Abstract
SUMMARY Purpose: In view of a putative role of oxidative stress in the pathophysiology of seizures, this study addressed the interactions between N‐acetylcysteine (NAC), a potent antioxidant and two antiepileptic drugs sodium valproate (SVP) and phenytoin (PHT) on experimental seizures in mice. Methods: The interaction was studied at three fixed ratio combinations (i.e., 1:1, 1:3, and 3:1) in the mouse maximal electroshock (MES) test using isobolographic analysis. Markers of oxidative stress (reduced glutathione [GSH] and malondialdehyde [MDA]) were estimated in the cortex of mice pretreated with either of these drugs alone or their 3:1 ratio combinations at the experimentally determined ED50 values (ED50 exp values). The grip strength and spontaneous alternation behavior (SAB) were also assessed. In addition, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and calcium levels were estimated. Results: We found an anticonvulsant action of NAC in the MES test. Further, the ED50 exp values for the combinations of PHT and NAC did not differ from the theoretically calculated ED50 values indicating additive effects. In case of SVP and NAC, however, the ED50 exp values were lower than the theoretically calculated ED50 values. The interaction of SVP with NAC at the fixed ratios of 1:3 and 3:1 was found to be synergistic. No significant changes were observed in the grip strength, SAB, cortical GSH and MDA levels, serum AST, ALT, ALP, or calcium levels. Conclusion: Our results thus hold promise for the use of NAC as an adjunct to PHT and SVP therapy.
Keywords: Electroshock seizures, N‐acetylcysteine, Oxidative stress, Phenytoin, Sodium valproate
Introduction
Oxidative stress and mitochondrial dysfunction are increasingly being recognized to have important roles in the pathophysiology of neurological diseases like epilepsy [1, 2, 3]. Reactive oxygen species have been implicated in the initial phases of seizure‐induced pathology [4] and several studies have reported oxidative stress in different brain regions after experimental seizures [5, 6, 7]. The ability of antioxidants to protect against the seizure manifestations and the accompanying biochemical changes further highlights a role of free radicals in seizures [8, 9].
Phenytoin (PHT) and sodium valproate (SVP) are among the widely used first‐line AEDs effective in the treatment of both generalized tonic–clonic (GTC) and focal onset seizures [10]. However, these drugs have a narrow margin of safety and their use in epileptic patients has occasionally been associated with disturbances in the blood antioxidant defense systems and increased lipid peroxidation [11, 12].
N‐acetylcysteine (NAC) is a thiol‐containing compound, which has been used in clinical practice for several years [13]. It has antioxidant properties and a few studies have reported the beneficial effects of NAC administration against lipid peroxidation both in the peripheral tissues and in the central nervous system (CNS) [14, 15]. NAC in high doses has been reported to improve and stabilize the neurological symptoms in patients with Unverricht–Lundborg disease, a type of progressive myoclonic epilepsy in which oxidative stress has been thought to be an important factor [16]. In addition, NAC administration has been reported to reverse the memory impairment in aged SAMP8 mice [17]. In preclinical studies, NAC has shown effectiveness against aminophylline [6] and pentylenetetrazole (PTZ)‐induced seizures [18]. We previously reported a facilitatory action of NAC on the anticonvulsant effects of SVP against the PTZ [18] and the electroconvulsive threshold model [19] of seizures. In view of above, this study was planned to analyze the type of interactions of NAC with PHT and SVP in the mouse maximal electroshock (MES) test and further to investigate the role of oxidative stress in the mediation of above effects. The latter is relevant in view of a role of oxidative stress in seizures and the evidence for the involvement of peroxidative injury in the adverse effects of AEDs. Further, the effects of PHT, SVP, NAC, and their combinations on neuromuscular function, memory, liver enzymes, alkaline phosphatase (ALP), and calcium levels were also investigated.
Methods
Animals
Swiss albino mice of either sex weighing between 24 and34 g, raised at the Central Animal House Facility of Jamia Hamdard were used. The animals were housed in polypropylene cages under controlled environmental conditions. The mice were maintained on a natural light and dark cycle, and had free access to food and water. The Jamia Hamdard Animal Ethics Committee approved the experimental protocol and procedures (Project number: 272).
Drugs
PHT was procured from Abbott, India; SVP from Sanofi Aventis, India; and NAC from Samarth Pharma, India.
Electroconvulsions
Electroconvulsions were produced in mice using a current (25 mA, 0.2 seconds) delivered via ear clip electrodes. Tonic hind limb extension was taken as the endpoint. The protective efficacy of the drugs under investigation (i.e., PHT, SVP, and NAC) was determined as their ability to protect 50% of mice against the MES‐induced tonic hind limb extension and expressed as respective median effective dose (ED50) values with 95% confidence limits [20].
Isobolographic Analysis
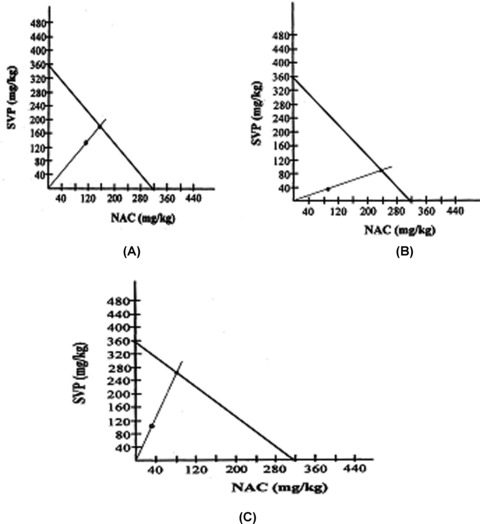
The type of drug interactions between antiepileptic drugs (AEDs) and NAC were determined using isobolographic analysis. The following dose ratios were used, i.e., 1:1, 1:3 and 3:1. Theoretical additive (ED50 add) and experimental (ED50 exp) values were determined for theoretic additivity and administered combination, respectively in the MES test in mice. The following equation was used to determine the ED50 add values: (ED50 add) = f1× (ED50) drug 1+ f2× (ED50) drug 2, where f1 is a fraction of drug 1 and f2 is a fraction of drug 2 in the total amount of drug combination. Each ED50 exp value was obtained from at least four to five groups of mice (10 animals per group) administered with different amounts of drugs at fixed ratio combination. From the dose–response curves of the combined drugs, the ED50 exp values with their standard error of mean (SEM) values were determined [20]. The SEM for the respective ED50 values was determined using the following equations: SEM (ED50) = 2.3 × (ED50) × SEM (log [ED50]) where logarithm is to base 10
SEM (log [ED50]) = s/sqrt (N′/2), where s is the difference between two log doses whose expected effects differ by 1 probit; N′ is the total number of animals tested between the log dose limits corresponding to expected probits 4 and 6; and sqrt is the square root of the expression in parentheses. The SEM for the ED50 add for the respective fixed fraction of drug dose combination was determined as follows:
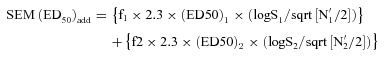 |
The interaction index, which indicates the strength of the interaction, was calculated as follows: interaction index = ED50 exp/ ED50 add. To visualize the type of interactions, the isoboles were drawn by plotting the points reflecting the respective doses of NAC (on the x‐axis) and doses of AEDs (i.e., PHT, SVP) on the y‐axis. The straight line connecting the ED50 values for the two tested drugs, administered alone, against the MES test, represents the theoretic isobole for the additive effect. If experimentally determined data points are placed on this line, then the drug effects are additive. When the points reflecting combinations of various fixed ratios are significantly below this line, the two component drugs act synergistically. Conversely, antagonism may be recognized if these points are located above the additive isobole.
Grip Strength and Spontaneous Alternation Behavior
Grip Strength was measured with the help of a grip strength meter [Panlab, Barcelona, Spain; 21]. In brief, the mouse was allowed to grip wire mesh of the apparatus by its front paws. The strength was recorded digitally in the instrument. For spontaneous alternation behavior, the method as described previously [21, 22] was employed. The mice were allowed to traverse a cross‐maze for 6 min. The number and sequence of arm entries were then recorded. An alternation was defined as entry into four different arms on overlapping quintuple sets. Five consecutive arm choices within the total set of arm choices made up a quintuple set. The percentage alternation score = (actual alternation/possible alternation) × 100, where possible alternations are number of arm entries minus 4.
Estimation of Tissue Glutathione (GSH) and Malondialdehyde (MDA)
GSH levels were estimated using a spectrophotometric procedure based on the method of Ellman [23]. Lipid peroxidation was assayed with the aid of thiobarbituric acid following the method of Konings and Drijver [24].
Estimation of Serum Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), ALP, and Calcium
The estimation of serum ALT and AST was based on the method of Reitman and Frankel [25] and was performed using a kit from Span Diagnostics, India. The estimation of serum ALP was done using a kit based on the method of Kind and King [26]. Calcium estimations were carried out using a reagent kit employing ortho‐cresolphthalein complexone as the color‐developing agent [27].
Statistical Analysis
In interaction studies, Student's t‐test was used to compare statistical significance between the ED50 add value and the ED50 exp value. An ED50 exp value significantly less than the ED50 add value was considered to indicate a synergistic interaction between the drugs used in combination. The results of grip strength test, spontaneous alternation behavior, and all biochemical parameters were analyzed by one‐way analysis of variance (ANOVA), Kruskal–Wallis test, or unpaired t‐test, wherever appropriate. The percentage incidence of seizures was analyzed using Fisher's exact test. P < 0.05 was considered significant.
Results
Anticonvulsant Effects of PHT, SVP, and NAC Administered Alone in the MES Test in Mice
The respective ED50 values for PHT, SVP, and NAC against MES‐induced seizures were found to be 10 mg/kg, 354.8 mg/kg, and 316.2 mg/kg.
Anticonvulsant Activity of the Combinations of PHT and NAC in the MES Test in Mice and Isobolographic Analysis of Interactions
The combinations of PHT and NAC at the three fixed ratios of 1:1, 1:3, and 3:1 exerted potent anticonvulsant activities in the mouse MES test (Table 1). With isobolographic analysis, it was observed that all combinations tested between PHT and NAC, i.e., at the fixed ratios of 1:1, 1:3, and 3:1 displayed additive interactions. The ED50 exp values for the three fixed ratios of 1:1, 1:3, and 3:1 were lower than the corresponding ED50 add values. However, the difference between the ED50 exp and the ED50 add values for the different ratios were not statistically significant, thus displaying only a slight tendency toward supraadditivity. The interaction index values are shown in Table 1. The isobolograms depicting the type of interactions between PHT and NAC at the three fixed ratios of 1:1, 1:3, and 3:1 are shown in Figure 1(A)–(C), respectively.
Table 1.
Anticonvulsant activity and analysis of interaction of the combinations of PHT and NAC in the MES test in mice
| FR (n = 10) | PHT (mg/kg) | NAC (mg/kg) | % Protection | % Mortality | ED50 add | ED50 exp | Interaction index |
|---|---|---|---|---|---|---|---|
| 1:1 | 1.25 | 40 | 20 | 20 | 163.1 ± 26.72 | 112.2 ± 33.55 | 0.69 |
| 2.5 | 80 | 60 | 10 | ||||
| 5 | 160 | 70 | 0 | ||||
| 10 | 320 | 80 | 0 | ||||
| 1:3 | 0.625 | 60 | 30 | 0 | 239.7 ± 38.81 | 177.8 ± 81.79 | 0.74 |
| 1.25 | 120 | 50 | 0 | ||||
| 2.5 | 240 | 60 | 0 | ||||
| 5 | 480 | 70 | 0 | ||||
| 3:1 | 1.875 | 20 | 10 | 0 | 86.5 ± 14.63 | 56.2 ± 14.22 | 0.65 |
| 3.75 | 40 | 30 | 0 | ||||
| 7.5 | 80 | 70 | 0 | ||||
| 15 | 160 | 90 | 0 |
Data are presented as median effective dose (ED50) values (in mg/kg) ± SEM.
Statistical analysis was performed with Student's t‐test.
n, number of animals per experimental group; PHT, phenytoin; NAC, N‐acetylcysteine; MES, maximal electroshock; ED50 add, theoretical additive ED50 value; ED50 exp, experimentally determined ED50 value.
Figure 1.
Isobologram displaying the type of interaction between phenytoin (PHT) and N‐acetylcysteine (NAC) at the fixed ratio of 1:1(A), 1:3(B), 3:1(C) in the mouse maximal electroshock model. The ED50 values for NAC and PHT are plotted on the x‐ and y‐axes, respectively.
Anticonvulsant Activity of the Combinations of SVP and NAC in the MES Test in Mice and Isobolographic Analysis of Interactions
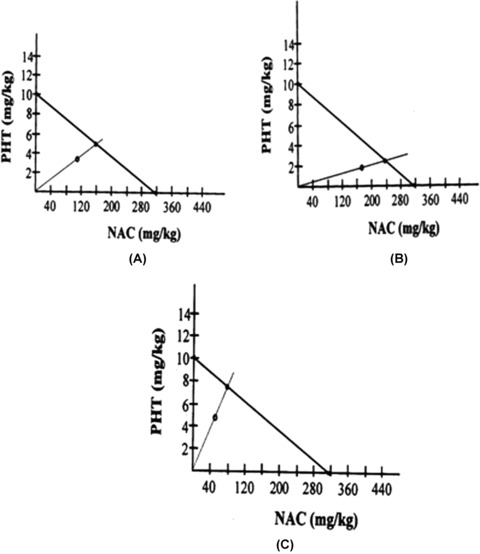
The combinations of SVP and NAC at the three fixed ratios of 1:1, 1:3, and 3:1 exerted clear‐cut anticonvulsant activities in the mouse MES test (Table 2). The combination of SVP with NAC at the fixed ratio of 1:1 exerted an additive interaction because the difference between the ED50 exp value and the ED50 add value was not statistically significant. However, the remaining combinations of SVP and NAC (i.e., at the fixed ratios of 1:3 and 3:1) exerted synergistic interactions. The interaction index values for the combinations of SVP and NAC are shown in Table 2. The isobolograms depicting the type of interactions between SVP and NAC at the three fixed ratios of 1:1, 1:3, and 3:1 are shown in Figure 2(A)–(C), respectively.
Table 2.
Anticonvulsant activity and analysis of interaction of the combinations of SVP and NAC in the MES test in mice
| FR (n = 10) | SVP (mg/kg) | NAC (mg/kg) | % Protection | % Mortality | ED50 add | ED50 exp | Interaction index |
|---|---|---|---|---|---|---|---|
| 1:1 | 45 | 40 | 20 | 10 | 335.5 ± 74.42 | 251.2 ± 69.33 | 0.75 |
| 90 | 80 | 50 | 10 | ||||
| 180 | 160 | 60 | 0 | ||||
| 360 | 320 | 80 | 0 | ||||
| 1:3 | 11.25 | 30 | 30 | 0 | 325.9 ± 62.66 | 125.9 ± 43.44* | 0.39 |
| 22.5 | 60 | 40 | 10 | ||||
| 45 | 120 | 60 | 0 | ||||
| 90 | 240 | 70 | 10 | ||||
| 3:1 | 67.5 | 20 | 30 | 20 | 345.1 ± 86.17 | 141.2 ± 45.47* | 0.41 |
| 135 | 40 | 60 | 0 | ||||
| 270 | 80 | 70 | 0 | ||||
| 540 | 160 | 90 | 0 |
Data are presented as median effective dose (ED50) values (in mg/kg) ± SEM.
Statistical analysis was performed with Student's t‐test.
n, number of animals per experimental group; SVP, Sodium valproate; NAC, N‐acetylcysteine; MES, maximal electroshock; ED50 add, theoretical additive ED50 value; ED50 exp, experimentally determined ED50 value.
*P < 0.05 between theoretical additive and experimental ED50 values.
Figure 2.
Isobologram displaying the type of interaction between sodium valproate (SVP) and N acetylcysteine (NAC) at the fixed ratio of 1:1(A), 1:3(B), 3:1(C) in the mouse maximal electroshock model. The ED50 values for NAC and SVP are plotted on the x‐ and y‐axes, respectively.
Effects of PHT, SVP, NAC and Their Combinations Alone and in the Presence of Electroshock on Cortical GSH and MDA Levels
The cortical GSH and MDA content of various groups are depicted in Table 3. In case of electroshock treated animals, no change in cortical GSH and MDA content was observed as compared to the control group. Compared to the control group, a significant reduction in cortical MDA level was observed in the group that was treated with PHT (10 mg/kg; KW = 48.098, P < 0.0001, Kruskal–Wallis ANOVA; P < 0.001, Dunn's Multiple Comparisons Test). The administration of SVP (110 mg/kg) and NAC (33 mg/kg) also produced a significant reduction in the cortical GSH (KW = 48.242, P < 0.0001, Kruskal–Wallis ANOVA; P < 0.01, Dunn's Multiple Comparisons Test) and MDA levels (P < 0.001, Dunn's Multiple Comparisons Test). However, none of the other treatments produced any significant effect on the cortical GSH and MDA levels except the groups that were pretreated with either PHT (10 mg/kg) or SVP + NAC (110 + 33 mg/kg) before electroshock. Although the former treatment produced a significant reduction in the cortical MDA levels (P < 0.001, Dunn's Multiple Comparisons Test), the latter significantly lowered the cortical GSH levels (P < 0.01, Dunn's Multiple Comparisons Test) compared to the control group.
Table 3.
Effects of PHT, SVP, NAC and their combinations alone and in the presence of electroshock on cortical GSH, MDA levels and serum ALT, AST, ALP, calcium levels
| Treatment | GSH (ng/mg protein) | MDA (nM/mg protein) | ALT (IU/L) | AST (IU/L) | ALP (KA Units) | Calcium (mg/dL) |
|---|---|---|---|---|---|---|
| Control | 4474 ± 125 | 3.46 ± 0.10 | 10.8 ± 2.82 | 40 ± 3.60 | 13.8 ± 1.38 | 8.2 ± 0.31 |
| Electroshock (ES) | 3580 ± 350 | 2.67 ± 0.12 | 12.6 ± 2.64 | 73.4 ± 8.49 | 18.8 ± 1.23 | 8.9 ± 0.18 |
| PHT (10) | 3808 ± 153 | 2.19 ± 0.01*** | 15.4 ± 2.17 | 74.6 ± 11.1 | 21.6 ± 1.44 | 9.4 ± 0.41 |
| SVP (360) | 4585 ± 123 | 2.88 ± 0.01 | 11.7 ± 2.37 | 49.4 ± 7.02 | 15.1 ± 0.29 | 10.8 ± 0.46** |
| NAC (320) | 4144 ± 100 | 2.64 ± 0.01 | 12.6 ± 2.34 | 48.3 ± 6.22 | 19.8 ± 1.59 | 9.4 ± 0.37 |
| PHT (5) + NAC (54) | 3458 ± 212 | 2.85 ± 0.13 | 11.4 ± 2.75 | 59.7 ± 8.41 | 20.1 ± 1.54 | 8.9 ± 0.27 |
| SVP (110) + NAC (33) | 3284 ± 110** | 2.18 ± 0.13*** | 9.4 ± 1.89 | 50.6 ± 3.82 | 17.7 ± 2.58 | 8.1 ± 0.13 |
| PHT (10) + ES | 3753 ± 244 | 2.17 ± 0.15*** | 13.1 ± 2.26 | 68.8 ± 8.01 | 17.9 ± 2.28 | 9.2 ± 0.32 |
| SVP (360) + ES | 4067 ± 140 | 3.06 ± 0.12 | 10 ± 2.18 | 63.7 ± 7.92 | 15.8 ± 1.62 | 10.1 ± 0.40 |
| NAC (320) + ES | 4468 ± 87 | 2.63 ± 0.11 | 13.4 ± 2.46 | 51.4 ± 6.07 | 17 ± 1.0 | 9 ± 0.22 |
| PHT (5) + NAC (54) + ES | 3544 ± 187 | 2.83 ± 0.12 | 10.8 ± 1.62 | 64.8 ± 8.39 | 23.9 ± 1.48* | 9.8 ± 0.19 |
| SVP (110) + NAC (33) + ES | 3121 ± 304** | 2.65 ± 0.01 | 8 ± 1.31 | 60.8 ± 5.98 | 20 ± 1.66 | 8.4 ± 0.16 |
Data are presented as mean ± SEM. Values in parentheses represent the dose in mg/kg, p.o. Treatment duration = 7 days. Number of animals per group = 7.
*P < 0.05, **P < 0.01 and ***P < 0.001 compared to control group. The results were analyzed with Kruskal–Wallis nonparametric ANOVA test followed by the post hoc Dunn's test.;
PHT, phenytoin; SVP, sodium valproate; NAC, N‐acetylcysteine; GSH, reduced glutathione; MDA, malondialdehyde; ALT, alanine aminotransferase; AST, aspartate aminotransferase; IU, International units; ALP, alkaline phosphatase; KA units, King Armstrong units.
Effects of PHT, SVP, NAC and Their Combinations Alone and in the Presence of Electroshock on Serum ALT and AST Levels
The results are presented in Table 3. None of the drug treatments at the dose levels investigated in this study affected the ALT (KW = 8.078, P= 0.7063) and AST activities (F [11,72]= 2.182, P= 0.0247, Tukey–Kramer Multiple Comparisons Test) significantly.
Effects of PHT, SVP, NAC and Their Combinations Alone and in the Presence of Electroshock on Serum ALP and Calcium Levels
Electroshock treatment caused a mild (nonsignificant) elevation in the serum ALP level. Compared to the normal control, a significant increase in the serum ALP level was observed in the group that was pretreated with the combination of PHT (5 mg/kg) and NAC (54 mg/kg) and subjected to electroshock (KW = 30.644, P= 0.0013, Kruskal–Wallis ANOVA; P < 0.05, Dunn's Multiple Comparisons Test). The serum calcium levels were not altered significantly after electroshock treatment. One‐week treatment with PHT (10 mg/kg), SVP (360 mg/kg), and NAC (320 mg/kg) caused an elevation in the serum calcium levels, however a significant increase was observed only after SVP treatment (KW = 42.655, P < 0.0001, Kruskal–Wallis ANOVA; P < 0.01, Dunn's Multiple Comparisons Test). None of the other treatments modified the serum ALP and calcium levels significantly (Table 3).
Discussion
The epilepsies are common CNS disorders that sometimes require a combined therapy, especially for patients with refractory seizures inadequately controlled with monotherapy. The primary aim of using such combinations is to enhance the efficacy and to minimize the adverse effects. At the preclinical stage, experimental seizure models not only provide an invaluable means of identifying potentially useful anticonvulsant agents but also aid in the bioevaluation of combinations of AEDs, which is of pivotal importance for their subsequent clinical application. In this study, the MES test was used because it is one of the standardized and most validated experimental models of GTC seizures [28]. Although PHT and SVP are the first‐line AEDs used in the management of GTC seizures [10], we had earlier found a facilitatory action of NAC, a potent antioxidant, on the anticonvulsant effects of SVP against the electroconvulsive threshold model of seizures in mice [19]. This work was, thus, undertaken to characterize the nature of interaction between standard AEDs (i.e., PHT, SVP) and NAC in the mouse MES test using isobolographic analysis. The latter is considered to be the optimal method to detect drug interactions of AEDs in animal models of epilepsy [29].
PHT, SVP, and NAC produced clear‐cut anticonvulsant effects against MES‐induced seizures in mice. The results obtained in this study with PHT and SVP are in accordance with the established evidence of the effectiveness of these agents against MES‐induced seizures [20, 30, 31]. However, ours is probably the first experimental evidence of an anticonvulsant activity being reported with NAC against MES‐induced seizures in mice. Our data further confirms the anticonvulsant properties of NAC that have been reported in other models of seizures viz aminophylline [6] and PTZ‐induced [19] seizures. Even clinically, NAC has been reported to exhibit beneficial effects in refractory epilepsies like progressive myoclonic epilepsies where other classical drugs like clonazepam, valproate, and zonisamide have failed to improve the manifestations of the disease [16].
In this study, PHT and NAC displayed additive interactions with a slight tendency toward supraadditivity for all the three fixed ratios (i.e., 1:1, 1:3, and 3:1) against MES‐induced seizures in mice. Isobolographic analysis of the interaction of SVP with NAC at the fixed ratio of 1:1 also revealed an additive interaction. However, SVP seems to act synergistically with NAC at the fixed ratios of 1:3 and 3:1 because the ED50 exp values were significantly lower than the ED50 addvalues. These results reveal beneficial pharmacodynamic interactions between AEDs (i.e., PHT, SVP) and NAC for the prevention of electroshock‐induced seizures.
PHT, SVP, NAC (ED50 doses), and their 3:1 fixed ratio combinations (at the ED50 exp values) did not produce any significant changes either in the grip strength or the spontaneous alternation behavior. This reflects that the doses employed were devoid of any adverse effects on the neuromuscular function and spatial memory.
Several lines of evidence including those from a variety of experimental models suggest a possible involvement of oxidative stress in the pathophysiology of epilepsy [1, 6]. However, with respect to electrically induced seizures, there are conflicting reports in literature. While Rola et al. [5] had found a significant increase in the levels of MDA in brain tissue of mice immediately after electroconvulsions, Barichello et al. [32] reported a significant decrease in the thiobarbituric acid reactive species in the hippocampus with no significant changes in the cortex, striatum, and cerebellum of rats immediately after a single electroconvulsive shock. Recently, Nieoczym et al. [33] too have reported a significant reduction in the lipid peroxidation intensity in the brain tissue of mice submitted to MES and decapitated 3 min after the electroshock. In our study, we did not find any significant change in GSH and MDA levels after electroshock seizures. Hence, the study doesn't provide support for activation of protective endogenous mechanisms immediately after seizures.
One‐week treatment with PHT alone significantly lowered the cortical MDA levels, thus signifying its neuroprotective potential, which is well documented by other workers [34, 35] have reported an increase in the GSH levels in primary cultured rat cerebral cortical cells after one‐week treatment with 0.6 mM valproate. In this study, however, we did not find any significant change in the cortical GSH levels after one‐week treatment with SVP alone. The nonsignificant changes in the cortical MDA levels observed in this study after treatment with SVP (360 mg/kg) and NAC (320 mg/kg) alone are in agreement with the findings of other investigators [15, 36, 37]. However, Kamboj et al. [15] have reported significant increases in the GSH levels in different brain regions including cerebral cortex of rats after NAC administration for 28 days. In our study, cortical GSH levels were not altered significantly after one‐week treatment with NAC alone. The difference in the duration of drug treatment might be an important variable responsible for the observed effects. Combinations of PHT and SVP with NAC (at the fixed ratio of 3:1) lowered the cortical GSH and MDA levels, with the latter combination exerting a statistically significant effect. These results reflect that probably the reduction in MDA levels may be a consequence of GSH utilization. However, compared to the electroshock group, none of the drugs alone or their combinations at their respective ED50 doses against MES‐induced seizures altered the cortical GSH and MDA levels significantly. Thus none of the drug treatments seem to adversely modulate the body's endogenous protective mechanisms. Although the results reveal the sensitizing effects of NAC on the anticonvulsant effects of PHT and SVP, the real mechanism(s) for these observed effects are at present not clearly understood.
Electroshock treatment caused an elevation in the ALT and AST levels compared to the control group; however the effect was not statistically significant. These findings are in accordance with our earlier observations in the PTZ‐induced model of seizures [18] and indicate the ability of seizures to produce mild stress on the liver. Akbas et al. [38] have reported enhanced oxidative stress in the liver of rats administered a convulsive dose of PTZ. Even clinically, fulminant hepatic failure and hepatomegaly have been considered as rare complications of seizures [39, 40]. One‐week treatment with PHT (10 mg/kg), SVP (360 mg/kg), and NAC (320 mg/kg) did not modify the serum ALT and AST levels significantly. Our results with SVP and NAC are in accordance with our earlier observations [18] and those of other investigators [41, 42, 43]. When PHT and SVP were administered in combination with NAC at doses corresponding to their ED50 exp values for the fixed ratio of 3:1, no significant alterations in serum ALT and AST levels were observed. Even in the groups that were pretreated with either of these drugs or their combinations and subjected to electroshock, no significant alterations in serum ALT and AST levels were evident. These results indicate that at optimal dose levels, the adverse effects of AEDs on liver function are rare. This is similar to the observations in the clinical settings where also only long‐term treatment with AEDs is occasionally associated with transient alterations of hepatic enzyme levels [44, 45, 46].
The mild although statistically nonsignificant, elevation in serum ALP levels after electroshock treatment might be because of the mild stress caused by electroshock on the liver. In the group that was pretreated with the combination of PHT (5 mg/kg) and NAC (54 mg/kg) and subjected to electroshock, a significant elevation in serum ALP activity was observed compared to the normal control group. This biochemical change may well be because of the influence of electroshock on liver because treatment with the combination of PHT and NAC alone was devoid of any significant effects on ALP activity. However, no such significant effects were observed in the group that was pretreated with the combination of SVP and NAC and subjected to electroshock, indicating that NAC may be a better adjunct with SVP than PHT.
Body electrolytes play a pivotal role in the development of seizure conditions [47]. Oladipo et al. [48] have reported significantly lower levels of serum calcium in epileptic children compared to the controls. However, in this study, no significant changes were observed in the serum calcium levels after electroshock treatment. This finding bears similarity to the observations of Papavasiliou et al. [49] and Hamed et al. [12]. Although the former had found nonsignificant alterations in serum calcium levels in patients with severe affective illness who had received electroconvulsive therapy, the observations of the latter were made in untreated epileptics. One‐week treatment with SVP (360 mg/kg) alone significantly elevated the serum calcium levels compared to the control group, however no significant differences in the levels of serum calcium were observed in the groups that received either PHT (10 mg/kg) or NAC (320 mg/kg). Our results with SVP, PHT, and NAC are in accordance with other clinical and experimental reports [50, 51]. Even in the groups that were pretreated with either of these drugs and subjected to electroshock, no significant alterations in serum calcium were seen. These results reveal that PHT and SVP, when used at optimal dose levels, can protect against seizures without altering calcium homeostasis. Clinically also, there are a few reports where no significant differences in serum calcium levels have been found in epileptic patients receiving either PHT or SVP [52, 53]. Even in the combination groups (at the fixed ratio of 3:1), no significant alterations in serum calcium levels were observed. These results indicate that NAC as an adjunct with either PHT or SVP might not produce any adverse effects on the serum calcium homeostasis, which may be an added advantage.
To conclude, our results suggest an inherent anticonvulsant activity of NAC, but also highlight the sensitizing action of NAC on the antiepileptic effects of PHT and SVP against MES‐induced seizures. Further, the combinations (at the dose ratio tested) produced minimal adverse effects on neuromuscular function, memory, and liver function. The serum calcium homeostasis was also not significantly affected. Our results thus hold promise for the use of NAC as an adjunct to PHT and SVP therapy. However, further pharmacological and biochemical investigations are required to understand the molecular mechanisms responsible for the beneficial effects.
Conflict of Interest
The authors state no conflicts of interest.
Acknowledgment
This work was supported by All India Council for Technical Education (AICTE) under the scheme Career Award for Young Teachers.
References
- 1. Patel M. Mitochondrial dysfunction and oxidative stress: Cause and consequence of epileptic seizures. Free Radic Biol Med 2004;37:1951–1962. [DOI] [PubMed] [Google Scholar]
- 2. Gao J, Chi ZF, Liu XW, Shan PY, Wang R. Mitochondrial dysfunction and ultrastructural damage in the hippocampus of pilocarpine‐induced epileptic rat. Neurosci Lett 2007;411:152–157. [DOI] [PubMed] [Google Scholar]
- 3. Devi PU, Manocha A, Vohora D. Seizures, antiepileptics, antioxidants and oxidative stress: An insight for researchers. Expert Opin Pharmacotherapy 2008;9:3169–3177. [DOI] [PubMed] [Google Scholar]
- 4. Bruce AJ, Baudry M. Oxygen free radicals in rat limbic structures after kainate‐induced seizures. Free Radic Biol Med 1995;18:993–1002. [DOI] [PubMed] [Google Scholar]
- 5. Rola R, Swiader M, Czuczwar SJ. Electroconvulsions elevate the levels of lipid peroxidation products in mice. Pol J Pharmacol 2002;54:521–524. [PubMed] [Google Scholar]
- 6. Gulati K, Ray A, Pal G, Vijayan VK. Possible role of free radicals in theophylline‐induced seizures in mice. Pharmacol Biochem Behav 2005;82:241–245. [DOI] [PubMed] [Google Scholar]
- 7. Tejada S, Sureda A, Roca C, Gamundí A, Esteban S. Antioxidant response and oxidative damage in brain cortex after high dose of pilocarpine. Brain Res Bull 2007;71:372–375. [DOI] [PubMed] [Google Scholar]
- 8. Barros DO, Xavier SML, Barbosa CO, et al Effects of the vitamin E in catalase activities in hippocampus after status epilepticus induced by pilocarpine in Wistar rats. Neurosci Lett 2007;416:227–230. [DOI] [PubMed] [Google Scholar]
- 9. Xavier SM, Barbosa CO, Barros DO, Silva RF, Oliveira AA, Freitas RM. Vitamin C antioxidant effects in hippocampus of adult Wistar rats after seizures and status epilepticus induced by pilocarpine. Neurosci Lett 2007;420:76–79. [DOI] [PubMed] [Google Scholar]
- 10. Lowenstein DH. Seizures and epilepsy In: Fauci AS, Martin JB, Braunwald E, et al editors. Harrison's principles of internal medicine, 14th ed. New York : McGraw‐Hill, 1998; 2315‐‐2316. [Google Scholar]
- 11. Mahle C, Dasgupta A. Decreased total antioxidant capacity and elevated lipid hydroperoxide concentrations in sera of epileptic patients receiving phenytoin. Life Sci 1997;61:437–443. [DOI] [PubMed] [Google Scholar]
- 12. Hamed SA, Abdullah MM, El‐Melegy N. Blood levels of trace elements, electrolytes, and oxidative stress/antioxidant systems in epileptic patients. J Pharmacol Sci 2004;96:465–473. [DOI] [PubMed] [Google Scholar]
- 13. Moldeus P, Cotgreave IA. N‐acetylcysteine. Methods Enzymol 1994;234:482–492. [DOI] [PubMed] [Google Scholar]
- 14. Demir S, Inal‐Erden M. Pentoxifylline and N‐acetylcysteine in hepatic ischemia/reperfusion injury. Clin Chim Acta 1998;275:127–135. [DOI] [PubMed] [Google Scholar]
- 15. Kamboj A, Kiran R, Sandhir R. N‐acetylcysteine ameliorates carbofuran‐induced alterations in lipid composition and activity of membrane bound enzymes. Mol Cell Biochem 2006a;286:107–114. [DOI] [PubMed] [Google Scholar]
- 16. Ben‐Menachem E, Kyllerman M, Marklund S. Superoxide dismutase and glutathione peroxidase function in progressive myoclonus epilepsies. Epilepsy Res 2000;40:33–39. [DOI] [PubMed] [Google Scholar]
- 17. Farr SA, Poon HF, Dogrukol‐AK D, et al The antioxidants alpha‐lipoic acid and N‐acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem 2003;84:1173–1183. [DOI] [PubMed] [Google Scholar]
- 18. Devi PU, Pillai KK, Vohora D. Modulation of pentylenetetrazole‐induced seizures and oxidative stress parameters by sodium valproate in the absence and presence of N‐acetylcysteine. Fundam Clin Pharmacol 2006a;20:247–253. [DOI] [PubMed] [Google Scholar]
- 19. Devi PU, Pillai KK, Vohora D. Facilitation action of N‐acetylcysteine on the anticonvulsant effect of sodium valproate in mice. Basic Clin Pharmacol Toxicol 2006b;98:521–522. [DOI] [PubMed] [Google Scholar]
- 20. Luszczki JJ, Borowicz KK, Swiader M, Czuczwar SJ. Interactions between oxcarbazepine and conventional antiepileptic drugs in the maximal electroshock test in mice: An isobolographic analysis. Epilepsia 2003;44:489–499. [DOI] [PubMed] [Google Scholar]
- 21. Ali A, Ahmad FJ, Pillai KK, Vohora D. Evidence of the antiepileptic potential of amiloride with neuropharmacological benefits in rodent models of epilepsy and behavior. Epilepsy Behav 2004;5:322–328. [DOI] [PubMed] [Google Scholar]
- 22. Vohora D, Pal SN, Pillai KK. Modulation of spontaneous alternation performance of mice treated with thioperamide and tacrine in a cross maze task. Fundam Clin Pharmacol 2005;19:531–532. [DOI] [PubMed] [Google Scholar]
- 23. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82:70–77. [DOI] [PubMed] [Google Scholar]
- 24. Konings AWT, Drijver EB. Radiation effects on membranes. I. Vitamin E deficiency and lipid peroxidation. Radiat Res 1979;80:494–501. [PubMed] [Google Scholar]
- 25. Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 1957;28:56–63. [DOI] [PubMed] [Google Scholar]
- 26. Kind PR, King EJ. Estimation of plasma phosphatase by determination of hydrolysed phenol with aminoantipyrine. J Clin Pathol 1954;7:322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pollard FH, Martin JV. The spectrophotometric determination of the alkaline‐earth metals with murexide, eriochrome black T and with o‐cresolphthalein complexone. Analyst 1956;81:348–353. [Google Scholar]
- 28. Loscher W, Fassbender CP, Nolting B. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. II. Maximal electroshock seizure models. Epilepsy Res 1991;8:79–94. [DOI] [PubMed] [Google Scholar]
- 29. Deckers CL, Czuczwar SJ, Hekster YA, et al Selection of antiepileptic drug polytherapy based on mechanisms of action: The evidence reviewed. Epilepsia 2000;41:1364–1374. [DOI] [PubMed] [Google Scholar]
- 30. Loscher W, Schmidt D. Which animal models should be used in the search for new antiepileptic drugs? A proposal based on experimental and clinical considerations. Epilepsy Res 1988;2:145–181. [DOI] [PubMed] [Google Scholar]
- 31. Joseph S, David J, Joseph T. Additive anticonvulsant effect of flunarizine and sodium valproate on electroshock and chemoshock induced seizures in mice. Indian J Physiol Pharmacol 1998;42:383–388. [PubMed] [Google Scholar]
- 32. Barichello T, Bonatto F, Agostinho FR, et al Structure‐related oxidative damage in rat brain after acute and chronic electroshock. Neurochem Res 2004;29:1749–1753. [DOI] [PubMed] [Google Scholar]
- 33. Nieoczym D, Albera E, Kankofer M, Wlaz P. Maximal electroshock induces changes in some markers of oxidative stress in mice. J Neural Transm 2008;115:19–25. [DOI] [PubMed] [Google Scholar]
- 34. Ates O, Cayli SR, Gurses I, et al Do sodium channel blockers have neuroprotective effect after onset of ischemic insult? Neurol Res 2007;29:317–323. [DOI] [PubMed] [Google Scholar]
- 35. Cui J, Shao L, Young LT, Wang JF. Role of glutathione in neuroprotective effects of mood stabilizing drugs lithium and valproate. Neuroscience 2007;144:1447–1453. [DOI] [PubMed] [Google Scholar]
- 36. Frey BN, Valvassori SS, Reus GZ, et al Effects of lithium and valproate on amphetamine‐induced oxidative stress generation in an animal model of mania. J Psychiatry Neurosci 2006;31:326–332. [PMC free article] [PubMed] [Google Scholar]
- 37. Kamboj A, Kiran R, Sandhir R. Carbofuran‐induced neurochemical and neurobehavioral alterations in rats: Attenuation by N‐acetylcysteine. Exp Brain Res 2006b;170:567–575. [DOI] [PubMed] [Google Scholar]
- 38. Akbas SH, Yegin A, Ozben T. Effect of pentylenetetrazole‐induced epileptic seizure on the antioxidant enzyme activities, glutathione and lipid peroxidation levels in rat erythrocytes and liver tissues. Clin Biochem 2005;38:1009–1014. [DOI] [PubMed] [Google Scholar]
- 39. Decell MK, Gordon JB, Silver K, Meagher‐Villemure K. Fulminant hepatic failure associated with status epilepticus in children: Three cases and a review of potential mechanisms. Intensive Care Med 1994;20:375–378. [DOI] [PubMed] [Google Scholar]
- 40. Ichai P, Huguet E, Guettier C, et al Fulminant hepatitis after grand mal seizures: Mechanisms and role of liver transplantation. Hepatology 2003;38:443–451. [DOI] [PubMed] [Google Scholar]
- 41. Loscher W, Wahnschaffe U, Honack D, Wittfoht W, Nau H. Effects of valproate and E‐2‐en‐valproate on functional and morphological parameters of rat liver. I. Biochemical, histopathological and pharmacokinetic studies. Epilepsy Res 1992;13:187–198. [DOI] [PubMed] [Google Scholar]
- 42. Korkmazer N, Vurucu S, Demirkaya E, Unay B, Kul M, Akin R, Gokcay E. Serum and liver tissue biotinidase enzyme activity in rats which were administered valproic acid. Brain Dev 2006;28:515–520. [DOI] [PubMed] [Google Scholar]
- 43. Lee MH, Kim M, Lee BH, et al Subchronic effects of valproic acid on gene expression profiles for lipid metabolism in mouse liver. Toxicol Appl Pharmacol 2008;226:271–284. [DOI] [PubMed] [Google Scholar]
- 44. Aiges HW, Daum F, Olson M, Kahn E, Teichberg S. The effects of phenobarbital and diphenylhydantoin on liver function and morphology. J Pediatr 1980;97:22–26. [DOI] [PubMed] [Google Scholar]
- 45. Verma NP, Haidukewych D. Differential but infrequent alterations of hepatic enzyme levels and thyroid hormone levels by anticonvulsant drugs. Arch Neurol 1994;51:381–384. [DOI] [PubMed] [Google Scholar]
- 46. Ahmed SN, Siddiqi ZA. Antiepileptic drugs and liver disease. Seizure 2006;15:156–164. [DOI] [PubMed] [Google Scholar]
- 47. Castilla‐Guerra L, del Carmen Fernandez‐Moreno M, Lopez‐Chozas JM, Fernandez‐Bolanos R. Electrolytes disturbances and seizures. Epilepsia 2006;47:1990–1998. [DOI] [PubMed] [Google Scholar]
- 48. Oladipo OO, Lesi FE, Ezeaka VC. Plasma magnesium and calcium levels in children with epilepsy in lagos. Niger Postgrad Med J 2007;14:26–29. [PubMed] [Google Scholar]
- 49. Papavasiliou PS, Miller ST, Palat G, Pleban P, Mostek W. Selective disturbances of serum mineral profiles by electroconvulsive therapy. J Nerv Ment Dis 1985;173:401–405. [DOI] [PubMed] [Google Scholar]
- 50. Hjortso E, Fomsgaard JS, Fogh‐Andersen N. Does N‐acetylcysteine increase the excretion of trace metals (calcium, magnesium, iron, zinc and copper) when given orally? Eur J Clin Pharmacol 1990;39:29–31. [DOI] [PubMed] [Google Scholar]
- 51. Onodera K, Takahashi A, Mayanagi H, Wakabayashi H, Kamei J, Shinoda H. Phenytoin‐induced bone loss and its prevention with alfacalcidol or calcitriol in growing rats. Calcif Tissue Int 2001;69:109–116. [DOI] [PubMed] [Google Scholar]
- 52. Ala‐Houhala M, Korpela R, Koivikko M, Koskinen T, Koskinen M, Koivula T. Long‐term anticonvulsant therapy and vitamin D metabolism in ambulatory pubertal children. Neuropediatrics 1986;17:212–216. [DOI] [PubMed] [Google Scholar]
- 53. Babayigit A, Dirik E, Bober E, Cakmakci H. Adverse effects of antiepileptic drugs on bone mineral density. Pediatr Neurol 2006;35:177–181. [DOI] [PubMed] [Google Scholar]


