Abstract
SUMMARY Aims: This meta‐analysis was undertaken to compare the three most common drug regimens of bupivacaine in spinal anesthesia for cesarean section: high‐dose bupivacaine (≥10 mg, HB), low‐dose bupivacaine (<10 mg, LB) and combination of low‐dose bupivacaine and opioids (LBO). Methods: Databases of MEDLINE, EMBASE, and Cochrane Library were searched (updated on October 30, 2011). Primary endpoints were the incidence of intraoperative hypotension and analgesia efficacy. Pooled risk ratio (RR) or standard mean difference and their 95% confidence intervals (95% CI) were calculated. A RR <1 indicates that LB or LBO regimen is associated with less intraoperative complications and better anesthesia or analgesia efficacy. Results: A total of 11 randomized controlled trials including 605 parturients were analyzed. Results of this meta‐analysis showed that compared with HB regimen, LB regimen decreased the incidence of intraoperative hypotension (RR = 0.64, 95% CI: 0.42–0.96) with less satisfactory analgesia (fixed model, RR = 1.50, 95% CI: 1.14–1.98). LBO regimen significantly reduced the incidence of intraoperative hypotension (RR = 0.52, 95% CI: 0.33–0.82) with reliable analgesia efficacy (RR = 2.56, 95% CI: 0.77–8.48). Conclusion: Compared with conventional HB regimen and LB regimen, LBO regimen not only reduced intraoperative hypotension but also provided reliable analgesia. Therefore, LBO regimen should be considered as the preferred drug combination for spinal anesthesia in cesarean section.
Keywords: Bupivacaine, Cesarean section, Hypotension, Opioids, Spinal anesthesia
Introduction
Spinal or combined spinal‐epidural (CSE) anesthesia is commonly used for elective cesarean sections (C‐sections). Spinal anesthesia induced hypotension, with an incidence of 30–100%, is the most common complication [1, 2]. Intraoperative hypotension can lead to many detrimental effects on both mother and neonate, such as nausea, vomiting, dizziness, decreased uteroplacental blood flow and fetal acidosis [3]. The incidence of hypotension can be decreased by preloading with fluids, administration of vasopressor drugs, and avoiding aortovacal compression [4, 5].
Bupivacaine is the most commonly used local anesthetic in spinal anesthesia and high dosage of bupivacaine is associated with the increased incidence of hypotension [2, 6]. Fan et al. [1], who compared the effects of bupivacaine dose on spinal anesthesia induced hypotension, found that: compared with low‐dose bupivacaine (LB), bupivacaine of 10 mg significantly increased the incidence of hypotension. Compared with high‐dose bupivacaine (HB), low dose has an advantage of less hypotension, although motor block and effective anesthesia time are less satisfactory [7]. LB combined with additional intrathecal injection of opioids such as fentanyl and sufentanil, however, could provide adequate anesthesia [8, 9]. LB combined with opioids (LBO) is accepted as a safe and effective drug regimen for spinal anesthesia. The aim of this meta‐analysis was to compare the three most frequently used drug regimens: LB, HB, and LBO to identify the most favorable regimen used in spinal anesthesia for C‐section.
Methods
Searching Strategy
Electronic databases of MEDLINE (1966–2011), EMBASE (1985–2011), and the Cochrane library were searched using medical subheadings or key words of “bupivacaine,”“caesarean section,”“spinal anesthesia,”“hypotension,”“CSE anesthesia,” and “randomized controlled trial”. Alternative spellings of above key words were considered when searching databases. Language and publishing time were not limited and the last search was performed on October 30, 2011.
Inclusion Criteria
Randomized controlled trials (RCTs) that compared the incidence of hypotension and analgesia effects of LB versus HB or HB versus LBO were included. Trials that included nonelective C‐sections were excluded. Only trials with spinal or CSE anesthesia were included. Trials with epidural anesthesia were excluded because epidural anesthesia was associated with less parturients who need to treat hypotension (risk ratio; RR = 1.23, 95% confidence intervals; CI 1.00–1.51) [10]. There was no limitation of bupivacaine's density, injection speed, or position.
Data Extraction
Two reviewers (Mantang Qiu, Hengbin Zhang) independently selected eligible trials and extracted data with a standard data collection form. Disagreement between the two reviewers was settled by discussing with the third reviewer (Fuqing Lin). The following data was collected: name of first author, journal, publishing time, number of parturients, baseline data (age, height, weight), anesthesia method, drug regimen of bupivacaine, hypotension, analgesia efficacy, other complications (nausea, vomiting, shivering, prurities), total dose of ephedrine, total dose of intraoperative analgesics, effective analgesia time. Bupivacaine with a dose of 10 mg or more was defined as high dose, and a dose of less than 10 mg was considered as low dose. Because a number of authors took 10 mg bupivacaine as a relatively high dose in trial designs [11, 12, 13], we used 10 mg bupivacaine as the cutoff point of HB and LB. All the data collected were defined according to the definition chosen by each trial and the data was not standardized. Trials including more than two groups were combined according to specific drug combination and our definition of LB, HB, and LBO. For example, the trial of Atalay et al. [14] included four groups, and we combined the three groups of 5 mg bupivacaine + 25 mg meperidine, 5 mg bupivacaine + 30 mg meperidine and 5 mg bupivacaine + 35 mg meperidine into one group categorized as LBO.
The quality of eligible trials was assessed using the tool of “risk of bias” according to the Cochrane Handbook V5.0.2. Sequence generation, allocation concealment, blinding, incomplete data, and selective reporting were assessed. Based on the method and design of the trials described, each of them was graded as “yes,”“no” or “unclear,” which represented high risk of bias, low risk of bias, and uncertain of bias respectively.
Statistical Methods
Primary endpoints of this meta‐analysis were the incidence of maternal hypotension and intraoperative analgesia effect, and secondary endpoints were spinal anesthesia induced complications, the total dose of ephedrine and effective analgesic time. Pooled RR or standard mean difference (SMD) and their 95% CI were calculated for each endpoint, and a RR <1 indicates that LB or LBO is associated with less intraoperative complications and better anesthesia; vice versa. A 95% CI did not include 1 for RR or did not include 0 for SMD was considered of statistical significance. Q‐test was used to analyze heterogeneity of trials. When P < 0.1, which was considered as heterogeneous, the source of heterogeneity was identified and Mantel–Haenszel random‐effects model was performed, otherwise, Mantel–Haenszel fixed‐effects model was used. Publication bias was tested by funnel plot in the analyses of hypotension and analgesia. Sensitivity analyses, in which one trial was deleted each time to identify its influence on the result, were performed when comparing the incidence of hypotension to identify the source of heterogeneity. Because intraoperative hypotension was associated with anesthetics, anesthesia techniques, and other factors [15]. All the data analyses were carried out using software Review Manager (V5.0.2).
Results
Characteristics of Eligible Trials and Quality Assessment
A total of 12 [1, 11, 12, 13, 14, 16, 17, 18, 19, 20, 21, 22] RCTs met the inclusion criteria and one trial [22] was excluded because of lack of available data (incidence of hypotension and analgesia assessment). Therefore, 11 trials [1, 11, 12, 13, 14, 16, 17, 18, 19, 20, 21] and 605 parturients were enrolled in our analyses (detail shown in Figure 1).
Figure 1.
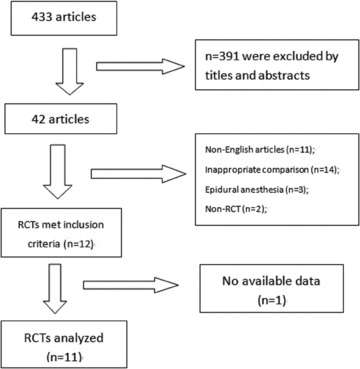
Flow chart.
Parturients enrolled in the 11 trials were described as ASA I, II or healthy without diabetes mellitus, infection, pregnancy induced hypertension, preplacental or other complications. CSE was used in three trials [1, 11, 20], and spinal anesthesia was used in the other eight trials. Opioids used in LBO regimen included fentanyl, sufentanil, and meperdine. Participants were all prehydrated with Ringer’ solution or saline before anesthesia. Spinal anesthesia was performed at L2/L3 or L3/L4 interspace and sitting position was most frequently used. Baseline data and other details were shown in Table 1.
Table 1.
Characteristics of analyzed trials
| Refs. | Year | Number of parturient | Drug regimen | Anesthesia | Age | Weight (kg) | Height (cm) | Primary endpoints |
|---|---|---|---|---|---|---|---|---|
| Atalay C (14) | 2010 | 20 | 10 mg bupivacaine | S | 27.8 ± 4.3 | 75.0 ± 3.4 | 160 ± 5.1 | Hypotensiona (MBP decreased by more than 15–20% from baseline), introoperative analgesia (0–10VAS), comfort (poor, fair, good, excellent) |
| 20 | 5 mg bupivacaine + 35 mg meperidine | 28.4 ± 5.4 | 74.0 ± 4.5 | 162 ± 4.3 | ||||
| 20 | 5 mg bupivacaine + 30 mg meperidine | 26.9 ± 6.8 | 73.0 ± 5.3 | 159 ± 5.2 | ||||
| 20 | 5 mg bupivacaine + 25 mg meperidine | 29.6 ± 5.4 | 75.0 ± 6.1 | 161 ± 5.4 | ||||
| Turhanoglu S (11) | 2009 | 20 | 10 mg bupivacaine | CSE | 28.2 ± 4.9 | 79.5 ± 7.7 | 162 ± 2.6 | Hypotension (SAP <90 mmHg or 30% decrease from the baseline), introoperative analgesia (a 10‐cm linear VAS), satisfaction with anesthetic (very bad, bad, good, very good) |
| 20 | 4 mg bupivacaine + 25 μg fentanyl | 27.3 ± 6.3 | 79.4 ± 5.3 | 161 ± 1.8 | ||||
| Agrawal A (16) | 2009 | 20 | 12 mg bupivacaine | S | 27.4 ± 4.49 | 57.5 ± 3.03 | 158 ± 3.30 | Hypotension (fall of blood pressure greater than 20% of baseline) |
| 20 | 7.5 mg bupivacaine + 25 μg fentanyl | 25.9 ± 3.32 | 55.9 ± 2.10 | 157 ± 2.96 | ||||
| 20 | 7.5 mg bupivacaine + 10 μg sufentanil | 25.7 ± 2.50 | 56.2 ± 4.55 | 156.2 ± 4.61 | ||||
| Tolia G (17) | 2008 | 25 | 11 mg bupivacaine | S | 25.1 ± 3.47 | 57.0 ± 7.40 | 157 ± 4.45 | Hypotension (SBP<95 mmHg or SBP decreased >25% from baseline value), quality of surgical anesthesia (poor, good, excellent) |
| 25 | 9 mg bupivacaine + 10 μg fentanyl | 24.1 ± 3.86 | 57.5 ± 5.51 | 156 ± 4.62 | ||||
| 25 | 7.5 mg bupivacaine + 10 μg fentanyl | 26.0 ± 4.36 | 58.4 ± 5.25 | 157 ± 4.83 | ||||
| Sivevski A (18) | 2006 | 20 | 13.5 mg bupivacaine | S | 26.0 ± 7.5 | 74.5 ± 10 | 164 ± 7 | Hypotension (SBP was lower than 95 mm Hg or 20% below the preinduction level), introoperative analgesia (VAS) |
| 20 | 9 mg bupivacaine+ 20μg fentanyl | 27.2 ± 4.7 | 76.0 ± 8 | 165 ± 5 | ||||
| Kiran S (12) | 2002 | 20 | 7.5 mg bupivacaine | S | 23.2 ± 3.03 | 59.3 ± 9.40 | 157 ± 6.24 | Hypotension (a decrease of SBP of 30% from baseline), introoperative analgesiab (poor, fair, good, excellent) |
| 20 | 8.75 mg bupivacaine | 24.8 ± 2.95 | 58.4 ± 9.07 | 62.7 ± 11.7 | ||||
| 20 | 10 mg bupivacaine | 25.4 ± 4.46 | 62.7 ± 11.7 | 9.82 ± 0.82 | ||||
| Ben‐David B (13) | 2000 | 16 | 10 mg bupivacaine | S | 32.6 ± 4.7 | 84.7 ± 9.0 | 164 ± 4.9 | Hypotension (SBP less than 95 mm Hg or a decrease of more than 25% from baseline), satisfaction of anesthetic (VAS) |
| 16 | 5 mg bupivacaine + 25 μg fentanyl | 29.0 ± 7.5 | 84.8 ± 13.8 | 164 ± 5.5 | ||||
| Chung CJ (19) | 1996 | 20 | 8–9 mg bupivacaine | S | 30.0 (23–34)c | 159.5 ± 2.4 | 159 ± 2.4 | Hypotension (SAP less than 100 mmHg or a 20% reduction of SAP), introoperative analgesiab (poor, fair, good, excellent) |
| 20 | 9–10 mg bupivacaine | 29.9(25–35)c | 159 ± 2.2 | 159 ± 2.2 | ||||
| 20 | 10–11 mg bupivacaine | 29.4 (24–36)c | 161 ± 2.7 | 161 ± 2.7 | ||||
| Thorén T (20) | 1994 | 21 | 12.5 mg bupivacaine | S | 28.8 ± 0.9 | 75.9 ± 2.2 | 164± 1.6 | Hypotension (a decrease in SBP of 20% or more from baseline value, or a SBP below 100 mm Hg), introoperative analgesiab (poor, fair, good, excellent) |
| 21 | 7.5 mg bupivacaine | CSE | 31.1 ± 1.0 | 75.1 ± 2.4 | 166 ± 1.3 | |||
| Fan SZ (1) | 1994 | 20 | 2.5 mg bupivacaine | CSE | 29 ± 3 | 68.4 ± 6.3 | 157 ± 6.5 | Hypotension (SBP less than 90 mm Hg or a 30% decrease from baseline level) |
| 20 | 5.0 mg bupivacaine | 28 ± 4 | 67.7 ± 5.8 | 159 ± 7.2 | ||||
| 20 | 7.5 mg bupivacaine | 28 ± 3 | 69.7 ± 6.3 | 157 ± 6.9 | ||||
| 20 | 10.0 mg bupivacaine | 27 ± 4 | 67.5 ± 5.8 | 157 ± 7.2 | ||||
| Pedersen H (21) | 1989 | 17 | 7.5–10 mg bupivacaine | S | 30.1 ± 1.2 | 78.2 ± 3.1 | 160 ± 1.8 | Hypotension (SBP below 100 mm Hg), introoperative analgesia (0–10 cm VAS) |
| 19 | 10–12.5 mg bupivacaine | 31.6 ± 1.1 | 78.2 ± 3.1 | 160 ± 1.5 |
S, spinal anesthesia; CSE, combined spinal‐anesthesia; VAS, visual analog scale; MBP, mean blood pressure; SAP, systolic arterial pressure; SBP, systolic blood pressure.
athe blood pressure at which vasopressor drugs were given was taken as definition.
bexcellent: no discomfort during the surgical procedure; good: mild discomfort but no systemic analgesics supplementation given; fair: pain that need additional analgesic supplementation; poor: moderate or severe pain that need ketamine more than 100 mg [Ref. 12] or fentanyl 100 μg [Ref. 19] or general anesthesia.
cvalues are mean (range), the others are mean ± SD.
Most of eligible trials did not describe the methods of sequence generation or allocation concealment because of publishing time; blinding methods were described in six trials [11, 13, 14, 17, 18, 19]; incomplete data was addressed in each trial; and all trials were considered as free of selective reporting (Figure 2).
Figure 2.
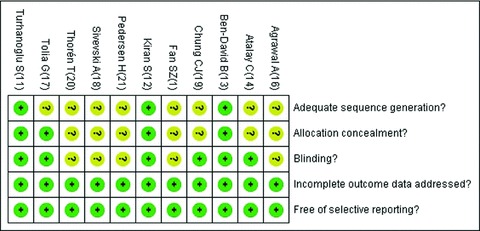
Risk of bias summary: Green for “yes”; yellow for “unknown.”
Main Results
-
1
HB vs. LB : Compared with HB, LB regimen decreased the incidence of maternal hypotension during C‐section significantly (RR = 0.64, 95% CI: 0.42–0.96) [1, 12, 19, 20, 21] (Figure 3). Intraoperative analgesia was graded as “excellent,”“good,”“fair,” or “poor” in three trials as shown in Table 1. LB and HB did not show significant difference (random model, RR = 1.50, 95% CI: 0.95–2.37) [12, 19, 20] (Figure 4) in the grade of “excellent” (Table 2). However, when analyzing by fixed‐effects model, LB showed insufficient analgesia efficacy (fixed model, RR = 1.50, 95% CI: 1.14–1.98), given that the P value of heterogeneity (P= 0.09) was close to the threshold value of 0.1.
-
2
HB vs. LBO: Compared with HB, LBO regimen showed a remarkable lower incidence of maternal hypotension (RR = 0.52, 95% CI: 0.33–0.82) [11, 13, 14, 16, 17, 18] (Figure 5). Intraoperative analgesia was evaluated by visual analog scale in four trials [11, 13, 14, 18] (Table 1), which was not appropriate for statistical analysis. However, the number of parturients who need additional analgesics, the indirect parameter to measure the intraoperative analgesia efficacy, did not differ significantly between two combinations (RR = 2.56, 95% CI: 0.77–8.48) [11, 13, 16, 18] (Figure 6). LBO regimen was associated with more prurities (RR = 16.13, 95% CI: 3.39–76.74), less vomiting (RR = 0.41, 95% CI: 0.23–0.74) and smaller dose of ephedrine (SMD =–0.88, 95% CI [–1.28, –0.49]) to maintain blood pressure when hypotension occurred. HB and LBO showed no significant differences in other complications (Table 2).
Figure 3.
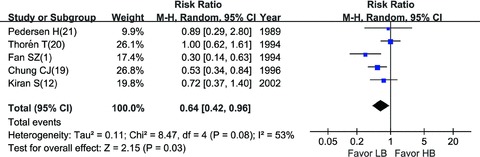
Incidence of hypotension: LB vs. HB.
Figure 4.
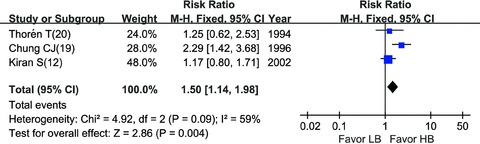
Intraoperative analgesia: LB vs. HB.
Table 2.
Summary of meta‐analysis results
| Comparison | Results | RR/SMD | 95% CI |
|---|---|---|---|
| HB vs. LB | Hypotension | 0.64 | [0.42, 0.96]a |
| Analgesia effects (excellent) | 1.5 | [0.95, 2.37] | |
| HB vs. LBO | Hypotension | 0.52 | [0.33, 0.82]a |
| Nausea | 0.54 | [0.23, 1.23] | |
| Vomiting | 0.41 | [0.23, 0.74]a | |
| Prurities | 16.13 | [3.39, 76.74]a | |
| Shivering | 0.25 | [0.06, 1.03] | |
| Paturients need analgesics | 2.56 | [0.77, 8.48] | |
| Total dose of ephedrine | (SMD) −0.88 | [–1.28, –0.49]b | |
| Effective analgesia time | (SMD) 3.86 | [–1.49, 9.22] |
aa 95% CI did not include 1 for RR.
ba 95% CI did not include 0 for SMD.
Figure 5.
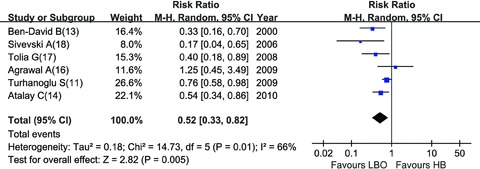
Incidence of hypotension: HB vs. LBO.
Figure 6.
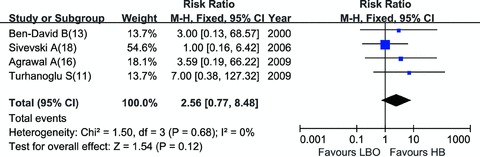
Parturients need analgesics: HB vs. LBO.
Discussion
Our meta‐analysis showed that LB could decrease the incidence of maternal hypotension but with an insufficient intraoperative analgesia effect, compared with conventional HB regimen. LBO regimen, however, was associated with less hypotension, reliable analgesia efficacy, and less vomiting at the same time.
Compared with conventional HB, LB was associated with less maternal hypotension (RR = 0.64, 95% CI: 0.42–0.96). Sensitivity analysis showed that trials of Thorén et al. [20] and Fan et al. [1] accounted for the heterogeneity, because the dosage of bupivacaine was relatively larger or smaller. And anesthesia techniques in the five trials [1, 12, 19, 20, 21] analyzed were similar. When came to intraoperative analgesia, there was slight heterogeneity (heterogeneity P= 0.09), however, it showed a statistical difference when analyzed using fixed‐effects model, which suggested that HB provided better analgesia. The source of heterogeneity may be due to the differences of analgesia efficacy assessing, because Kiran and Singal [12] and Chung et al. [19] assessed the overall intraoperative analgesia, wheras Thorén et al. [20] assessed intraoperative analgesia both before and after delivery (the data after delivery was analyzed; and when using the data before delivery, HB also showed advantage over LB). Intraoperative analgesia was assessed using a subjective, nonquantitative grading system, which may also lead to bias. Nevertheless, HB regimen still showed a trend of better analgesia effect than LB regimen (Figure 4).
When comparing the analgesia efficacy of HB and LBO, there was no direct parameter suitable for analysis in all the six trials [11, 13, 14, 16, 17, 18], therefore we used the number of parturients who needed additional analgesics as an indirect parameter to evaluate intraoperative analgesia efficacy. LBO regimens decreased the incidence of hypotension and achieved reliable analgesia effect (the number need additional analgesics had no significant differences) compared with HB regimen, in addition the result was stable (heterogeneity P= 0.68). As for the heterogeneity in the comparison of hypotension, sensitivity analyses suggested that the trial of Turhanoglu et al. [11] was responsible for the heterogeneity, because 4 mg of bupivacaine was relatively a small dose compared to other trials.
As shown in these trials [11, 13, 14, 16, 17, 18], the addition of opioids allows a reduced dose of local anesthetic and enhanced analgesia. Opioids of fentanyl, sufentanil, and meperdine were used in combination with bupivacaine according to including trials. Fentanyl is considered as an ideal opioid in obstetrics because it is more liquid soluble than morphine and does not tend to spread intrathecally to the fourth ventricle in sufficient concentrations to cause respiratory depression [23]. Meperdrine, however, is also preferred due to its long duration of action, good postoperative effects, low cost [24] and meperdine is hyperbaric [25]. As for which is the best opioids in LBO for C‐section, this should be answered by further clinical trials.
Ephedrine was used in all of 11 trials to maintain blood pressure but the dosage of ephedrine administrated differed when hypotension occurred. Turhanoglu et al. [11] and Sivevski [18] used a dose of 5 mg; Atalay et al. [14] used a dose of 10 mg, and Ben‐David et al. [13] preferred 5–10 mg. In addition, ephedrine was given when bradycardia occurred by Turhanoglu et al. [11]. Because of less intraoperative hypotension, LBO was associated with less consumption of ephedrine.
Because the kinds of operative complications, definitions, and data form reported by trials varied, no adequate data was allowed to compare the difference of complications between HB and LB. When came to the comparison of HB and LBO, less vomiting and more prurities occurred with LBO regimen. Because the incidence of vomiting is dependent on hypotension [14], less vomiting may explain by that LBO decreased the incidence of hypotension [26]. Prurities was more frequent with LBO and it was reported in all trials [11, 14, 16] analyzed, and the reason may be the addition of opioids. HB and LBO therapies showed no difference in nausea and shivering.
There were some limitations in our meta‐analysis. At first, a number of trials in non‐English (most were Spanish and Japanese) were excluded, which may lead to ethic bias. Second, four funnel plots for the main comparison of hypotension and analgesia were a little asymmetrical. However, when examined by quantitative Egger or Begg test with the software of Stata, the P values were all larger than 0.05, showing that there is no significant publication bias. This may be explained by the reason that number of trials in each comparison was relatively small. Third, due to the lack of standardization of data with different definitions and various technical details, the heterogeneity for comparison cannot be eliminated. In terms of the definition of hypotension, Klöhr et al. [27], after reviewing 63 articles, found that there existed 15 kinds of definitions in literature about maternal hypotension during C‐section and there was not a widely accepted definition. The last, we did not report the effects of neonatal outcomes such as Apgar scores, umbilical blood pH values, because these data was not reported in an appropriate form.
In conclusion, during C‐section under spinal or CSE anesthesia, both LB and LBO regimens could decrease the incidence of maternal hypotension. But LB was associated with insufficient analgesia compared with HB regimen; whereas LBO showed equal analgesia compared with HB. Therefore, we conclude that LBO regimen is the preferred drug regimen of bupivacaine in C‐section.
Authors’ Contributions
Man‐Tang Qiu has designed the study, seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Fu‐Qing Lin has designed the study, seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Shu‐Kun Fu has designed the study, seen the original study data, and approved the final manuscript.
Heng‐Bin Zhang has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Hui‐Hua Li approved the final manuscript.
Li‐Ming Zhang reviewed and proofread the manuscript.
Quan Li has designed the study, seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Conflict of Interest
The authors have no conflicts of interest.
Disclosure
Abstract of this meta‐analysis has been published on the 2011 annual meeting of American Society of Anesthesiologists.
Acknowledgments
We thank Dr. Yu Bai (Second Military Medical University, Shanghai, CHINA) for guidance in methods of meta‐analysis. This research was partly supported by Shanghai Natural Science Foundation [10411951400] (to Shukun Fu), [11ZR1428100] (to Quan Li).
References
- 1. Fan SZ, Susetio L, Wang YP, Cheng YJ, Liu CC. Low dose of intrathecal hyperbaric bupivacaine combined with epidural lidocaine for cesarean section: A balance block technique. Anesth Analg 1994;78:474–477. [DOI] [PubMed] [Google Scholar]
- 2. Yu SC, Ngan Kee WD, Kwan AS. Addition of meperidine to bupivacaine for spinal anesthesia for Cesarean section. Br J Anaesth 2002;33:379–383. [DOI] [PubMed] [Google Scholar]
- 3. Rout CC, Rocke DA. Prevention of hypotension following spinal anesthesia for cesarean section. Int Anesthesiol Clin 1994;32:117–135. [PubMed] [Google Scholar]
- 4. Kansal A, Mohta M, Sethi AK, Tyagi A, Kumar P. Randomised trial of intravenous infusion of ephedrine or mephentermine for management of hypotension during spinal anesthesia for caesarean section. Anesthesia 2005;60:28–34. [DOI] [PubMed] [Google Scholar]
- 5. Cooper DW, Carpenter M, Mowbray P, Desira WR, Ryall DM, Kokri MS. Fetal and maternal effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology 2002;97:1582–1590. [DOI] [PubMed] [Google Scholar]
- 6. Kang FC, Tsai YC, Chang PJ, Chen TS. Subarachnoid fentanyl with diluted small‐dose bupivacaine for cesarean section delivery. Acta Anaesthesiol Sin 1998;36:207–214. [PubMed] [Google Scholar]
- 7. Roofthooft E, Van de Velde M. Low‐dose spinal anesthesia for Caesarean section to prevent spinal‐induced hypotension. Curr Opin Anaesthesiol 2008;21:259–262. [DOI] [PubMed] [Google Scholar]
- 8. Dahlgren G, Hultstrand C, Jakobsson J, Norman M, Errikson EW, Martin H. Intrathecal sufentanil, fentanyl, or placebo added to bupivacaine for cesarean section. Anesth Analg 1997;85:1288–1293. [DOI] [PubMed] [Google Scholar]
- 9. Hunt CO, Naulty S, Bader AM, et al Perioperative analgesia with subarachnoid fentanyl‐bupivacaine for cesarean delivery. Anesthesiology 1989;71:535–540. [DOI] [PubMed] [Google Scholar]
- 10. Ng K, Parsons J, Cyna AM, Middleton P. Spinal versus epidural anesthesia for caesarean section. Cochrane Database Syst Rev 2004;2:CD003765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Turhanoglu S, Kaya S, Erdogan H. Is there an advantage in using low‐dose intrathecal bupivacaine for cesarean section? J Anesth 2009;23:353–357. [DOI] [PubMed] [Google Scholar]
- 12. Kiran S, Singal NK. A comparative study of three different doses of 0.5% hyperbaric bupivacaine for spinal anesthesia in elective caesarean section. Int J Obstet Anesth 2002;11:185–189. [DOI] [PubMed] [Google Scholar]
- 13. Ben‐David B, Miller G, Gavriel R, Gurevitch A. Low‐dose bupivacaine‐fentanyl spinal anesthesia for cesarean delivery. Reg Anesth Pain Med 2000;25:235–239. [PubMed] [Google Scholar]
- 14. Atalay C, Aksoy M, Aksoy AN, Dogan N, KÜrasd H. Combining intrathecal bupivacaine and meperidine during caesarean section to prevent spinal anesthesia‐induced hypotension and other side‐effects. J Int Med Res 2010;38:1626–1636. [DOI] [PubMed] [Google Scholar]
- 15. Benhamou D, Wong C. Neuraxial anesthesia for cesarean delivery: What criteria define the “optimal” technique? Anesth Analg 2009;109:1370–1373. [DOI] [PubMed] [Google Scholar]
- 16. Agrawal A, Agrawal S, Asthana V, Payal YS, Sharam J, Gupta V. Comparison of intrathecal fentanyl and sufentanil in addition to bupivacaine for caesarean section under spinal anesthesia. J of Anaesth Clin Pharma 2009;25:154–156. [Google Scholar]
- 17. Tolia G, Kumar A, Jain A, Pandey M. Low dose intrathecal bupivacaine with fentanyl for cesarean delivery. J of Anaesth Clin Pharma 2008;24:201–204. [Google Scholar]
- 18. Sivevski A. Spinal anesthesia for cesarean section with reduced dose of intrathecal bupivacaine plus fentanyl. Prilozi 2006;27:225–236. [PubMed] [Google Scholar]
- 19. Chung CJ, Bae SH, Chae KY, Chin YJ. Spinal anesthesia with 0.25% hyperbaric bupivacaine for Caesarean section: Effects of volume. Br J Anaesth 1996;77:145–149. [DOI] [PubMed] [Google Scholar]
- 20. Thorén T, Holmström B, Rawal N, Schollin J, Lindeberg C, Skeppner G. Sequential combined spinal epidural block versus spinal block for cesarean section: Effects on maternal hypotension and neurobehavioral function of the newborn. Anesth Analg 1994;78:1087–1092. [PubMed] [Google Scholar]
- 21. Pedersen H, Santos AC, Steinberg ES, Schapiro HM, Harmon TW, Finster M. Incidence of visceral pain during cesarean section: The effect of varying doses of spinal bupivacaine. Anesth Analg 1989;69:46–49. [PubMed] [Google Scholar]
- 22. Seyedhejazi M, Madarek E. The effect of small dose bupivacaine‐fentanyl in spinal anesthesia on hemodynamic nausea and vomiting in cesarean section. Pak J Med Sci 2007;23:747–750. [Google Scholar]
- 23. Gunusen I, Karaman S, Sargin A, Firat V. A randomized comparison of different doses of intrathecal levobupivacaine combined with fentanyl for elective cesarean section: Prospective, double‐blinded study. J Anesth 2011;25:205–212. [DOI] [PubMed] [Google Scholar]
- 24. Kafle SK. Intrathecal meperidine for elective Caesarean section: A comparison with lidocaine. Can J Anaesth 1993;40:718–721. [DOI] [PubMed] [Google Scholar]
- 25. Lui AC, Polis TZ, Cicutti NJ. Densities of cerebrospinal fluid and spinal anaesthetic solutions in surgical patients at body temperature. Can J Anaesth 1998;45:297–303. [DOI] [PubMed] [Google Scholar]
- 26. Balki M, Carvalho JC. Intraoperative nausea and vomiting during cesarean section under regional anesthesia. Int J Obstet Anesth 2005;14:230–241. [DOI] [PubMed] [Google Scholar]
- 27. Klöhr S, Roth R, Hofmann T, Rossaint R, Heesen M. Definitions of hypotension after spinal anesthesia for caesarean section: Literature search and application to parturients. Acta Anaesthesiol Scand 2010;54:909–921. [DOI] [PubMed] [Google Scholar]


