Summary
Background and purpose
Few reports concerned on recombinant tissue plasminogen activator (rt‐PA) treatment in stroke patients with decreased consciousness. This study assesses the efficacy and safety of intravenous rt‐PA administration within 4.5 h in stroke patients with decreased consciousness.
Methods
A total of 136 stroke patients with decreased consciousness, who received or not rt‐PA intravenously within 4.5 h after stroke onset from Jiangsu province of China from 2009 to 2012, were reviewed retrospectively. Glasgow Coma Scale (GCS), National Institute of Health Stroke Scale (NIHSS), intracranial hemorrhage rate, and mortality were used to determine patient outcome when discharged. A 3‐month outcome was calculated by modified Rankin Scale (mRS) with score 0 to 1 considered favorable outcome.
Results
Baseline characteristics of two groups were similar. When discharged, no significant differences were observed regarding NIHSS score (P = 0.994) or GCS score (P = 0.591) between groups. After 3 months, 22.8% patients in rt‐PA group had favorable outcome as compared with 7.5% patients in control group (P = 0.014). Treatment with rt‐PA did not significantly increase incidence of hemorrhage (P = 0.494) or mortality (P = 0.169).
Conclusions
Intravenous rt‐PA administration within 4.5 h after onset of symptoms benefited stroke patients with abnormal consciousness.
Keywords: Decreased level of consciousness, Efficacy and safety, Intravenous thrombolysis, Stroke
Introduction
Ischemic stroke patients with decreased consciousness often have poor clinical prognosis 1, 2. Some reports have even proposed that coma is an independent predictor of poor stroke outcome 3. However, current randomized trials of thrombolytic stroke treatment often used inclusion criteria which made enrollment of these patients less likely 4, 5, 6. Thus, little data are available concerning about applying rt‐PA to this special ischemic stroke subgroup.
Intravenous thrombolysis by rt‐PA is a well‐known effective treatment for acute ischemic stroke. In 1995, the first multi‐center randomized study conducted by the National Institute of Neurological Disorders and Stroke (NINDS) confirmed the effectiveness of intravenous thrombolytic therapy in the treatment of acute stroke within 3 h of stroke onset 7. The recently published European Cooperative Acute Stroke Study III (ECASS III) demonstrated that intravenous alteplase administered between 3.0 and 4.5 h after stroke onset significantly improved clinical outcomes in patients with acute ischemic stroke 8. The third international stroke trial (IST‐3) indicated that intravenous thrombolysis had benefit until 6 h even for elderly patients 9.
Despite proven efficacy in general stroke patients within 4.5 h, worries remain regarding the safety and efficacy of rt‐PA for acute ischemic stroke accompanied with low level of consciousness. Unfortunately, demographic characteristics of this patient subgroup are lacking. In practice, compared with general stroke patients, the neurological status of this subgroup is much more severe, as manifested by higher NIHSS score and lower GCS score. Many studies indicate that severity of neurological deficit at baseline is a risk factor for intracranial hemorrhage 10, 11. The efficacy and safety of administrating rt‐PA to stroke patients with decreased level of consciousness is unknown.
In this study, rt‐PA at a dose of 0.9 mg/kg was administered to stroke patients with impaired level of consciousness for the purpose of exploring reasonable therapeutic strategies for this special group.
Patients and Methods
Patients
This retrospective study was proved by the ethics committee of the Affiliated Drum Tower hospital of Nanjing University, Medical School. All data were obtained from the Jiangsu Stroke Research Collaborative Group including eight hospitals in Jiangsu Province of China. Neurologists in this group are well experienced in applying rt‐PA for acute ischemic stroke. Records were reviewed of stroke patients who presented to each hospital between January 2009 and June 2012. The patients got the rt‐PA treatment if they agree to receive thrombolysis, and patients were used as control if not. A standardized form was used to systematically collect data, including demographic characteristics, risk factors, time delay from stroke onset to hospital arrival, circulation territory, CT scan, complications, and so on. The data from both groups got optimum balance by statistics analysis, to minimize selection bias.
The patients treated with thrombolysis were signed the informed consent. The patients treated with intravenous rt‐PA, in a dose of 0.9 mg/kg to a maximum of 90 mg (10% bolus with the remainder over 1 h).
Patient inclusion criteria: (1) over 18 years old; (2) clinical diagnosis of stroke with a clinically meaningful neurologic deficit; (3) CT or MRI brain scanning reliably excluded intracranial hemorrhage; (4) time between onset of stroke and initiation of rt‐PA was <4.5 h; and (5) decreased level of consciousness on admission (decreased level of consciousness is defined as GCS score <15).
Exclusion criteria were as follows: (1) minor or rapidly improving symptoms or signs; (2) a history of intracranial hemorrhage; (3) stroke, myocardial infarction, or serious head trauma within the previous 3 months; major surgery or serious trauma within 2 weeks; gastrointestinal or urinary tract hemorrhage within 3 weeks; arterial puncture at a noncompressible site or lumbar puncture within 1 week; (4) administration of heparin within 48 h preceding the onset of stroke; (5) elevated activated partial thromboplastin time or international normalized ratio >1.5; (6) platelet count <100,000/mm3; (7) systolic BP >185 mm Hg, diastolic BP >110 mm Hg; (8) blood glucose <50 or >400 mg/dL; symptoms suggestive of subarachnoid hemorrhage, even if CT scan was normal; and (10) pregnant women.
Measurement of Outcome
The neurological function of each patient at the time of admission and discharge was assessed by National Institutes of Health Stroke Scale (NIHSS) and Glasgow Coma Scale (GCS). The scales were assessed and recorded at admission by the attending neurologist who treated the patients in each medical center. NIHSS is a 42‐point scale quantifying neurologic impairment in 11 categories, with higher values reflecting more severe neurological deficit. GCS is a 15‐point scale with lower score denoting lower consciousness level. The modified Rankin Scale (mRS) is a simplified overall assessment of function, in which a score of 0 indicates the absence of symptoms, 5 indicates severe disability, and 6 indicates death. The 3‐month outcome was evaluated by mRS, dichotomized as favorable outcome (score of 0–1) or unfavorable outcome (score of 2–6) 8, 12. The mRS scores at 90 days were performed by telephone interview.
Safety parameters included mortality, intracranial hemorrhage and symptomatic intracranial hemorrhage (SICH) at indicated time points. SICH was defined as appearance of new hemorrhage on brain imaging contemporaneous with any neurological deterioration >1 point on the NIHSS. To detect intracranial hemorrhage, CT or MRI scans were performed at any time after the onset of stroke when clinical signs suggested hemorrhage.
Statistical Analysis
All data were entered and calculated using SPSS Version 17.0 (SPSS, Chicago, IL, USA). The significance of intergroup differences was assessed using the chi‐squared test for categorical variables and a univariate logistic regression analysis for continuous variables. The Mann–Whitney U‐test was performed to determine stratified analysis of the mRS score distribution. Survival rate was calculated using the Kaplan–Meier method.
Results
Baseline Characteristics of Patients
The study identified 136 stroke patients with decreased level of consciousness. Among them, 70 patients received IV rt‐PA (rt‐PA group) and 66 did not receive any thrombolytic therapy (untreated or control group). The two groups had similar demographics and basic characteristics (Table 1). The mean GCS score was 9.50 in the rt‐PA group and 9.67 in the untreated group (P = 0.728). 27(38.6%) of rt‐PA patients had a GCS score <8 compared with 25(37.9%) of untreated (P = 0.934). The two groups had similar mean NIHSS scores on admission (20.1 ± 7.20 vs. 19.2 ± 6.28; P = 0.442). With the exception of hypertension (72.9% vs. 56.1%; P = 0.042), there were no statistically significant differences between groups in other baseline characteristics, including age, gender, diabetes, atrial fibrillation, hypercholesterolemia, history of stroke, coronary artery disease, treatment duration, and stroke location.
Table 1.
Demographic and baseline characteristics of the patients
| Baseline parameters | IV rt‐PA (n = 70) | Untreated (n = 66) | P Value |
|---|---|---|---|
| Age, years, mean ± SD | 72.1 ± 10.8 | 74.1 ± 13.4 | 0.068 |
| Males | 45 (64.3%) | 36 (54.5%) | 0.247 |
| Hypertension | 51 (72.9%) | 37 (56.1%) | 0.042 |
| Diabetes | 11 (15.7%) | 9 (13.6%) | 0.733 |
| Atrial fibrillation | 26 (37.1%) | 32 (48.5%) | 0.181 |
| Hypercholesterolemia | 16 (22.9%) | 10 (15.2%) | 0.256 |
| History of stroke | 18 (25.7%) | 22 (33.3%) | 0.331 |
| Coronary artery disease | 16 (22.9%) | 7 (10.6%) | 0.057 |
| Time to treatment initiation | |||
| Mean ± SD | 196 ± 64.3 | 203 ± 59.2 | 0.485 |
| Median(range) | 190 (75–230) | 195 (60–270) | |
| NIHSS score | |||
| Mean ± SD | 20.1 ± 7.20 | 19.2 ± 6.28 | 0.442 |
| Median (range) | 20 (7–38) | 19 (8–36) | |
| GCS score | |||
| Mean ± SD | 9.50 ± 3.05 | 9.67 ± 2.54 | 0.728 |
| Median (range) | 10 (4–14) | 10 (5–14) | |
| ≤8 | 27 (38.6%) | 25 (37.9%) | 0.934 |
| 9–15 | 43 (61.4%) | 41 (62.1%) | |
| Circulation territory | 0.094 | ||
| TACI | 40 (57.1%) | 47 (71.2%) | |
| PACI | 18 (25.7%) | 15 (22.7%) | |
| POCI | 12 (17.1%) | 4 (6.1%) | |
| LACI | 0 | 0 | |
IV, intravenous; rt‐PA, reconstructive tissue plasminogen activator; SD, standard deviation; NIHSS, National Institutes of Health Stroke Scale; GCS, Glasgow Coma Scale; TACI, total anterior circulation infarct; PACI, partial anterior circulation infarct; POCI, posterior circulation infract; LACI, lacunar infarct.
Data are n (%) unless otherwise indicated.
Efficacy of rt‐PA in the Treatment of Stroke Patients with Decreased Level of Consciousness
The comparisons of efficacy between the rt‐PA and untreated groups are summarized in Table 2. In‐hospital outcomes, measured by NIHSS and GCS at discharge, showed no significant improvement in patients treated with rt‐PA compared with controls.
Table 2.
Efficacy of rt‐PA at discharge and 3 months
| Scale | IV rt‐PA (n = 70) | Untreated (n = 66) | Odds ratio (95% CI) | P Value |
|---|---|---|---|---|
| NIHSS score at discharge | ||||
| Mean ± SD | 17.2 ± 15.6 | 17.2 ± 12.0 | 1.00 (0.976–1.03) | 0.994 |
| Median (range) | 10 (0–38) | 14 (0–37) | ||
| GCS score at discharge | ||||
| Mean ± SD | 11.1 ± 4.95 | 11.5 ± 3.99 | 0.980 (0.908–1.06) | 0.591 |
| Median (range) | 14 (3–15) | 13 (3–15) | ||
| Favorable outcome (mRS score of 0–1) | 16 (22.8%) | 5 (7.5%) | 3.62 (1.24–10.5) | 0.014 |
IV, intravenous; rt‐PA, reconstructive tissue plasminogen activator; SD, standard deviation; NIHSS, National Institutes of Health Stroke Scale; GCS, Glasgow Coma Scale; mRS, Modified Rankin scale:0, no symptoms at all; 1, no significant disability despite symptoms (able to carry out all usual duties and activities); 2, slight disability (unable to carry out all previous activities but able to look after own affairs without assistance); 3, moderate disability (requiring some help but able to walk without assistance); 4, moderately severe disability (unable to walk without assistance and unable to attend to own bodily needs without assistance); 5, severe disability (bedridden, incontinent, and requiring constant nursing care and attention); 6, death.
Data are n (%) unless otherwise indicated.
At 3 months, 16 patients (22.8%) in the rt‐PA group had a favorable outcome (defined as a score of 0–1 on mRS) compared with five patients (7.5%) in the control group, representing an absolute improvement of 15.3 percentage points (odds ratio, 3.62; 95% CI, 1.24–10.5; P = 0.014). The overall distribution of mRS scores is shown in Figure 1. An ordinal analysis showed a favorable shift in mRS scores with rt‐PA treatment (P = 0.049).
Figure 1.
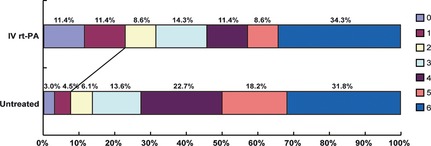
Distribution of scores on the modified Rankin Scale at 3 month. An ordinal analysis of the score distribution showed a significant difference between the study groups (P = 0.042).
Safety of rt‐PA in the Treatment of Stroke Patients with Decreased Level of Consciousness
Mortality within 7 days in rt‐PA group was not significantly higher than that in untreated (21.4% vs. 18.2%; odds ratio, 1.23; 95% CI, 0.526–2.86; P = 0.635) (Table 3). In addition, there was no significant difference in 3‐month mortality between two groups. 24 of 70 rt‐PA treated patients died (34.3%), as compared with 21 of 66 untreated patients (31.8%) (odds ratio, 1.12; 95% CI, 0.547–2.29; P = 0.760). Of these 45 deaths, 27 died between days 1 and 7 (15 [21.4%] in the rt‐PA group and 12 [18.2%] in the untreated group), 13 between days 8 and 30 (7 [10.0%] in the rt‐PA group and 6 [9.1%] in the untreated group), and 5 between days 31 and 90 (2 [2.9%] in the rt‐PA group and 3 [4.5%] in the untreated group).
Table 3.
Safety of rt‐PA at 3 months
| Events | IV rt‐PA (n = 70) | Untreated (n = 66) | Odds ratio (95% CI) | P Value |
|---|---|---|---|---|
| Death within 7 days | 15 (21.4%) | 12 (18.2%) | 1.23 (0.526–2.86) | 0.635 |
| Death within 3 months | 24 (34.3%) | 21 (31.8%) | 1.12 (0.547–2.29) | 0.760 |
| ICH | ||||
| Symptomatic | 8 (11.4%) | 3 (4.5%) | 2.71 (0.687–10.7) | 0.141 |
| Total | 21 (30.0%) | 12 (18.2%) | 1.93 (0.860–4.33) | 0.108 |
IV, intravenous; rt‐PA, reconstructive tissue plasminogen activator; SD, standard deviation; ICH, any intracranial hemorrhage.
Data are n (%) unless otherwise indicated.
The rate of SICHs in the rt‐PA group was higher, but not statistically significant, than the untreated group (11.4% vs. 4.5%; odds ratio, 2.71; 95% CI, 0.687–10.7; P = 0.141). Similarly, the frequency of total intracranial hemorrhage was higher, but not statistically significant, in the rt‐PA group (30.0% vs. 18.2%; odds ratio, 1.93; 95% CI, 0.860–4.33; P = 0.108).
Discussion
In this study, it was found that patients with severe ischemic stroke could benefit from treatment with intravenous rt‐PA administrated within 4.5 h after the onset of stroke. Specifically, at 3 months, 22.8% of patients in rt‐PA group had a favorable outcome compared with 7.5% in untreated group representing an absolute improvement of 15.3%. The overall incidence of SICH appeared a little higher, but not statistically significant, in the rt‐PA group. Mortality in each group was similar. These data are encouraging for clinical therapy for stroke patients with decreased level of consciousness. There are few clinic trials of thrombolysis involving consciousness problem. Most of them concerned severe stroke patients (NIHSS score >20). Sylaja et al. 11 showed that patients aged >70 years, admission NIHSS score >20, congestive heart failure or diabetes had a higher risk of SICH, increased mortality and significantly lower rate of functional recovery than patients without these risk factors, when treated within 3 h with rt‐PA. Subgroup analysis of the NINDS t‐PA stroke trial indicated that the 49 patients, who was >75 years and admission NIHSS >20, had no favorable response to treatment 13. However, IST‐3 manifested that the patients older than 80 years could get similar benefits to those aged 80 years or younger, suggesting that do not need any restriction of thrombolysis on the basis of stroke severity 9.
Consciousness is defined as a general awareness of oneself and surrounding environment. Two functional brain regions are responsible for consciousness in the human brain. One is the reticular activating system (RAS) in the brainstem. The other is the higher cortical areas of the cerebral cortex activated via the thalamic portion of the RAS. Regarding the nutrition and blood supply of the two territories, the former area relies on the vertebral‐basilar artery belonging to posterior circulation, and the later depends on anterior circulation. Based on the Oxfordshire community stroke project (OCSP) classification, CT or MRI data demonstrated that 82.8% rt‐PA treated patients were affected by total anterior circulation infarct (TACI) and partial anterior circulation infarct (PACI), while only 17.1% were affected by posterior circulation infract (POCI). Correspondingly, 93.9% rt‐PA untreated patients were affected by TACI and PACI, while only 6.1% were affected by POCI. This spectrum suggests that most of the study participants were affected by the occlusion of anterior circulation. This information bears noting. Posterior circulation thrombosis has been considered to be complicated and have poor prognosis 1, 14. It is therefore concluded that intravenous administration of rt‐PA mainly benefits unconscious stroke patients with anterior circulatory occlusion.
Most large clinical trials indicate that rt‐PA treatment does not have any detrimental effect on the mortality of stroke patients. Consistent with these observations, data of the current study showed that the overall mortality in rt‐PA treated group and control group during the 3 month‐ and 7 day‐follow‐up were statistically similar. However, mortality of patients in the current study was much higher than previous studies 8, 9. For instance, 3‐month mortality in the current study was 34.3% in the rt‐PA group compared with 31.8% in the control group, while, in ECASS III, 3‐month mortality was 7.7% in the rt‐PA group compared with 8.4% in control 8. This high mortality in the current study may be due to the relatively high NIHSS scores of recruited patients. Similarly, the patient percentage with favorable outcome in the current study is also much less than ECASS III. The highest risk of thrombolytic therapy is SICH and most studies demonstrate that rt‐PA increases the incidence of intracranial hemorrhage 8, 9. The risk could be outweighed by improved functional outcome if rt‐PA is applied at appropriated time. In the current study, intracranial hemorrhage and SICH rates in the rt‐PA group were higher than control, although the difference did not achieve statistical significance.
Overall, the current study suggests a potentially effective treatment for stroke patients with impaired consciousness within 4.5 h. However, there are several limitations. For instance, the study is a retrospective and nonrandomized study, which means that the individual or real efficacy of rt‐PA in each patient is hard to assess. Additionally, the study evaluated all the stroke patients with decreased level of consciousness. Actually, stroke patients with severe unconscious status are predicted to have worse outcome than stroke patients with mild unconscious status. A very good example is that GCS ≤8 on admission is an independent predictor of 1‐month stroke mortality and the mortality in patients with GCS ≤8 was nearly eight times that of patients with better GCS 15. Further studies are needed to detail the association of GCS score and the efficacy of rt‐PA in stroke patients. Also, a recent study proposed that patients treated with rt‐PA up to 6 h after stroke survived with less disability 9, which supports the need for expanding thrombolysis in the special stroke group beyond 4.5 h. Clarification of these issues may help develop accurate and safe therapeutic strategies for stroke patients accurately and safely.
Conflict of Interest
The authors have no conflict of interest.
Acknowledgments
We thank Li‐Wen Zhang, Zheng‐Xiang Zhang, Peng Zhang, and Hui Li for information collection of patients. We also thank Brad Peterson for revising the article. This study was supported by the National Natural Science Foundation of China (81230026 and 81171085), the National Natural Science Foundation of Jiangsu Province of China (BL2012013), 973 Fund from the Ministry of Science and Technology in China (2009CB521906), the Medical Leading Talent and Innovation Team Project of Jiangsu Province (LJ201101).
The first three authors contributed equally to this work.
References
- 1. Voetsch B, DeWitt LD, Pessin MS, Caplan LR. Basilar artery occlusive disease in the New England Medical Center Posterior Circulation Registry. Arch Neurol 2004;61:496–504. [DOI] [PubMed] [Google Scholar]
- 2. Tsao JW, Hemphill JC 3rd, Johnston SC, Smith WS, Bonovich DC. Initial Glasgow Coma Scale score predicts outcome following thrombolysis for posterior circulation stroke. Arch Neurol 2005;62:1126–1129. [DOI] [PubMed] [Google Scholar]
- 3. Grond M, Rudolf J, Schmulling S, Stenzel C, Neveling M, Heiss WD. Early intravenous thrombolysis with recombinant tissue‐type plasminogen activator in vertebrobasilar ischemic stroke. Arch Neurol 1998;55:466–469. [DOI] [PubMed] [Google Scholar]
- 4. Hacke W, Kaste M, Fieschi C, et al. Randomised double‐blind placebo‐controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European‐Australasian Acute Stroke Study Investigators. Lancet 1998;352:1245–1251. [DOI] [PubMed] [Google Scholar]
- 5. Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue‐type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA 1999;282:2019–2026. [DOI] [PubMed] [Google Scholar]
- 6. Clark WM, Albers GW, Madden KP, Hamilton S. The rtPA (alteplase) 0‐ to 6‐hour acute stroke trial, part A (A0276g): results of a double‐blind, placebo‐controlled, multicenter study. Thromblytic therapy in acute ischemic stroke study investigators. Stroke 2000;31:811–816. [DOI] [PubMed] [Google Scholar]
- 7. Tissue Plasminogen Activator for Acute Ischemic Stroke . The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group. N Engl J Med 1995;333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 8. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–1329. [DOI] [PubMed] [Google Scholar]
- 9. Sandercock P, Wardlaw JM, Lindley RI, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST‐3]): a randomised controlled trial. Lancet [Multicenter Study Randomized Controlled Trial Research Support, Non‐U.S. Gov't] 2012;379:2352–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Larrue V, von Kummer RR, Muller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European‐Australasian Acute Stroke Study (ECASS II). Stroke 2001;32:438–441. [DOI] [PubMed] [Google Scholar]
- 11. Sylaja PN, Dong W, Grotta JC, et al. Safety outcomes of Alteplase among acute ischemic stroke patients with special characteristics. Neurocrit Care 2007;6:181–185. [DOI] [PubMed] [Google Scholar]
- 12. Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010;375:1695–1703. [DOI] [PubMed] [Google Scholar]
- 13. Generalized Efficacy of t‐PA for Acute Stroke . Subgroup analysis of the NINDS t‐PA Stroke Trial. Stroke 1997;28:2119–2125. [DOI] [PubMed] [Google Scholar]
- 14. Bruckmann H, Ferbert A, del Zoppo GJ, Hacke W, Zeumer H. Acute vertebral‐basilar thrombosis. Angiologic‐clinical comparison and therapeutic implications. Acta Radiol Suppl 1986;369:38–42. [PubMed] [Google Scholar]
- 15. Ong TZ, Raymond AA. Risk factors for stroke and predictors of one‐month mortality. Singapore Med J 2002;43:517–521. [PubMed] [Google Scholar]


