Conflict of Interest
The authors have no conflict of interest.
Tardive dyskinesia (TD) is a side effect of long‐term administration of typical neuroleptics such as haloperidol, and is characterized by excessive and involuntary movements of the face, mouth, tongue as well as other parts of the body 1. In contrast, atypical antipsychotic drugs such as clozapine exhibit a reduced risk of TD 2. However, the neurochemical mechanisms that underlie the differential effects between typical and atypical antipsychotic drugs on TD remain unclear 3.
Abnormalities in dopaminergic function, specifically, the supersensitivity of dopamine (DA) receptors, have been demonstrated to involved in the pathophysiology of TD 4. Behavioral evidence of dopamine hypersensitivity is convinced in many different animal models. The up‐regulation of D2 dopamine receptors was thought to be the primary mechanism responsible for the development of behavioral sensitivity 4. Apomorphine (APO), a nonselective D1/D2 agonist, can induce stereotyped behavior and vacuous chewing movements (VCMs) following chronic treatment with neuroleptics and routinely has been used as an experimental model of TD 5.
Aquaporin‐4 (AQP4), a predominant water channel in brain, is highly expressed in perivascular astrocyte endfeet and plays crucial roles in central nervous system 6. AQP4 knockout increases DA synthesis and turnover in various brain regions. Currently, multiple lines of evidence suggest that AQP4 is involved in anomalous dopaminergic neurotransmission‐induced diseases, such as Parkinson's disease 7, addiction 8 and mood disorders. However, the implication of AQP4 in TD remains unclear. In the present study, AQP4 knockout mice (AQP4−/−) were applied to define the roles of AQP4 in TD.
Twelve‐week‐old male AQP4+/+ and AQP4−/− mice were injected with haloperidol (2 mg/kg i.p.), clozapine (10 mg/kg i.p.), or saline once a day for 21 days. Locomotor activities were monitored after the final injections by using ZIL‐2 activity meter (Chinese Academy of Medical Sciences). Mice were placed into activity monitor chambers (20 cm × 20 cm) for 10 min, and then activities were measured at 10‐min intervals. Subsequently, mice received an injection of APO (1 mg/kg, s.c.). Following this injection, the numbers of activities were recorded for the next 10‐min intervals. As shown in Figure 1, both haloperidol and clozapine showed a significant inhibitory effect on locomotor activities in AQP4+/+ and AQP4−/− mice. The typical antipsychotic haloperidol was much more potent in inhibiting locomotor activity than the atypical antipsychotic clozapine in both genotypes of mice (Figure 1A). AQP4 knockout did not influence these behavioral differences in locomotor activity. AQP4+/+ mice displayed marked hyperlocomotion after APO‐stimulation. Notably, AQP4 knockout abolished APO‐induced hyperlocomotion following both chronic neuroleptics and saline treatment (Figure 1B). The results indicate that AQP4 knockout may directly block psychomotor stimulant action of APO.
Figure 1.
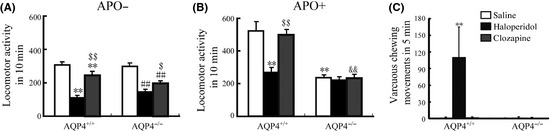
Effect of haloperidol (2 mg/kg, i.p., 21 days) or clozapine (10 mg/kg i.p., 21 days) on spontaneous (A) and apomorphine (APO, 1 mg/kg, s.c.)‐induced (B) locomotor activity and vacuous chewing movements (VCMs) (C) in AQP4+/+ and AQP4−/− mice. The experiment of VCMs was carried out 5 min after a single injection of apomorphine (APO, 1 mg/kg, s.c.). Samples were analyzed by the Student's t‐test for comparison of two groups and analysis of variance (ANOVA) for comparison of multiple groups. P‐values of <0.05 were considered statistically significant. Data present mean ± SEM. n = 14. **P < 0.01 versus saline‐treated AQP4+/+ mice; ## P < 0.01 versus saline‐treated AQP4−/− mice; $$ P < 0.01, $ P < 0.05 versus corresponding haloperidol‐treated mice; && P < 0.01 versus clozapine‐treated AQP4+/+ mice.
Vacuous chewing movements induced by a long‐term neuroleptic administration in rodent animals have been the most extensively used phenomenological animal model of TD5. VCMs were referred to as single mouth openings in the vertical plane not directed toward physical material. The numbers of VCMs were counted three days after the final injections. Mice were placed individually into clear observation cages (16 cm × 30 cm × 19 cm) without food for a 1‐hour habituation period. VCMs were measured in 5‐min intervals 5 min after the injection of APO (1 mg/kg, s.c.). We found AQP4+/+ mice that stimulated by APO displayed a significant increase in VCMs in haloperidol pretreated group, but not in clozapine pretreated group. However, no change of VCMs in respond to APO was observed in AQP4−/− mice following treated with haloperidol, clozapine or saline (Figure 1C). In agreement with previous reports, our results showed that chronic administration of haloperidol produced behavioral supersensitivity to stimulant action of APO. AQP4 knockout abolished APO‐induced VCMs following chronic haloperidol treatment. This result implicates that AQP4 plays an important role in the occurrence of APO‐stimulated TD.
Recent evidence suggests astrocytes may be involved in the pharmacological action of antipsychotic drugs. AQP4 is predominantly expressed in astrocyte. To elucidate the mechanism of AQP4 involved in TD, we observed the effects of haloperidol and clozapine on astrocyte proliferation in medial prefrontal cortex (mPFC) and striatum from AQP4+/+ and AQP4−/− mice. Mice were killed after the final injection of neuroleptics. Mice were perfused with 4% paraformaldehyde (PFA), and brains were dissected and maintained in 4% PFA overnight. Following the gradient dehydration, the brain tissues were sectioned by Leica freezing microtome at 30 μm. Sections were incubated overnight with mouse anti‐GFAP antibody (1:1000; Chemicon), followed by goat anti‐mouse antibody (chemicon, 1:800). Immunoreactivity was visualized by incubation with DAB. GFAP‐positive cells were counted using the Optical Fractionator method with Stereo Investigator software (Microbrightfield, VT, USA) on a Z‐series of sections of 180 μm apart. As shown in Figure 2, haloperidol failed to alter the numbers of astrocytes in mPFC and striatum in two genotypes. In contrast, atypical drug clozapine significantly diminished the number of GFAP‐positive cells in mPFC and striatum in AQP4+/+ mice. AQP4 knockout did not alter the numbers of astrocytes in mPFC and striatum in basal state, but abolished the inhibition on astrocyte in mPFC (Figure 2B) and striatum (Figure 2D) following chronic treatment of clozapine. Glial cells have been suggested to play important roles in the pathophysiology of schizophrenia‐like psychotic disorders. Astrocyte activation was confirmed in schizophrenia patients, and in animal model induced by MK‐801 9, 10. It has been postulated that MK‐801‐induced 5‐HT release and resultant activation of 5‐HT2A receptors can stimulate the release of glutamate from the nerve terminals, which in turn might promote the activation of glial cells. The inhibition of astrocyte proliferation by clozapine may block the 5‐HT2A receptors. AQP4 deficiency reversed suppression on astrocyte, suggesting that AQP4 may be involved in astroglial dysregulation and psychiatric disorders. But the exact mechanisms are in need of further investigations.
Figure 2.
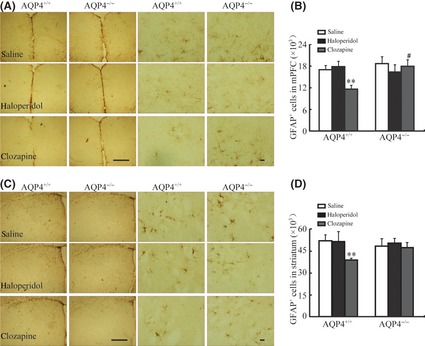
Effect of haloperidol (2 mg/kg, i.p., 21 days) or clozapine (10 mg/kg, i.p., 21 days) on immunohistochemistry of GFAP in medial prefrontal cortex (mPFC) (A) and in striatum (B) of AQP4+/+ and AQP4−/− mice. Scale bar 40 μm for left line and scale bar 200 μm for right line. Quantitative analysis of GFAP + astrocytes in mPFC (C) and in striatum (D). Samples were analyzed by the Student's t‐test for comparison of two groups and analysis of variance (ANOVA) for comparison of multiple groups. P‐values of <0.05 were considered statistically significant. Data present mean ± SEM. n = 4. **P < 0.01 versus saline‐treated AQP4+/+ mice; # P < 0.05 versus haloperidol‐treated AQP4+/+ mice.
In conclusion, we found that AQP4 knockout abolished APO‐induced hyperlocomotion and VCMs following chronic treatment of neuroleptics. These data indicate that AQP4 participates in the sensitivity to APO‐stimulated stereotyped behavior induced by chronic neuroleptic treatment and may contribute to the therapeutic roles and side effects of antipsychotic agents.
Acknowledgments
This study was supported by the grants from the National Key Program of Basic Research of China (No. 2011CB504103), the National Natural Science Foundation of China (No. 30973517), and the National Science & Technology Major Project (No. 2012ZX09304‐001).
References
- 1. Baldessarini RJ, Gardner DM. Incidence of extrapyramidal syndromes and tardive dyskinesia. J Clin Psychopharmacol 2011;31:382–384. [DOI] [PubMed] [Google Scholar]
- 2. Pena MS, Yaltho TC, Jankovic J. Tardive dyskinesia and other movement disorders secondary to aripiprazole. Mov Disord 2011;26:147–152. [DOI] [PubMed] [Google Scholar]
- 3. Nord M, Farde L. Antipsychotic occupancy of dopamine receptors in schizophrenia. CNS Neurosci Ther 2011;17:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perreault ML, O'Dowd BF, George SR. Dopamine receptor homooligomers and heterooligomers in schizophrenia. CNS Neurosci Ther 2011;17:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Turrone P, Remington G, Nobrega JN. The vacuous chewing movement (VCM) model of tardive dyskinesia revisited: is there a relationship to dopamine D(2) receptor occupancy. Neurosci Biobehav Rev 2002;26:361–380. [DOI] [PubMed] [Google Scholar]
- 6. Zeng XN, Xie LL, Liang R, Sun XL, Fan Y, Hu G. AQP4 knockout aggravates ischemia/reperfusion injury in mice. CNS Neurosci Ther 2012;18:388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chi Y, Fan Y, He L, et al. Novel role of aquaporin‐4 in CD4+ CD25+ T regulatory cell development and severity of Parkinson's disease. Aging Cell 2011;10:368–382. [DOI] [PubMed] [Google Scholar]
- 8. Liu L, Lu Y, Kong H, et al. Aquaporin‐4 deficiency exacerbates brain oxidative damage and memory deficits induced by long‐term ovarian hormone deprivation and D‐galactose injection. Int J Neuropsychopharmacol 2012;15:55–68. [DOI] [PubMed] [Google Scholar]
- 9. Weis S, Llenos IC. GFAP‐immunopositive astrocytes in schizophrenia. Schizophr Res 2004;67:293–295. [DOI] [PubMed] [Google Scholar]
- 10. Arif M, Chikuma T, Ahmed MM, Yoshida S, Kato T. Suppressive effect of clozapine but not haloperidol on the increases of neuropeptide‐degrading enzymes and glial cells in MK‐801‐treated rat brain regions. Neurosci Res 2007;57:248–258. [DOI] [PubMed] [Google Scholar]


