Summary
Aims
Prior studies have demonstrated the involvement of leptin and cannabinoids in food intake and metabolism. However, the interaction between leptin and cannabinoids in epilepsy has not been studied. This study elucidated the relationship between leptin and cannabinoids in penicillin‐induced epileptiform activity in rats.
Methods
The CB1 receptor agonist, arachidonyl‐2‐chloroethylamide (ACEA), at doses of 2.5 and 7.5 μg, the CB1 receptor antagonist, [N‐(piperidine‐1‐yl)‐5‐(4‐iodophenyl)‐1‐(2,4‐dichlorophenyl)‐4‐methyl‐1H‐pyrazole‐3 carboxamide] (AM‐251), at doses of 0.125 and 0.25 μg, and leptin, at the dose of 1 μg, were administered intracerebroventricularly (i.c.v.) 30 min after intracortical penicillin (i.c.) application.
Results
Leptin caused proconvulsant activity in all groups. The administration of AM‐251, at a dose of 0.25 μg, increased the frequency of penicillin‐induced epileptiform activity by producing status epilepticus‐like activity, whereas AM‐251, at a dose of 0.125 μg, was not effective when applied alone. ACEA, at a dose of 7.5 μg, decreased the frequency of epileptiform activity. Leptin reversed the anticonvulsant activity of ACEA and enhanced the proconvulsant activity of AM‐251.
Conclusions
This study provides electrophysiological evidence that the proconvulsant activity of leptin is mediated, at least in part, by inhibition of cannabinoids in the experimental model of epilepsy.
Keywords: Cannabinoids, Epilepsy, Epileptiform activity, Leptin, Penicillin
Introduction
Epilepsy represents a chronic neurological disease characterized by recurrent spontaneous seizures that have been associated with an imbalance between excitatory and inhibitory systems in selected regions of the brain 1. Several mechanisms have been proposed to explain why excitability of neurons increases in epilepsy.
Several lines of evidence suggest that leptin and the cannabinoid system play key roles in regulating seizure activity in the brain 2, 3, 4. Most experimental studies have revealed an inhibitory role for leptin in preventing seizures 2, 3, 5. Leptin inhibited epileptiform‐like activity in rat hippocampal cultures, 4‐aminopyridine‐induced convulsions, and pentylenetetrazole‐induced convulsions 2, 6, 7. In contrast, a few studies showed that leptin can cause an increase in the excitability of neurons 8, 9. Leptin increased the frequency of action potentials of proopiomelanocortin neurons 9 and of penicillin‐induced epileptiform activity in rats 10. Lynch III et al. 11 demonstrated that leptin has proconvulsant activity with at least two glutamate receptor agonists: NMDA and kainate.
On the other hand, cannabinoids have been used for the treatment of seizure for many years. The anticonvulsant effects of cannabinoids are mediated through activation of the cannabinoid CB1 receptors according to various models of experimental epilepsy, such as the maximal electroshock of grand‐mal seizure 12, the rat pilocarpine model of acquired epilepsy 13, the in vitro hippocampal neuronal culture models of acquired epilepsy and status epilepticus 14, the pentylenetetrazole (PTZ) model of myoclonic seizures in mice 15 and the penicillin‐induced model of epileptiform activity in rats 16. Some evidence also exists for a functional relationship between leptin and the cannabinoid system in the brain 17, 18, 19, 20. The first study of endocannabinoid level regulation in the hypothalamus was carried out by Di Marzo et al. 17 who showed that leptin inhibits endocannabinoid biosynthesis in the rat brain. Leptin also blocks glucocorticoid‐mediated endocannabinoid release in the paraventricular nucleus of the hypothalamus 19.
Little evidence is available to show the relationship between leptin and cannabinoid system in the regions associated with the different aspects of food intake and metabolism, and no reports exist regarding their interaction in the experimental models of epilepsy. Therefore, we decided to initiate the first investigation into the effects of intracerebroventricular (i.c.v.) injection of the CB1 agonist, ACEA, and the CB1 antagonist, AM‐251, on leptin interactions in penicillin‐induced epileptiform activity in the rats.
Materials and Methods
Animals
Male Wistar rats (230–260 g) were purchased from The Animal House of Ondokuz Mayis University and kept in a temperature controlled (22 ± 1°C) environment on a 12‐h light/dark cycle. All experimental procedures were approved by the local ethics committee (2009/61). Rats were assigned to the following experiments and groups for intracortical (i.c.) delivery of
2.5 μL artificial cerebrospinal fluid [aCSF containing the following(mM): NaCl, 124; KCl, 5; KH2PO4, 1.2; CaCl2, 2.4; MgSO4, 1.3; NaHCO3, 26; glucose, 10; HEPES, 10; pH 7.4 when saturated with 95% O2 and 5% CO2] (i.c.)
500 units penicillin (2.5 μL, i.c.)
500 units penicillin (2.5 μL, i.c.) + 7.5 μg ACEA (i.c.v.)
500 units penicillin (2.5 μL, i.c.) + 0.25 μg AM‐251 (i.c.v.)
500 units penicillin (2.5 μL, i.c.) + 1 μg leptin (i.c.v.)
500 units penicillin (2.5 μL, i.c.) + 1 μg leptin (i.c.v.) + 0.25 μg AM‐251 (i.c.v.)
500 units penicillin (2.5 μL, i.c.) + 1 μg leptin (i.c.v.) + 7.5 μg ACEA (i.c.v.)
500 units penicillin (2.5 μL, i.c.) + 0.125 μg AM‐251 (i.c.v.)
500 units penicillin (2.5 μL, i.c.) + 1 μg leptin (i.c.v.) + 0.125 μg AM‐251 (i.c.v.)
500 units penicillin (2.5 μL, i.c.) + 2.5 μg ACEA (i.c.v.)
500 units penicillin (2.5 μL, i.c.) + 1 μg leptin (i.c.v.) + 2.5 μg ACEA (i.c.v.)
7.5 μg ACEA (i.c.v.)
0.25 μg AM‐251 (i.c.v.)
1 μg leptin (i.c.v.)
1 μL dimethylsulfoxide (DMSO)/saline (3:7 volume/volume, i.c.v.).
Each animal group was composed of seven rats.
Placement of Electrodes for Electrocorticography (ECoG) Recordings
The animals were anesthetized with urethane (1.25 g/kg, i.p.) and placed in a rat stereotaxic apparatus. Under stereotaxic guidance, four screw electrodes were placed bilaterally over the somatomotor cortex along with a ground lead positioned over the nasal sinus. Bipolar two Ag–AgCl ball electrodes were placed over somatomotor cortex of each hemisphere 16. The electrocorticographic activity was continuously monitored on an eight‐channel recorder (PowerLab, 4/SP, AD Instruments, Castle Hill, NSW, Australia). The frequency and amplitude of epileptiform ECoG activity were analyzed off‐line.
Drug and Drug Administration
AM‐251 (N‐(piperidine‐1‐yl)‐5‐(4‐iodophenyl)‐1‐(2,4‐dichlorophenyl)‐4‐methyl‐1H‐pyrazole‐3‐carboxamide) and ACEA (arachidonyl‐2‐chloroethylamide) (Sigma Chemical Co., St. Louis, MO, USA) were used in the experiments. AM‐251 and ACEA were dissolved in dimethylsulfoxide (DMSO) with added sterile physiological saline (final solution DMSO/saline 3:7 volume/volume, respectively), and the requisite doses were administered intracerebroventricularly in a volume of 1 μL. Intracerebroventricular injections were administered into the left lateral ventricle of each rat through a stereotaxic apparatus, with the coordinates of 0.8 mm posterior to the bregma, 2.0 mm lateral to the midline, and 4.2 mm ventral to the surface of the skull, based on the atlas of the rat brain 21. All drugs were injected in an infusion rate of 0.5 μL/min using a Hamilton microsyringe type 701N, and an additional minute was allowed to elapse before removal of needle to avoid backflow of the drug.
In the first set of experiments, ACEA, at doses of 2.5 and 7.5 μg (i.c.v.), AM‐251, at doses of 0.125 and 0.25 μg, or leptin, at the dose of 1 μg were administered 30 min after penicillin (i.c.) application. In the second set of experiments, the animals received leptin at the dose of 1 μg 10 min before either an effective dose of ACEA (7.5 μg, i.c.v.) or a non‐effective dose of ACEA (2.5 μg, i.c.v.) or an effective dose of AM‐251 (0.25 μg, i.c.v.), or a non‐effective dose of AM‐251 (0.125 μg, i.c.v.).
The epileptic focus was produced by injection of 500 units of penicillin G potassium (1 mm beneath the brain surface).
Statistical Analysis
Statistical comparisons were made using the GraphPad Instat (v3.06) software (GraphPad Software, San Diego, CA, USA). All data were expressed as means ± standard error of the mean (SEM). Data from electrophysiological recordings were analyzed by one‐way analysis of variance (anova) and Tukey–Kramer post hoc tests for comparisons. For all statistical tests, P < 0.05 was considered statistically significant.
Results
Intracortical injection of penicillin (500 units) induced an epileptiform activity characterized by bilateral high frequency and/or polyspike complexes and high voltage synchronized spike activity in all four recording leads (Figure 1A). Epileptiform activity began within 5 min and reached a constant level by 30 min after penicillin injection. The means of spike frequency and amplitude of epileptiform activity were 31.4 ± 5.1 and 28.2 ± 4.3 spike/min and 963 ± 137 and 807 ± 104 μV, in the left and right sides of the brain, respectively. The intracerebroventricular injections of leptin, at a dose of 1 μg, significantly increased the frequency of epileptiform activity in the 80 min after leptin injection, without changing the amplitude (Figure 2). The means of spike frequency and amplitude of epileptiform activity were 60.2 ± 7.4 and 57.2 ± 6.8 spike/min and 1006 ± 107 and 920 ± 94 μV in the left and right sides of the brain in the presence of leptin, respectively (Figure 1B).
Figure 1.
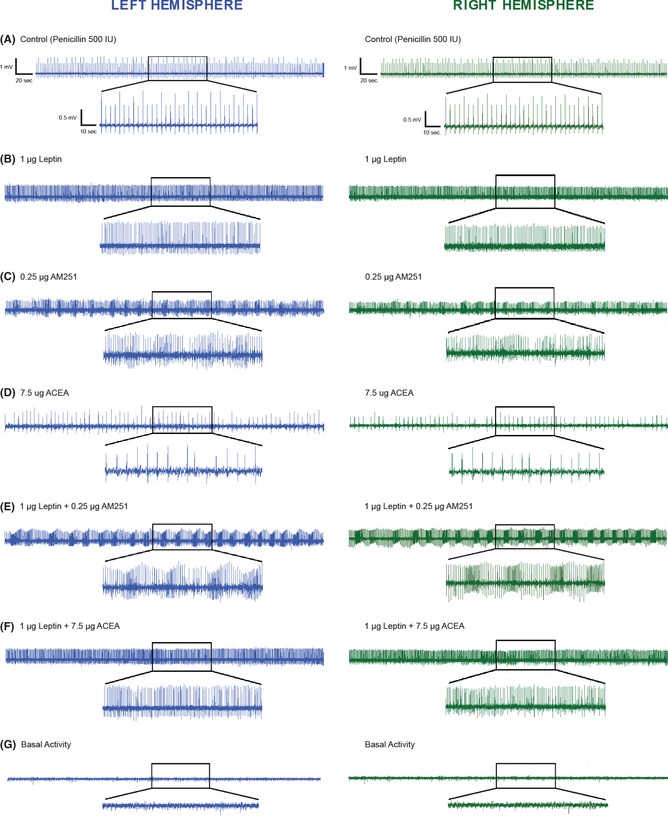
(A) The intracortical injection of penicillin (500 IU) induced epileptiform activity on ECoG. (B) The intracerebroventricular (i.c.v.) administration of leptin, at a dose of 1 μg, increased the mean frequency of penicillin‐induced epileptiform activity without changing the amplitude. (C) Administration of the CB1 receptor antagonist, AM‐251 (i.c.v.), at a dose of 0.25 μg, caused a marked increase in the frequency of penicillin‐induced epileptiform activity within 30 min after AM‐251 injection. (D) Administration of the CB1 receptor agonist, ACEA (i.c.v.), at a dose of 7.5 μg, significantly decreased the mean frequency of epileptiform activity within 50 min after ACEA injection. (E) The administration of AM‐251 (0.25 μg, i.c.v.) 10 min after leptin injection (1 μg, i.c.v.) increased the frequency of epileptiform activity within 40 min after leptin injection. (F) The administration of leptin (1 μg, i.c.v.) 10 min before ACEA (7.5 μg, i.c.v.) injection fully inhibited the anticonvulsant effects of ACEA. (G) Baseline ECoG activity before penicillin or the injection of other substances. Representative ECoGs are presented for the 120 min after penicillin administration.
Figure 2.
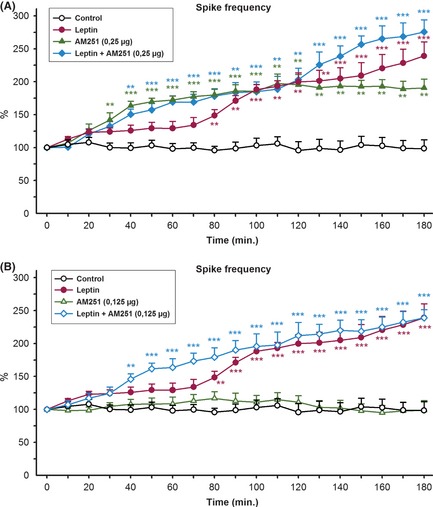
We used the most effective dose and a non‐effective dose of AM‐251 and ACEA in this study 16. The effective dose of AM‐251(0.25 μg, i.c.v.) caused a significant increase in the mean frequency of epileptiform activity in the 30 min after AM‐251 injection, without changing amplitude (Figure 2A). It also triggered the development of status epilepticus‐like activity, characterized by bursting spiking activity (Figure 1C). The means of spike frequency and amplitude of epileptiform activity were 58.2 ± 5.8 and 55.8 ± 6.2 spike/min and 893 ± 78 and 819 ± 66 μV in the left and right sides of the brain, respectively, in the presence of AM‐251 (0.25 μg, i.c.v.) (Figure 1C). The administration of AM‐251, at a dose of 0.125 μg, did not significantly change the frequency or amplitude of epileptiform activity (Figure 2B). Statistical analysis revealed that the intracerebroventricular injections of ACEA (7.5 μg, i.c.v.) protected against the penicillin‐induced epileptiform activity (Figure 3A). The anticonvulsant activity appeared in the 50 min after ACEA injection (Figure 3A). The means of spike frequency and amplitude of epileptiform activity were 10.8 ± 2.5 and 9.3 ± 2.2 spike/min and 901 ± 94 and 860 ± 87 μV in the left and right sides of the brain, respectively, in the presence of ACEA (7.5 μg, i.c.v.) (Figure 1D). The administration of ACEA (2.5 μg, i.c.v.) was not effective against penicillin‐induced epileptiform activity (Figure 3B).
Figure 3.
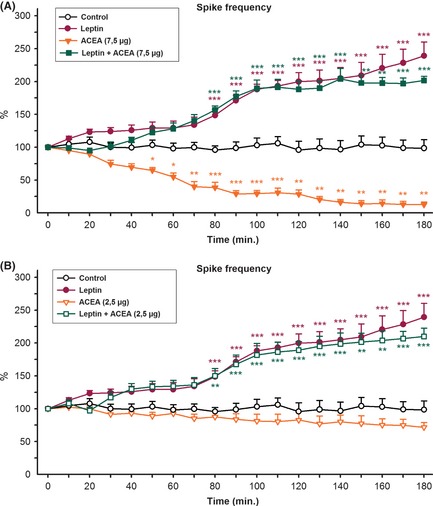
We decided to investigate the role of ACEA and AM‐251 in the proconvulsant effects of leptin on the penicillin‐induced epileptiform activity. For this purpose, the most effective dose and a noneffective dose of AM‐251 and ACEA were administered intracerebroventricularly 10 min after leptin (1 μg, i.c.v.) injection. The administration of leptin (1 μg, i.c.v.) and AM‐251 (0.25 μg, i.c.v.) significantly increased the mean frequency of epileptiform activity 40 min after leptin injection compared to application of leptin alone (Figure 2A). The means of spike frequency and amplitude of epileptiform activity were 62.3 ± 6.4 and 57.7 ± 5.6 spike/min and 862 ± 99 and 792 ± 92 μV in the left and right sides of the brain, respectively, in the presence of leptin (1 μg, i.c.v.) and AM‐251 (0.25 μg, i.c.v.) (Figure 1E). Nevertheless, the administration of a non‐effective dose of AM‐251 (0.125 μg, i.c.v.) 10 min after leptin injection exacerbated the frequency of epileptiform activity compared with the activity in the penicillin + AM‐251 (0.125 μg, i.c.v.) group (Figure 2B). Status epilepticus‐like activity also developed in the presence of leptin and AM‐251 (0.125 μg, i.c.v.). The proconvulsant effects appeared within 40 min after of leptin injection (Figure 2B). The administration of the most effective dose of ACEA (7.5 μg, i.c.v.) with leptin reversed the anticonvulsant activity of ACEA (Figure 3A). The proconvulsant activity appeared within 70 min after ACEA (7.5 μg, i.c.v.) administration in the presence of leptin (1 μg, i.c.v.) (Figure 3A). The mean frequency and amplitude of epileptiform activity were similar to those observed in the penicillin + leptin group (Figure 1F). The administration of a non‐effective dose of ACEA (2.5 μg, i.c.v.) did not affect proconvulsant activity of leptin (Figure 3B). The proconvulsant activity appeared within 70 min after ACEA administration (Figure 3B).
High frequency and amplitude of epileptiform activity were detected at the site of injection but this activity was also propagated to the contralateral cerebral cortex. No statistically significant difference was noted between the mean frequency and amplitude of epileptiform activity of left and right sides of the brain. As expected, intracortical injection of aCSF (2.5 μL), 1 μL dimethylsulfoxide (DMSO)/saline), and intracerebroventricular injection of the most effective of ACEA and AM‐251 did not cause any change in the frequency or amplitude of ECoG activity with respect to control baseline in non‐penicillin injected animals. The baseline ECoG activity before injection of penicillin or other substances is shown in Figure 1G.
Discussion
A link appears to exist between the cannabinoid system and leptin in the modulation of hypothalamic neuronal activity, which is associated with food intake and metabolism 17, 20. This study demonstrates that leptin reversed the anticonvulsant effect of the cannabinoid CB1 receptor agonist, ACEA, whereas it enhanced the proconvulsant effect of the cannabinoid CB1 receptor antagonist, AM‐251. In agreement with previous data, this study extends the connection between the cannabinoid system and leptin signaling in this experimental model of epilepsy.
The comparative analysis of epileptiform ECoG recordings in the right and left sides of the cerebral cortex in rats revealed that the differences between the frequency and amplitude of epileptiform activity in the right and left sides of the brain were not statistically significant in all experimental groups. This may suggest the involvement of all structures of both hemispheres in the mutual positive interaction during penicillin‐induced epileptic activity.
In general, leptin is used to test its effect on metabolic disorders in both in vitro and in vivo experimental studies. In addition to its potential metabolic effects, leptin has shown anticonvulsant activity in different studies 2, 3, 7. Mutant ob/ob mice were more likely to die and were more susceptible to generalized clonic and clonic–tonic seizures than were wild‐type mice when given submaximal PTZ doses 6. Direct injection of leptin partially decreased the cumulative seizure duration of ictal activity 7. In contrast, data from a number of experimental studies suggest that leptin may have proconvulsant properties 10, 11, 22. Cowley et al. 9 showed that leptin increases the frequency of action potentials in the proopiomelanocortin neurons, suggesting either depolarization caused by non‐specific cation channel or inhibition by neuropeptide Y/GABA neurons. Intracerebroventricular injection of leptin also increased the frequency of penicillin‐induced epileptiform activity in rats 10, 22. Leptin selectively increased the convulsion‐related signs induced by activation of the NMDA subtype of glutamate receptors over the AMPA subtype in the glutamate‐induced seizure model in mice 11. The results of the present study confirm that the administration of leptin, at a dose of 1 μg, significantly increased the mean frequency of epileptiform activity without changing the amplitude.
In contrast, cannabinoids are well documented to exhibit anticonvulsive and neuroprotective properties 12, 23, 24, 25. Doses of the cannabinoid selective agonist ACEA (2–8 mg/kg, i.p.) significantly increased the seizure threshold in the mouse PTZ model of myoclonic seizures 26. ACEA, at doses of 5 and 7.5 mg/kg, i.p., increased the electroconvulsive threshold whereas a dose of 15 mg/kg, i.p., did not significantly alter the threshold level in mice 23. The cannabimimetic compound, WIN 55,212‐2, suppressed spontaneous recurrent epileptiform discharges via CB1 receptor activation in the rat pilocarpine model of acquired epilepsy 27. WIN 55, 212‐2 also stereoselectively inhibited spontaneous recurrent epileptiform discharges in the in vitro hippocampal neuronal culture model of acquired epilepsy 14. The CB1 antagonist, AM‐251, produced continuous epileptiform discharges in the epileptic neurons, causing the development of status epilepticus‐like activity 28. Recently, Wendt et al. 24 suggested that WIN 55,212‐2 significantly delayed kindling acquisition. In the present study, ACEA, at dose of 7.5 μg, was effective at reducing the frequency of epileptiform activity whereas ACEA, at dose of 2.5 μg, had no anticonvulsive action against penicillin‐induced epileptiform activity. AM‐251, at dose of 0.25 μg, caused status epilepticus‐like activity and increased the mean frequency of epileptiform activity. The lower dose of AM‐251 (0.125 μg) did not cause either status epilepticus‐like activity or proconvulsant activity in this study. These results confirm those of previous studies, which suggested that CB1 receptors have a role in regulating the seizure frequency.
On the other hand, a possible interaction between leptin and the cannabinoid CB1 receptor has been suggested 17, 20. Leptin inhibits CB1 receptor expression in regions that process the rewarding and motivational signals associated with food intake 29. Jo et al. 18 demonstrated that the interaction between the leptin receptor and endocannabinoid signaling is responsible for the maintenance of weight balance in genetically modified mice. Leptin also inhibits glucocorticoid‐mediated endocannabinoid release in the hypothalamic paraventricular nucleus 19. Furthermore, Thanos et al. 20 showed differences in CB1 receptor binding expression in the brain of Ob and Le rats. They suggest that leptin receptor activity plays a role in inhibiting CB1 receptor in the brain, because low leptin levels and a restricted diet result in increases in CB1 receptor expression in Ob rats but not in the Le rats 20.
In agreement with previous studies, the present study showed that leptin blocked the anticonvulsant activity of ACEA against penicillin‐induced epileptiform activity. The proconvulsant activity started within 80 min in the presence of leptin and ACEA. Leptin also enhanced AM‐251 responsiveness in the penicillin model of epilepsy. Interestingly, leptin showed its own effect in the presence of leptin + ACEA. Exacerbated proconvulsant activity and the development of status epilepticus‐like activity were seen in the presence of leptin + AM‐251. The underlying molecular mechanism of both the enhanced AM‐251 responsiveness and the inhibition by ACEA effects remains speculative.
Leptin causes depolarization by inhibiting neuropeptide Y/GABA neurons, and it inhibits endocannabinoid synthesis in the brain, while cannabinoids are known to have CB1 receptor dependent anticonvulsant effects in various models of epilepsy. Therefore, it is logical to expect a proconvulsant effect in the presence of leptin, as was observed in the present study.
Conclusion
The administration of leptin caused proconvulsant activity in the penicillin‐induced epileptiform activity in the rats. The CB1 receptor agonist, ACEA, showed anticonvulsant activity while the CB1 receptor antagonist, AM‐251, showed proconvulsant activity with a status epilepticus‐like activity. Administration of leptin reversed the anticonvulsant activity of ACEA and enhanced the proconvulsant activity of AM‐251. The present study provides electrophysiological evidence for the involvement of the cannabinoid system and leptin as modulators of the neuron excitability that underlies seizure in this experimental model of epilepsy.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
The authors would like to thank Drs. S. Andronati, L. Godlevsky, T. Karasyeva and P. Antonenko for the contributions have been made during our visit to Odessa/Ukraine. This work was supported by grant 110S266 from TUBITAK.
References
- 1. Dichter MA. The epilepsies and convulsive disorders In: Isselbacher KJ, editor. Harrison's Principles of Internal Medicine. New York: McGraw‐ Hill, 1994;2223–2233. [Google Scholar]
- 2. Shanley LJ, O'Malley D, Irving AJ, Ashford ML, Harvey J. Leptin inhibits epileptiform‐like activity in rat hippocampal neurones via PI 3‐kinase‐driven activation of BK channels. J Physiol 2002;545:933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harvey J. Leptin regulation of neuronal excitability and cognitive function. Curr Opin Pharmacol 2007;7:643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Messer RD, Levine ES. Epileptiform activity in the CA1 region of hippocampus becomes refractory to attenuation by cannabinoids in part because of endogenous γ‐ aminobutyric acid type B receptor activity. J Neurosci Res 2012;90:1454–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Obeid M, Frank J, Medina M, et al. Neuroprotective effects of leptin following kainic acid‐induced status epilepticus. Epilepsy Behav 2010;19:278–283. [DOI] [PubMed] [Google Scholar]
- 6. Erbayat‐Altay E, Yamada KA, Wong M, Thio LL. Increased severity of pentylenetetrazole induced seizures in leptin deficient ob/ob mice. Neurosci Lett 2008;433:82–86. [DOI] [PubMed] [Google Scholar]
- 7. Xu L, Rensing N, Yang XF, et al. Leptin inhibits 4‐aminopyridine‐ and pentylenetetrazole‐induced seizures and AMPAR‐mediated synaptic transmission in rodents. J Clin Invest 2008;118:272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Powis JE, Bains JS, Ferguson AV. Leptin depolarizes rat hypothalamic paraventricular nucleus neurons. Am J Physiol 1998;274:R1468–R1472. [DOI] [PubMed] [Google Scholar]
- 9. Cowley MA, Smart JL, Rubinstein M, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 2001;411:480–484. [DOI] [PubMed] [Google Scholar]
- 10. Ayyildiz M, Yildirim M, Agar E, Baltaci AK. The effect of leptin on penicillin‐induced epileptiform activity in rats. Brain Res Bull 2006;68:374–378. [DOI] [PubMed] [Google Scholar]
- 11. Lynch JJ III, Shek EW, Castagné V, Mittelstadt SW. The proconvulsant effects of leptin on glutamate receptor‐mediated seizures in mice. Brain Res Bull 2010;82:99–103. [DOI] [PubMed] [Google Scholar]
- 12. Wallace MJ, Wiley JL, Martin BR, DeLorenzo RJ. Assessment of the role of CB1 receptors in cannabinoid anticonvulsant effects. Eur J Pharmacol 2001;428:51–57. [DOI] [PubMed] [Google Scholar]
- 13. Falenski KW, Blair RE, Sim‐Selley LJ, Martin BR, DeLorenzo RJ. Status epilepticus causes a long‐lasting redistribution of hippocampal cannabinoid type 1 receptor expression and function in the rat pilocarpine model of acquired epilepsy. Neuroscience 2007;146:1232–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blair RE, Deshpande LS, Sombati S, Falenski KW, Martin BR, DeLorenzo RJ. Activation of the cannabinoid type‐1 receptor mediates the anticonvulsant properties of cannabinoids in the hippocampal neuronal culture models of acquired epilepsy and status epilepticus. J Pharmacol Exp Ther 2006;317:1072–1078. [DOI] [PubMed] [Google Scholar]
- 15. Gholizadeh S, Shafaroodi H, Ghasemi M, Bahremand A, Sharifzadeh M, Dehpour AR. Ultra‐low dose cannabinoid antagonist AM251 exchanges cannabinoid anticonvulsant effects in the pentylenetetrazole‐induced seizure in mice. Neuropharmacology 2007;53:763–770. [DOI] [PubMed] [Google Scholar]
- 16. Kozan R, Ayyildiz M, Agar E. The effects of intracerebroventricular AM‐251, a CB1‐receptor antagonist, and ACEA, a CB1‐receptor agonist, on penicillin‐induced epileptiform activity in rats. Epilepsia 2009;50:1760–1767. [DOI] [PubMed] [Google Scholar]
- 17. Di Marzo V, Goparaju SK, Wang L, et al. Leptin‐regulated endocannabinoids are involved in maintaining food intake. Nature 2001;410:822–825. [DOI] [PubMed] [Google Scholar]
- 18. Jo YH, Chen YJ, Chua SC Jr, Talmage DA, Role LW. Integration of endocannabinoid and leptin signaling in an appetite‐related neural circuit. Neuron 2005;48:1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malcher‐Lopes R, Di S, Marcheselli VS, et al. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci 2006;26:6643–6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thanos PK, Ramalhete RC, Michaelides M, Piyis YK, Wang GJ, Volkow ND. Leptin receptor deficiency is associated with upregulation of cannabinoid 1 receptors in limbic brain regions. Synapse 2008;62:637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney: Academic Press, 1986. [Google Scholar]
- 22. Aslan A, Yildirim M, Ayyildiz M, Güven A, Agar E. Interaction of leptin and nitric oxide pathway on penicillin‐induced epileptiform activity in rats. Brain Res 2010;1321:117–124. [DOI] [PubMed] [Google Scholar]
- 23. Luszczki JJ, Czuczwar P, Cioczek‐Czuczwar A, Czuczwar SJ. Arachidonyl‐2‐chloroethylamide, a highly selective cannabinoid CB1 receptor agonist enhances the anticonvulsant action of valproate in the mouse maximal electroshock‐induced seizure model. Eur J Pharmacol 2006;547:65–74. [DOI] [PubMed] [Google Scholar]
- 24. Wendt H, Soerensen J, Wotjak CT, Potschka H. Targeting the endocannabinoid system in the amygdala kindling model of temporal lobe epilepsy in mice. Epilepsia 2011;52:e62–e65. [DOI] [PubMed] [Google Scholar]
- 25. Cakil D, Yildirim M, Ayyildiz M, Agar E. The effect of co‐administration of the NMDA blocker with agonist and antagonist of CB1‐receptor on penicillin‐induced epileptiform activity in rats. Epilepsy Res 2011;93:128–137. [DOI] [PubMed] [Google Scholar]
- 26. Bahremand A, Shafaroodi H, Ghasemi M, Nasrabady SE, Gholizadeh S, Dehpour AR. The cannabinoid anticonvulsant effect on pentylenetetrazole‐induced seizure is potentiated by ultra‐low dose naltrexone in mice. Epilepsy Res 2008;81:44–51. [DOI] [PubMed] [Google Scholar]
- 27. Wallace MJ, Blair RE, Falenski KW, Martin BR, DeLorenzo RJ. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J Pharmacol Exp Ther 2003;307:129–137. [DOI] [PubMed] [Google Scholar]
- 28. Deshpande LS, Sombati S, Blair RE, Carter DS, Martin BR, DeLorenzo RJ. Cannabinoid CB1 receptor antagonist cause status epilepticus‐like activity in the hippocampal neuronal culture model of acquired epilepsy. Neurosci Lett 2007;411:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav 2005;86:773–795. [DOI] [PubMed] [Google Scholar]


