Summary
Aims
Working memory (WM) impairments are considered to be a main feature of mild cognitive impairment (MCI). Functional brain imaging studies have revealed evidence of alterations in the frontal and temporal cortices associated with WM in MCI patients. However, some imaging methods are too expensive for routine clinical use and have a low temporal resolution.
Methods
Using a newly developed near‐infrared spectroscopy (NIRS) system, we studied the spatiotemporal dynamics of oxygenated hemoglobin (oxy‐Hb) during a WM task in eight patients with mild cognitive impairment (MCI) and 16 age‐ and gender‐matched healthy controls.
Results
We performed temporal and spatial correlation analyses on each group during their WM tasks. These results consistently demonstrated that, when compared with the healthy controls, the MCI patients exhibited significantly decreased activation in the left frontal, right superior frontal and left temporal lobes. We found evidence of altered frontal and temporal processing during WM tasks in the MCI patients.
Conclusions
These results confirm the functional deficits in the frontal and temporal cortices and the impairment of WM and cognitive abilities in MCI patients and suggest that fNIRS may be a useful tool for evaluating brain activation in cognitive disorders.
Keywords: Frontal and temporal lobe, Mild cognitive impairment, Near‐infrared spectroscopy (NIRS), Oxygenated hemoglobin, Working memory
Introduction
The term “mild cognitive impairment” (MCI) is used to characterize individuals who have memory or other cognitive impairments beyond those expected for their age. In MCI individuals, there are subjective memory complaints that are documented by abnormal performances on objective cognitive tests. These deficits have little or no effect on the ability to perform daily living activities, and the patient should not display any evidence of dementia 1. MCI is commonly thought to be a transitional state between healthy aging and Alzheimer's disease (AD) 2. MCI is a part of AD symptom development and progression. The yearly probability of developing or “converting” to AD is higher in MCI patients than in normal aging individuals 3. Because detecting patients in the preclinical phase of AD offers the best potential for intervention 4, it is important to distinguish early AD and MCI from normal aging using various diagnostic means.
Previous research has focused on using brain imaging to better understand the neural networks involved in MCI. Numerous functional magnetic resonance imaging (fMRI) studies using a range of working memory (WM) tasks have demonstrated that there are different brain activity patterns in MCI patients when compared with healthy elderly subjects 5, 6. Working memory, the ability to hold information in memory while performing another mental operation 7, has been suggested to be an essential component of higher cognition skills, such as decision making, reasoning, problem solving, learning, and language. WM is one aspect of memory that is affected in the early stages of AD and MCI 8, 9, 10.WM has also been investigated in MCI, and MCI patients appear to recruit alternate WM networks compared with healthy elderly subjects. Saykin et al. examined a verbal 0‐, 1‐, and 2‐back WM task and found that MCI patients had reduced bilateral activation in the parietal and frontal regions compared with healthy elderly subjects 11. Other researchers have also found significant frontoparietal differences between MCI patients and healthy elderly subjects 5, 6. These findings suggest that reduced cortical activation in this neural network during WM may be associated with the neurodegenerative processes in MCI individuals.
A wide variety of brain functions have been evaluated using fMRI for research and clinical purposes. However, fMRI devices are too expensive for routine clinical use 12, and researchers require alternative imaging techniques. Functional near‐infrared spectroscopy (fNIRS) is an optical method that allows noninvasive, in vivo measurement of the concentrations of oxygenated and deoxygenated hemoglobin in cortical areas 13. fNIRS is a recently developed and relatively new optical imaging technology that uses light in the near‐infrared spectrum (670–900 nm) to noninvasively monitor the hemodynamic responses evoked by brain activity and to measure quantitative changes in the concentrations of two blood chromophores: oxygenated hemoglobin (oxy‐Hb) and deoxygenated hemoglobin (deoxy‐Hb) 14, 15. fNIRS has several unique advantages over functional neuroimaging methodologies, such as fMRI, including portability, fewer constraints, low susceptibility to movement artifacts and a high temporal sampling rate (≥10 Hz). fNIRS is considered to be a more convenient and promising technique for studying special populations, such as infants and other patients who are considered to be unsuitable for fMRI studies. To date, fNIRS has been frequently used to investigate focal brain activation during cognitive engagement in healthy participants 16, 17, 18, 19 and in patients with psychiatric or cognitive disorders, such as schizophrenia, depression, bipolar disorder, and MCI 12. However, most of these studies have used verbal fluency tests (VFTs) 5 as their activation task, and only a limited number of studies have used the n‐back task to assess memory and manipulation ability using the fNIRS technique. Because performance on WM tasks reflects both memory and cognitive ability, it is a more meaningful measurement of cognitive degeneration in MCI patients.
In the present study, therefore, we combined the fNIRS technique and the n‐back memory task to investigate cortical activation in MCI patients. We hypothesized that the MCI patients would have reduced activation in their frontal and temporal activation patterns and would have poorer behavioral performances compared with healthy controls.
Materials and Methods
Participants
The present study included eight amnestic MCI patients and 16 sociodemographically matched healthy controls. The participants were recruited from three resources: the Neurology Department Clinic of the Beijing Hospital, the Clinic of the China Academy of Chinese Medical Sciences, and the research center for cognitive aging and brain health at the State Key Laboratory for Cognitive Neuroscience and Learning, Beijing Normal University. The participants were all right handed and were native Chinese speakers. All of the participants were selected according to the following criteria: (1) aged 57–70 years old, (2) completed no <6 years of education, (3) scored 24 or higher on the Chinese version of the Mini‐Mental‐Status Examination (MMSE) 20, (4) had no history of neurological, psychiatric, or systemic illness known to influence cerebral function, such as serious vascular diseases, head trauma, tumors, current depression, alcoholism, and epilepsy, (5) had no history of psychoactive medication use, and (6) met the physical demands of the imaging scanner. All of the MCI patients were diagnosed using the Petersen criteria for amnestic MCI 2, 21, 22, including subjective memory complaints, cognitive memory impairment (scoring more than 1.5 standard deviations below the age‐ and education‐adjusted norm on the Auditory Verbal Learning Test (AVLT) 23), normal general cognitive function (scoring no <24 on the MMSE, except for two subjects who scored 22 and 23), and preserved activities of daily living (scoring 0 on the Activities of Daily Living (ADL) scale 24). The demographic information for each group and the between‐group comparisons are presented in Table 1. The study was approved by the Institutional Review Board of the Beijing Normal University Imaging Center for Brain Research. Written informed consent was obtained from all participants.
Table 1.
The demographics and neuropsychological tests of all the participants
| Characteristic,Mean ± SD | Control (n = 16) | MCI (n = 8) | T or χ2 statistic | P‐value |
|---|---|---|---|---|
| Age (years) | 63.1 ± 5.3 | 64.8 ± 7.2 | 0.97 | 0.38 |
| Education (years) | 11.2 ± 2.5 | 11.2 ± 3.0 | 0.11 | 0.90 |
| General mental status | ||||
| MMSE | 28.4 ± 1.1 | 26.3 ± 2.3 | 3.04 | <0.006 |
| Memory | ||||
| AVLT‐delay recall | 6.8 ± 2.5 | 3.6 ± 2.6 | 2.79 | <0.011 |
| AVLT‐total | 35.3 ± 10.4 | 23.4 ± 7.9 | 2.82 | <0.010 |
| Executive function | ||||
| Stroop test time (s) | 29.4 ± 6.2 | 38.4 ± 11.5 | −2.50 | <0.020 |
| Stroop test correct answers | 49.4 ± 0.7 | 46.3 ± 5.6 | 2.29 | <0.032 |
| Language ability | ||||
| BNT | 25.9 ± 2.2 | 23.9 ± 3.7 | 1.70 | NS |
The neuropsychological scores were compared between the MCI and control groups using t‐tests. P < 0.05 was considered to be significant. NS, not significant; MMSE, the Chinese version of the Mini‐Mental‐Status Examination; AVLT, Auditory Verbal Learning Test; BNT, Boston Naming Test.
Neuropsychological Testing
All of the participants received a battery of four neuropsychological tests assessing general mental status and other cognitive domains, such as episodic memory, executive function, and language ability. As mentioned previously, general mental status was assessed using the MMSE. The episodic memory tests also included the AVLT 23. Executive function was assessed with the Stroop Test 25. Finally, language ability was assessed with the Boston Naming Test (BNT) 26. The neuropsychological characteristics for each group are presented in Table 1.
Experimental Protocol
A blocked periodic design that incorporated alternating 0‐ and 1‐back tasks was used during the WM task. There were three blocks in each condition. Within each block, 10 single digits were pseudo randomly shown to the participants. Every block contained 20 trials. Each digit was presented for 1000 ms, followed by an interstimulus interval of 1000 ms. Each block started with a 2 s cue presentation that indicated the 0‐ or 1‐back. During the 0‐back task, the participants were asked to decide whether the digit currently on the screen was the digit “4”. During the 1‐back task, the participants were asked to decide whether the current digit had appeared one position back in the sequence. Each sequence contained three targets, and the participants were asked to press a button with their right index finger as fast as possible when detecting a target. The stimuli were presented on a personal computer using E‐Prime (version 1.0; Psychology Software Tools, Inc., Pittsburgh, PA, USA). To ensure that each participant understood the instructions and performed the task correctly, they were asked to practice all blocks of the task for 10–15 min before the experiment.
fNIRS Data Acquisition and Data Processing
We used an ETG‐4000 (Hitachi Medical Co., Tokyo, Japan) Optical Topography system to measure the concentration changes in oxy‐Hb and deoxy‐Hb. We used the “3 × 11” measurement patches provided by Hitachi. Each patch included 17 emitters and 16 detector probes that were alternately positioned at inter‐optode distances of 30 mm, resulting in a total of 52 measurement channels (Figure 1). The sampling frequency was 10 Hz.
Figure 1.
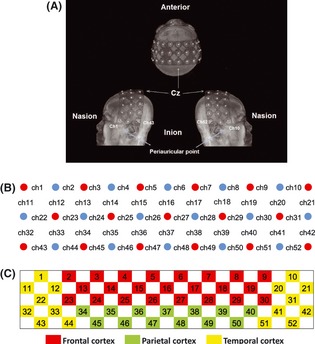
Schematic arrangement of the fNIRS probe array. (A) MRI image of the probe holder position; (B) the arrangement of the probe array of 17 emitters and 16 detectors and the international 10–20 system markers; (C) anatomical areas covered by the probe array according to the AAL template. Note that areas that were not of interest to this study are not highlighted in this figure.
Band‐pass filtering was applied to the raw data before further processing. The fNIRS data from each individual subject were processed channel by channel. High‐frequency physiological noise and low‐frequency baseline drift were removed using band‐pass filtering with cutoff frequencies of 0.3 and 0.01 Hz. The changes in the oxy‐Hb and deoxy‐Hb hemodynamic concentrations were then calculated using the modified Beer–Lambert law 27. Because oxy‐Hb signals can reflect changes in regional cerebral blood oxygenation and provide a better signal‐to‐noise ratio than can deoxy‐Hb signals 28, 29, we only investigated the oxy‐Hb variations during the WM task.
The data analysis was performed at the individual level first. For each subject, the individual hemodynamic concentration of oxy‐Hb was separately obtained by averaging the six blocks of the time series. A grand‐average of oxy‐Hb concentrations at the group level was then obtained by averaging all the of individual oxy‐Hb variations. The oxy‐Hb waveform changes for each subject were acquired from all 52 channels (Figure 1). A two‐sample t‐test was applied at the group level to determine the spatial locations that differed significantly between the MCI and the healthy control groups during the WM task.
Statistical Analysis
To test the group differences in age, years of education and neuropsychological scores, we analyzed the data using t‐tests. For the group oxy‐Hb changes, the comparisons between the MCI and control groups were performed using t‐tests. The mean value of the oxy‐Hb change was obtained for each measurement channel of each participant during the WM task. Finally, we investigated the relationship between oxy‐Hb and neuropsychological performance. Pearson correlation analyses were performed separately for the MCI and control groups.
Results
Demographics and Neuropsychological Testing
The demographic data are shown in Table 1. There were no significant differences in age or years of education between the MCI patients and the healthy controls (P > 0.05). As expected, the MMSE scores were significantly higher in the controls than in the MCI patients (P < 0.05). The neuropsychological characteristics of each group are shown in Table 1. The controls performed significantly better than the MCI patients on executive function and memory tasks, but the differences in language ability were not significant.
Behavioral Performance during Scanning
During the 0‐back test, the MCI group performed less well (mean of 90.7% correct, SD = 0.06) than the healthy control group (mean of 93.5% correct, SD = 0.13). However, both groups performed with a high accuracy and did not differ significantly (t = 0.58, P = 0.569). The MCI patients did not perform the task significantly slower than healthy controls (t = −1.675, P = 0.108). During the 1‐back test, the MCI group performed less well (mean of 88.7% correct, SD = 0.12) than the healthy control group (mean of 96.3% correct, SD = 0.06). However, both groups performed with a high accuracy, and the difference was marginally significant (t = 2.02, P = 0.055). The MCI patients did not perform the task significantly slower than did the healthy controls (t = −1.421, P = 0.169).
Temporal Correlates during WM
To observe the overall responses from the MCI and healthy control groups, the temporal oxy‐Hb profiles were grand‐averaged across the eight MCI patients and 16 healthy controls. Figure 2 shows the subject‐averaged oxy‐Hb temporal variation profiles on the 52 measurement channels. The grand‐averaged oxy‐Hb profile from healthy controls shows large activations across most of the channels during the WM task (Figure 2). The result of the independent t‐test for the between‐group comparisons of the [oxy‐Hb] changes during the WM task showed that the MCI patients had significantly reduced activation when compared with healthy controls in Channel 2 (t = 2.067, P = 0.050), Channel 4 (t = 2.023, P = 0.055, marginally significant difference), Channel 18 (t = 3.069, P = 0.006), and Channel 22 (t = 2.397, P = 0.025). Moreover, most of the channels that appeared to be deactivated in MCI patients were located in the frontal and temporal lobes, especially in the frontal lobe (Figure 3).
Figure 2.
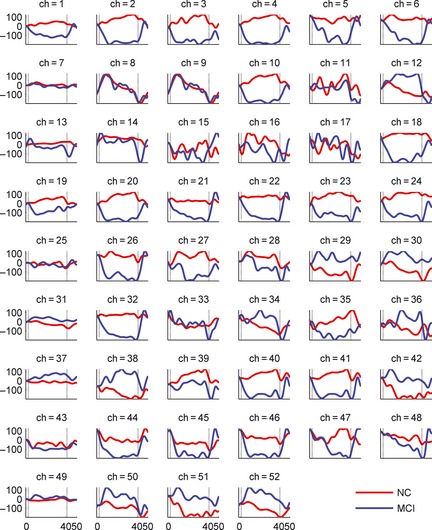
Grand‐average waveforms of oxy‐Hb concentration changes in 52 measurement channels during n‐back. The horizontal axis in each graph expresses the block time (s), and the vertical axis expresses hemoglobin concentration change (mM mm). The start time of the stimulation period was defined as 0 s. The red and blue lines express the averaged [Oxy‐Hb] changes in healthy controls (HC) and MCI, respectively.
Figure 3.
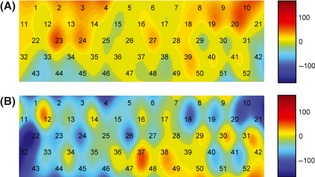
Topographical images of [oxy‐Hb] concentration. (A) activation pattern of the healthy controls during the n‐back task. (B) activation pattern of the MCI patients during the n‐back task. The numbers in the image indicate the measurement channels.
Spatial Correlates during WM
To investigate the spatial correlates in the MCI and healthy control groups during the WM task performance, a two‐dimensional topographical image of the calculated oxy‐Hb concentrations from all 52 channels was created using a nearest‐neighbor interpolation algorithm. The data were first averaged across all of the healthy control and MCI subjects, followed by a temporal average over the first 10–30 s of the task for each channel. Figure 3 shows the oxy‐Hb topographical images for the MCI and healthy control groups. It was found that the control image had brighter colors than the MCI image in most of the topographical areas, indicating that the healthy controls had increased brain activation and the MCI patients had decreased brain activation when performing the WM task. This finding is also consistent with the temporal correlates between the two groups when looking at all 52 measurement channels, as shown in Figure 2. Specifically, the hypoactivity observed in the MCI images is mostly located in the left brain areas.
The Correlation between the Oxy‐Hb Concentration and Neuropsychological Test Variables
We further tested whether there was a relationship between the variation of participants' oxy‐Hb concentrations in the frontal‐temporal lobes and their performances on the neuropsychological tests. The mean oxy‐Hb concentrations for Channels 2 (r = 0.643, P < 0.001), Channel 4 (r = 0.689, P < 0.001), Channel 18 (r = 0.613, P < 0.001), and Channel 22 (r = 0.740, P < 0.001) during the WM task were positively correlated with the number of correct answers on the Stroop test. No significant correlations between the mean oxy‐Hb concentration and other neuropsychological parameters (the MMSE score, AVLT, Stroop time and BNT) were found in either group (Figure 4).
Figure 4.
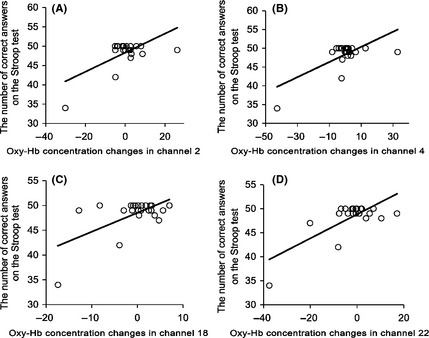
The correlations between the number of correct answers on the Stroop test and the oxy‐Hb concentration changes in (A) channels 2, (B) channels 4, (C) channels 18, and (D) channels 22. The relationships were caused by strong correlations in (A) channel 2 (r = 0.643, P < 0.001), (B) channel 4 (r = 0.689, P < 0.001), (C) channel 18 (r = 0.613, P < 0.001), and (D) channel 22 (r = 0.740, P < 0.001).
Discussion
We studied the fNIRS of the cortical hemoglobin oxygenation of areas that process WM in MCI patients and healthy controls. The statistical parameter mapping documented significant changes in brain activation across all channels. The MCI patients had lower oxy‐Hb concentrations in the left dorsolateral prefrontal area, right supplementary motor area and left superior temporal regions compared with the healthy subjects; additionally, the oxy‐Hb concentrations in these regions were significantly correlated with behavioral performance in both groups.
The regions observed to be engaged during the WM task were consistent with the findings of previous functional neuroimaging studies 5, 6, 11. Although a wide variety of stimuli, such as words, figures and pictures of faces, scenery, and objects, have been used to investigate WM in previous research, the frontal, parietal, and temporal regions have been identified as the key neural networks 30. The n‐back WM task requires the participants to use temporary information storage and manipulation during complex cognitive tasks 7. During the n‐back task used in this study, the participants were required to decide whether the current digit matched the one from n steps earlier in the sequence. The results confirm that the prefrontal lobes play a crucial role when performing n‐back tasks that require high levels of executive and memory function, such as the ability to remember and manipulate tasks. They also emphasize the value of multi‐channel NIRS for monitoring the brain activation associated with these cognitive progresses. This characteristic of the task may recruit the frontal and temporal cortex in normal aging individuals 31, 32.
Compared with the healthy controls, the MCI patients showed significantly reduced activation in the prefrontal region and supplementary motor area during the n‐back task. This result is consistent with several previous studies that have used other WM tasks 11. Saykin found that drug‐naive MCI patients had reduced fronto‐parietal network activity when compared with controls during an n‐back task. The results of our study suggest that the MCI patients failed to recruit sufficient frontal resources for the task and did not show the expected task‐related activation areas exhibited by the control subjects. However, some fMRI studies have reported that MCI patients had increased activation compared with the controls in the middle temporal, frontal 5, and parietal lobes 33. These results may reflect compensatory mechanisms in the brains of MCI patients, and the level of compensation may be an index of disease progression 34. Meanwhile, the variation in the results of fMRI studies may be partially due to differences in the cognitive tasks and processing methods. To the best of our knowledge, only a few NIRS studies on MCI have been published. Arai et al. 12 reported a decrease in the right parietal cerebral Hb oxygenation in MCI patients and a decrease in the frontal area and the bilateral parietal areas in the AD group during a Verbal Fluency Task (VFT) using fNIRS. A deficit in verbal fluency mainly appeared in Alzheimer disease patients but not in MCI patients. Our study used fNIRS to measure changes in cortical hemoglobin oxygenation in MCI patients during a WM task. We found MCI patients exhibited significantly decreased activation in the prefrontal lobes, which is different from the results of the VFT studies. Further studies using discriminative analysis are needed to determine whether these findings can be extended to the individual level. Our results confirm the dysfunction of the frontal and temporal cortices in MCI patients.
Task‐induced brain activation depends on the ability to perform the task 35. Successful performance is an influential factor in brain activation as measured by functional imaging 36, 37. The number of regions engaged in a task and the extent of the activation increase as the task difficulty and cognitive effort increases 38, 39. Therefore, we designed a simple WM (0‐ and 1‐back) task that could be a low WM load condition. Both the MCI patients and the controls were able to perform these tasks successfully enough to maintain a high accuracy rate. Because the ability to perform the task was similar between MCI patients and controls, it can be assumed that the effect of the performance level on brain function was minimized. Due to the simplicity of the task, the MCI patients and the controls could both perform the n‐back task accurately.
In the present study, the oxy‐Hb concentrations during the WM task were positively correlated with the accuracy rate of the Stroop task. WM tasks emphasize the mechanisms or processes that control, regulate, and maintain the activation of task‐relevant information 40. The capabilities of WM are related to process speed and executive function. Individual differences in WM capacity are also reflected in Stroop task performances, and the Stroop task also requires the maintenance of a single crucial goal in WM 41.
Some limitations of the present study must be mentioned. First, all of our MCI patients had taken Memantine before they participated in the study. However, to the best of our knowledge, they were not currently taking the anti‐AD medication, and there is no evidence in the literature of a direct relationship between anti‐AD medication (e.g., Memantine) effectiveness and oxy‐Hb concentration. A review has suggested that treatment with anti‐AD medication seems to normalize brain function and make the brain function of MCI patients more similar to that observed in healthy individuals 42, 43. Therefore, the findings in our study were likely caused by the disease rather than the medication, although we cannot completely rule out medication effects. Future studies with drug naïve patients are required to rule out medication effects and confirm the findings of this study. Second, because of the limited number of channels, the area of the NIRS measurement was restricted to the frontal cortex, parietal cortex, and parts of the temporal cortex. Simultaneous whole‐head NIRS measurements and other neuroimaging methodologies might be used to clarify the association of the frontal lobe with other brain regions. Finally, the spatial resolution of fNIRS is approximately 10–20 mm, which is lower than the spatial resolution of fMRI (1–3 mm). Limited spatial resolution may directly result in coarse spatial localization of brain regions. In the activation map (Figure 1), for example, it is difficult to accurately locate the corresponding functional brain area for the cognitive task.
In conclusion, this is the first study to use an n‐back task to evaluate cortex dysfunction in individuals with MCI using multi‐channel fNIRS. The results confirm functional deficits in the left dorsolateral prefrontal area, right supplementary motor area, and left superior temporal regions and the impaired cognitive ability of MCI patients, and these results suggest that fNIRS may be a useful clinical tool for evaluating cortex activation in cognitive disorders.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the Natural Science Foundation of China (Nos. 30873458 and 81173460), the Fundamental Research Funds for the Central Universities (No. 248‐105102), the Program for New Century Excellent Talents in University (No. NCET‐10‐0249), the Program for Excellent Doctoral Dissertation Foundation (No. 2007B7), and the Project of the Institute of Basic Research in Clinical Medicine (No. Z0175). The authors would like to thank the participating sites in the Clinic at the Neurology Department of Beijing Hospital and the Clinic at the China Academy of Chinese Medicine, all the volunteers and patients for their participation in our study.
The first two authors contributed to the work equally.
References
- 1. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303. [DOI] [PubMed] [Google Scholar]
- 2. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–94. [DOI] [PubMed] [Google Scholar]
- 3. Lopez OL, Becker JT, Jagust WJ, et al. Neuropsychological characteristics of mild cognitive impairment subgroups. J Neurol Neurosurg Psychiatry 2006;77:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ernst RL, Hay JW, Fenn C, Tinklenberg J, Yesavage JA. Cognitive function and the costs of Alzheimer disease. An exploratory study. Arch Neurol 1997;54:687–93. [DOI] [PubMed] [Google Scholar]
- 5. Yetkin FZ, Rosenberg RN, Weiner MF, Purdy PD, Cullum CM. FMRI of working memory in patients with mild cognitive impairment and probable Alzheimer's disease. Eur Radiol 2006;16:193–206. [DOI] [PubMed] [Google Scholar]
- 6. Goekoop R, Rombouts SA, Jonker C, et al. Challenging the cholinergic system in mild cognitive impairment: a pharmacological fMRI study. Neuroimage 2004;23:1450–9. [DOI] [PubMed] [Google Scholar]
- 7. Baddeley A. The episodic buffer: a new component of working memory? Trends Cogn Sci 2000;4:417–23. [DOI] [PubMed] [Google Scholar]
- 8. Rochon E, Waters GS, Caplan D. The relationship between measures of working memory and sentence comprehension in patients with Alzheimer's disease. J Speech Lang Hear Res 2000;43:395. [DOI] [PubMed] [Google Scholar]
- 9. Germano C, Kinsella GJ. Working memory and learning in early Alzheimer disease. Neuropsychol Rev 2005;15:1–10. [DOI] [PubMed] [Google Scholar]
- 10. Economou A, Papageorgiou SG, Karageorgiou C, Vassilopoulos D. No episodic memory deficits in amnestic MCI. Cogn Behav Neurol 2007;20:99–106. [DOI] [PubMed] [Google Scholar]
- 11. Saykin AJ, Wishart HA, Rabin LA, et al. Cholinergic enhancement of frontal lobe activity in mild cognitive impairment. Brain 2004;127:1574–83. [DOI] [PubMed] [Google Scholar]
- 12. Arai H, Takano M, Miyakawa K, et al. A quantitative near‐infrared spectroscopy study: a decrease in cerebral hemoglobin oxygenation in Alzheimer's disease and mild cognitive impairment. Brain Cogn 2006;61:189–94. [DOI] [PubMed] [Google Scholar]
- 13. Yamashita Y, Maki A, Ito Y, Watanabe E, Mayanagi Y, Koizumi H. Noninvasive near‐infrared topography of human brain activity using intensity modulation spectroscopy. Opt Eng 1996;35:1046–9. [Google Scholar]
- 14. Jobsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 1977;198:1264–7. [DOI] [PubMed] [Google Scholar]
- 15. Villringer A, Chance B. Non‐invasive optical spectroscopy and imaging of human brain function. Trends Neurosci 1997;20:435–42. [DOI] [PubMed] [Google Scholar]
- 16. Gervain J, Macagno F, Cogoi S, Pena M, Mehler J. The neonate brain detects speech structure. Proc Natl Acad Sci U S A 2008;105:14222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakano T, Watanabe H, Homae F, Taga G. Prefrontal cortical involvement in young infants' analysis of novelty. Cereb Cortex 2009;19:455–63. [DOI] [PubMed] [Google Scholar]
- 18. Sugiura L, Ojima S, Matsuba‐Kurita H, et al. Sound to language: different cortical processing for first and second languages in elementary school children as revealed by a large‐scale study using fNIRS. Cereb Cortex 2011;21:2374–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zeff BW, White BR, Dehghani H, Schlaggar BL, Culver JP. Retinotopic mapping of adult human visual cortex with high‐density diffuse optical tomography. Proc Natl Acad Sci USA 2007;104:12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang M, Katzman R, Salmon D, et al. The prevalence of dementia and Alzheimer's disease in Shanghai, China: impact of age, gender, and education. Ann Neurol 1990;27:428–37. [DOI] [PubMed] [Google Scholar]
- 21. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer disease: recommendations from the National Institute on Aging‐Alzheimer Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morris JC. Revised criteria for mild cognitive impairment may compromise the diagnosis of Alzheimer disease dementia. Arch Neurol 2012; 69:700–8: archneurol. 2011.3152 v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo Q, Zhao Q, Chen M, Ding D, Hong Z. A comparison study of mild cognitive impairment with 3 memory tests among Chinese individuals. Alz Dis Assoc Dis 2009;23:253. [DOI] [PubMed] [Google Scholar]
- 24. Zhang M, He Y. Activities of daily living scale. Shanghai Archives of Psychiatry 1995;7:5–6. [Google Scholar]
- 25. Guo Q, Hong Z, Lv C, Zhou Y, Lu J, Ding D. Application of Stroop color‐word on Chinese elderly patients with mild cognitive impairment and mild Alzheimer's dementia. Chin J Neuromed 2005;4:701–4. [Google Scholar]
- 26. Guo Q, Hong Z, Shi W, Sun Y, Lv C. Boston naming test in Chinese elderly, patient with mild cognitive impairment and Alzheimer's dementia. Chin Ment Health J 2006;20:81–5. [Google Scholar]
- 27. Franceschini MA, Joseph DK, Huppert TJ, Diamond SG, Boas DA. Diffuse optical imaging of the whole head. J Biomed Opt 2006;11:054007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Homae F, Watanabe H, Otobe T, et al. Development of global cortical networks in early infancy. J Neurosci 2010;30:4877–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hoshi Y. Functional near‐infrared optical imaging: utility and limitations in human brain mapping. Psychophysiology 2003;40:511–20. [DOI] [PubMed] [Google Scholar]
- 30. Hautzel H, Mottaghy FM, Schmidt D, et al. Topographic segregation and convergence of verbal, object, shape and spatial working memory in humans. Neurosci Lett 2002;323:156–60. [DOI] [PubMed] [Google Scholar]
- 31. Grady CL, McIntosh AR, Bookstein F, Horwitz B, Rapoport SI, Haxby JV. Age‐related changes in regional cerebral blood flow during working memory for faces. Neuroimage 1998;8:409–25. [DOI] [PubMed] [Google Scholar]
- 32. Grady CL, Yu H, Alain C. Age‐related differences in brain activity underlying working memory for spatial and nonspatial auditory information. Cereb Cortex 2008;18:189–99. [DOI] [PubMed] [Google Scholar]
- 33. Bokde ALW, Karmann M, Born C, et al. Altered brain activation during a verbal working memory task in subjects with amnestic mild cognitive impairment. J Alzheimers Dis 2010;21:103–18. [DOI] [PubMed] [Google Scholar]
- 34. Dickerson BC, Sperling RA. Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer's disease: insights from functional MRI studies. Neuropsychologia 2008;46:1624–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maguire EA, Vargha‐Khadem F, Mishkin M. The effects of bilateral hippocampal damage on fMRI regional activations and interactions during memory retrieval. Brain 2001;124:1156–70. [DOI] [PubMed] [Google Scholar]
- 36. Smith EE, Geva A, Jonides J, Miller A, Reuter‐Lorenz P, Koeppe RA. The neural basis of task‐switching in working memory: effects of performance and aging. Proc Natl Acad Sci USA 2001;98:2095–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mencl WE, Pugh KR, Shaywitz SE, et al. Network analysis of brain activations in working memory: behavior and age relationships. Microsc Res Tech 2000;51:64–74. [DOI] [PubMed] [Google Scholar]
- 38. McAllister TW, Sparling MB, Flashman LA, Guerin SJ, Mamourian AC, Saykin AJ. Differential working memory load effects after mild traumatic brain injury. Neuroimage 2001;14:1004–12. [DOI] [PubMed] [Google Scholar]
- 39. Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD. Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia 1997;35:1373–80. [DOI] [PubMed] [Google Scholar]
- 40. Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci 2003;4:829–39. [DOI] [PubMed] [Google Scholar]
- 41. Long DL, Prat CS. Working memory and stroop interference: an individual differences investigation. Mem Cognit 2002;30:294–301. [DOI] [PubMed] [Google Scholar]
- 42. Petrella JR, Prince SE, Krishnan S, Husn H, Kelley L, Doraiswamy PM. Effects of donepezil on cortical activation in mild cognitive impairment: a pilot double‐blind placebo‐controlled trial using functional MR imaging. AJNR Am J Neuroradiol 2009;30:411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bentley P, Driver J, Dolan RJ. Cholinesterase inhibition modulates visual and attentional brain responses in Alzheimer's disease and health. Brain 2008;131:409–24. [DOI] [PMC free article] [PubMed] [Google Scholar]


