The contribution of vasculature in adult neural stem cell niches has been well acknowledged in both hippocampus (HP) and subventricular zone (SVZ) 1, 2, as well as the postinjury neurogenesis processes 3. In the neurogenic zones, the specifically organized vasculature shows enhanced blood–brain barrier (BBB) permeability, and neural stem/progenitor cells locate in adjacent to blood vessels, with contacting processes. Notably, adult neural stem cells are astrocytes in nature 4. On the other hand, tumor stem cells, such as glioma stem cells also reside in the specialized vasculature niche within the brain to maintain their stem cell‐like characteristics 5, and can also actively modify the microenvironment themselves, for instance, by generating vascular pericytes to support vessel growth 6. In both cases, the stem cells can be astrocytes, and locate in adjacent to vasculature during cell proliferation. In a recent article from Nature Neuroscience, with liver two‐photon imaging of astrocytes expressing fluorescent proteins, Bardehle et al. showed that the vasculature can also function as the “progenitor cell” niche for astrocyte proliferation upon brain injury (Figure 1) 7.
Figure 1.
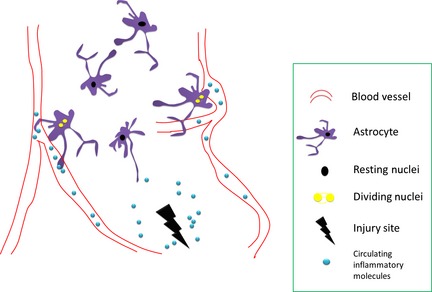
Brain astrocytes respond heterogeneously to circulating injury signals: resident, polarized, and dividing. The proliferating astrocytes locate in adjacent to blood vessels, and generate two daughter cells at one time.
In this recent study, Bardehle et al. employed transgenic mice expressing green fluorescent protein (GFP) underlying GLutamate ASpartate Transporter (GLAST), which is mainly expressed by astrocytes in the brain 7. They found that in response to acute brain injury, astrocytes reacted heterogeneously, while the proliferating astrocytes mainly locate in close to blood vessels. Under electronic microscopic examinations, these proliferating astrocytes showed direct contact to the blood vessel, which is also the case for neural stem cells. Finally, the genetic deletion of Cdc42, a small RhoGTPase that is important for cell proliferation, led to diminished astrocyte proliferation in responses to brain injury 7.
One surprising finding is that each astrocyte divides limitedly. In most of their preparations (stab wound), less than 20% astrocytes of the total population proliferate, and generate only two daughter cells (single division) 7. This could not fully explain the significant increase in glial fibrillary acidic protein (GFAP) positive cells in the injury site. One possibility is that resident astrocyte express minimal GFAP protein, and reactive astrocytes upregulate their GFAP expression; the other possibility is that in mild injury conditions, the BBB was not severely disrupted, and therefore, the circulating cytokines can only reach those juxtavascular astrocytes which are in tight contact with the vessels.
Interestingly, the author did not observe clear signs for astrocyte migration or recruitment to wound site in their preparation. This is consistent with previous studies showing that in postnatal cortex, the astrocyte expand in number by local proliferation 8, rather than long‐distance migration. The immobility of these reactive astrocytes argued for regional repair after mild brain injury, although some distant astrocyte extend prolonged processes toward the injury site, which might guide the migration of other types of glial cells, such as microglial cells 9. Future studies combining the live imaging of astrocytes and microglial cells would further elucidate this question.
This finding indicated that vasculature could function as the niche allowing cell proliferation for astrocytes as well. There are several potential explanations. First, cell division consumes lots of energy. For instance, it has been found that S‐phase entry of neural progenitor cells is correlated with increased blood flow in the SVZ 10. Secondly , juxtavascular astrocytes can sense the circulating pro‐inflammatory signals in response to brain injury. Thirdly, circulating mononuclear cells may infiltrate and exhibit glial fate. Last but not least, there are different molecules secreted into the perivascular region, such as the nitric oxide and other growth factors. The microenvironment therefore is critical to maintain the progenitor cell property and to initialize the cell proliferation.
The study further proved the functional heterogeneity of astrocytes in vivo. Not all astrocytes react to mild brain injury and are proliferative as the response. Whether these juxtavascular astrocytes are naturally “mother” astrocytes, or own molecular characteristics of stem/progenitor cells are unknown. Cortical astrocytes can be induced into neural progenitor cells 11, while cortical injury can induce local neurogenesis as well 12. It is possible that these juxtavascular astrocytes can be induced for a neuronal fate, or even contributes to the formation of glioma. Further molecular and microanatomical dissections of these juxtavascular astrocytes would help to answer these questions. Notably, these glial cells maintain their morphology during cell proliferation 13, and the functions of these extended processes during cell division are to be determined.
This discovery certainly raised possibilities for targeted brain rehabilitation therapies over glial cells. For instance, if the circulating cytokines are critical in triggering astrocytes proliferation, the peripheral administration of antiinflammatory agents might be enough to control astrocyte proliferation. On the other hand, if the slow and delayed astrocyte proliferation is beneficial for brain recovery (e.g., removal of debris), the circulation system can act as the intervention route for the CNS system given the increased BBB permeability at the stem cell niches. It will also be possible to target different populations of astrocytes considering their heterogeneities, such as utilizing the potentially different molecules of these cells.
In summary, the recent study argued for the importance of vasculature in astrocyte proliferation. The neural regulation of peripheral blood vessels was well recognized since decades ago 14. In the central nervous system, the blood vessel dilation and increased local perfusion are controlled by astrocytes, termed as neurovascular coupling 15: active neuron release substances that lead to astrocyte calcium rise, which then dilate the coupled vessel via direct or indirect mechanisms 16. In addition, the vessel permeability is regulated by the blood–brain barrier (BBB), formed by astrocyte endfeets together with perivascular cells. The loss of endfeet during brain injury could increase the vasculature permeability and contribute to the neuroinflammation. With all previous studies showing that astrocytes control the blood flow and BBB permeability, the interaction is now found to be vice versa. How could blood vessel participate in other types of physiological activities of astrocytes is yet to be studied.
Conflicts of interest
The authors declare no conflict of interest.
References
- 1. Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol 2000;425:479–494. [DOI] [PubMed] [Google Scholar]
- 2. Tavazoie M, Van der Veken L, Silva‐Vargas V, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell 2008;3:279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saghatelyan A. Role of blood vessels in the neuronal migration. Semin Cell Dev Biol 2009;20:744–750. [DOI] [PubMed] [Google Scholar]
- 4. Doetsch F, Caille I, Lim DA, Garcia‐Verdugo JM, Alvarez‐Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999;97:703–716. [DOI] [PubMed] [Google Scholar]
- 5. Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell 2007;11:69–82. [DOI] [PubMed] [Google Scholar]
- 6. Cheng L, Huang Z, Zhou W, et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell 2013;153:139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bardehle S, Kruger M, Buggenthin F, et al. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci 2013;16:580–586. [DOI] [PubMed] [Google Scholar]
- 8. Ge WP, Miyawaki A, Gage FH, Jan YN, Jan LY. Local generation of glia is a major astrocyte source in postnatal cortex. Nature 2012;484:376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005;308:1314–1318. [DOI] [PubMed] [Google Scholar]
- 10. Lacar B, Herman P, Hartman NW, Hyder F, Bordey A. S phase entry of neural progenitor cells correlates with increased blood flow in the young subventricular zone. PLoS ONE 2012;7:e31960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kriegstein A, Alvarez‐Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 2009;32:149–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang Z, Covey MV, Bitel CL, Ni L, Jonakait GM, Levison SW. Sustained neocortical neurogenesis after neonatal hypoxic/ischemic injury. Ann Neurol 2007;61:199–208. [DOI] [PubMed] [Google Scholar]
- 13. Ge WP, Zhou W, Luo Q, Jan LY, Jan YN. Dividing glial cells maintain differentiated properties including complex morphology and functional synapses. Proc Natl Acad Sci U S A 2009;106:328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peterson LH. Regulation of blood vessels. Circulation 1960;21:749–759. [DOI] [PubMed] [Google Scholar]
- 15. Roy CS, Sherrington CS. On the Regulation of the Blood‐supply of the Brain. J Physiol 1890;11:85–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron 2011;71:782–797. [DOI] [PubMed] [Google Scholar]


