacement of dead or damaged neuron fibers after spinal cord injury (SCI). Recently, induced pluripotent stem cells (iPSCs) without use of viruses or need for genomic integration explored onto the stem cell field as a repair strategy for SCI 25. Our data supported transplant iPSCs immigrate toward the damage at the time window for efficacy (7 days) and derived loss of neurons is possible after spinal cord hemisection (unpublished).
Biological stimuli such as Nogo‐A, motor recovery, and the sprouting of corticospinal axons were enhanced in monkey's SCI model treated with Nogo‐A‐specific antibody 26, 27, from research patient study; epidural stimulation enabled the man to achieve voluntary movement, standing and assisted stepping after complete paraplegia 27, 28, 29; Harkema clinical investigation demonstrated that neural interface system and rehabilitation engineers could be a viable clinical approach for functional recovery after severe paralysis 30, 31. All these interventions provided encouraged outcomes to enhance spinal function recovery and living/life support.
How to Make the Real AHBR in Human
A feasible preclinical CTA model was conducted successfully with the first human hand transplantation in the world 24 as direct result. Although several “head or body biological transplant models” have been attempted since Guthrie in 1908 1, AHBR still remains a dream for surgeons in the present. To make the dream a reality, an idealized biological model must first be created. This model must provide the scientific community with complete or partial spinal recovery data and present evidence for long‐term survival while preventing rejection, either acute or chronic.
This will also require a leadership platform that consists of experts in the relevant fields. The development of an efficacy executive committee and opening a national or international symposium are other critical factors to consider. This would be similar to adopting the structure used by the Louisville Hand Transplant Committee in the middle of 1990s that resulted in the first national hand transplant as well as the development of successful pig models 16.
Future Priorities: What is Needed to Achieve the Clinical Potential of AHBR
The advancement of AHBR will require a biological model that represents a reproducible, cost‐effective, clinical strategy to monitor rejection and observe spinal functional recovery. The model has to meet the following criteria:
Address “immunological barriers” and drug‐related toxicity
Contain all of the individual components of an AHBR including composite tissues (skin, muscle, bone, vessels, bone marrow, peripheral nerves, and CNS, etc.)
Allow evaluation of peripheral and central nerve function to the body and head
Preserve maximal body function to reduce mortality and provide long‐term clinical follow up.
All outcomes will be priority investigated prior to the final preclinical model using a large primate animal.
Conclusion
Spinal regeneration remains a major barrier to the advancement of the field. But while transferring a person's head to another body would not allow them to move or walk again at present time, it could prolong their life. The importance of AHBR is that, if performed in humans, it has the potential to save lives from almost any disease excepting those that afflict the central nervous system.
Who might benefit from a head transplant? Dr. White pointed out: the first candidates for the procedure will probably be people who have been paralyzed from the neck down because of an accident 7, who for reasons that are still largely unclear, often die prematurely of multiple‐organ failure. Although reconstructing a paralyzed person's head to another body would not–at least at this point in the development of the technology–allow them to move or walk again, it could prolong their life.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This study was supported by Scientist Foundation of HMU (To Dr. Xiaoping Ren), Harbin, China.
Reference
- 1. Guthrie CC. Blood vessel surgery and its applications. London and New York: Edward Arnold; Longmans, Green & Co., 1912. [Google Scholar]
- 2. Demikhov VP. Experimental transplantation of vital organs. New York: Consultants Bureau, 1962. [Google Scholar]
- 3. Dog‐Head Transplant Claimed by Chinese. Washington, DC: The Washington Post, Times Herald 1, 1959. [Google Scholar]
- 4. White RK, Albin MS, Locke GE, Davidson E. Brain transplantation: Prolonged survival of brain after carotid‐jugular interposition. Science 1965;150:779–781. [DOI] [PubMed] [Google Scholar]
- 5. White RJ, Wolin LR, Massopust LC Jr., Taslitz N, Verdura J. Cephalic exchange transplantation in the monkey. Surgery 1971;70:135–139. [PubMed] [Google Scholar]
- 6. White RJ, Wolin LR, Massopust LC Jr., Taslitz N, Verdura J. Primate cephalic transplantation: Neurogenic separation, vascular association. Transplant Proc 1971;3:602–604. [PubMed] [Google Scholar]
- 7. White RJ, Albin MS, Verdura J, et al. The isolation and transplantation of the brain. An historical perspective emphasizing the surgical solutions to the design of these classical models. Neurol Res 1996;18:194–203. [DOI] [PubMed] [Google Scholar]
- 8. White RJ, Albin MS, Verdura J. Isolation of the monkey brain: In vitro preparation and maintenance. Science 1963;141:1060–1061. [DOI] [PubMed] [Google Scholar]
- 9. White RJ, Albin MS, Verdura J. Preservation of viability in the isolated monkey brain utilizing a mechanical extracorporeal circulation. Nature 1964;202:1082–1083. [DOI] [PubMed] [Google Scholar]
- 10. Lee S. Historical events on development of experimental microsurgical organ transplantation. Yonsei Med J 2004;45:1115–1120. [DOI] [PubMed] [Google Scholar]
- 11. Takaoka Y, White RJ, Likavec MJ. A high performance isolated rat brain preparation. Part I: Operative technique and recipient extracorporeal perfusion. Resuscitation 1985;12:253–259. [DOI] [PubMed] [Google Scholar]
- 12. Hirabayashi S, Harii K, Sakurai A, Takaki EK, Fukuda O. An experimental study of craniofacial growth in a heterotopic rat head transplant. Plast Reconstr Surg 1988;82:236–243. [DOI] [PubMed] [Google Scholar]
- 13. Francois CG, Breidenbach WC, Maldonado C, et al. Hand transplantation: Comparisons and observations of the first four clinical cases. Microsurgery 2000;20:360–371. [DOI] [PubMed] [Google Scholar]
- 14. Jones JW, Gruber SA, Barker JH, Breidenbach WC. Successful hand transplantation. One‐year follow‐up. Louisville Hand Transplant Team. N Engl J Med 2000;343:468–473. [DOI] [PubMed] [Google Scholar]
- 15. Lanzetta M, Petruzzo P, Margreiter R, et al. The international registry on hand and composite tissue transplantation. Transplantation 2005;79:1210–1214. [DOI] [PubMed] [Google Scholar]
- 16. Gander B, Brown CS, Vasilic D, et al. Composite tissue allotransplantation of the hand and face: A new frontier in transplant and reconstructive surgery. Transpl Int 2006;19:868–880. [DOI] [PubMed] [Google Scholar]
- 17. Jiang HQ, Wang Y, Hu XB, Li YS, Li JS. Composite tissue allograft transplantation of cephalocervical skin flap and two ears. Plast Reconstr Surg 2005;115:31e–35e discussion 36e–37e. [DOI] [PubMed] [Google Scholar]
- 18. The first facial transplant. Lancet 2005;366:1984. [DOI] [PubMed] [Google Scholar]
- 19. Butler PE, Hettiaratchy S, Clarke A. Facial transplantation: A new gold standard in facial reconstruction? J Plast Reconstr Aesthet Surg 2006;59:211–212. [DOI] [PubMed] [Google Scholar]
- 20. Conolly B, Benanzio M. Cosmas and damian revisited In: Lanzetta M, Dubernard J‐M, Petruzzo P, editors. Hand Transplantation. Milan: Springer, 2007;3–10. [Google Scholar]
- 21. Ustuner ET, Zdichavsky M, Ren X, et al. Long‐term composite tissue allograft survival in a porcine model with cyclosporine/mycophenolate mofetil therapy. Transplantation 1998;66:1581–1587. [DOI] [PubMed] [Google Scholar]
- 22. Shirbacheh MV, Ren X, Jones JW, et al. Pharmacokinetic advantage of intra‐arterial cyclosporin A delivery to vascularly isolated rabbit forelimb. I. Model development. J Pharmacol Exp Ther 1999;289:1185–1190. [PubMed] [Google Scholar]
- 23. Jones JW Jr., Ustuner ET, Zdichavsky M, et al. Long‐term survival of an extremity composite tissue allograft with FK506‐mycophenolate mofetil therapy. Surgery 1999;126:384–388. [PubMed] [Google Scholar]
- 24. Ren X, Shirbacheh MV, Ustuner ET, et al. Osteomyocutaneous flap as a preclinical composite tissue allograft: Swine model. Microsurgery 2000;20:143–149. [DOI] [PubMed] [Google Scholar]
- 25. Fujimoto Y, Abematsu M, Falk A, et al. Treatment of a mouse model of spinal cord injury by transplantation of human induced pluripotent stem cell‐derived long‐term self‐renewing neuroepithelial‐like stem cells. Stem Cells 2012;30:1163–1173. [DOI] [PubMed] [Google Scholar]
- 26. Freund P, Schmidlin E, Wannier T, et al. Nogo‐A‐specific antibody treatment enhances sprouting and functional recovery after cervical lesion in adult primates. Nat Med 2006;12:790–792. [DOI] [PubMed] [Google Scholar]
- 27. Buchli AD, Rouiller E, Mueller R, Dietz V, Schwab ME. Repair of the injured spinal cord. A joint approach of basic and clinical research. Neurodegener Dis 2007;4:51–56. [DOI] [PubMed] [Google Scholar]
- 28. Harkema S, Gerasimenko Y, Hodes J, et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet 2011;377:1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van den Brand R, Heutschi J, Barraud Q, et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science 2012;336:1182–1185. [DOI] [PubMed] [Google Scholar]
- 30. Hochberg LR, Serruya MD, Friehs GM, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 2006;442:164–171. [DOI] [PubMed] [Google Scholar]
- 31. Hochberg LR, Bacher D, Jarosiewicz B, et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 2012;485:372–375. [DOI] [PMC free article] [PubMed] [Google Scholar]


