Summary
Aims
To investigate whether electroacupuncture (EA) produced analgesic effect and whether nuclear factor kappa B (NF‐κB) and cystathionine β synthase (CBS) involved in EA‐mediated analgesia in painful diabetic neuropathy in rats.
Methods
Diabetes was induced by an intraperitoneal injection of streptozotocin (STZ) in adult female rats. Mechanical pain threshold was measured by von Frey filaments. EA was applied at acupoint Zu‐San‐Li (ST‐36) in both hindlimbs. Western blot analysis was employed to detect changes in protein levels of NF‐κB and CBS in spinal dorsal root ganglion (DRGs).
Results
Mechanical allodynia was developed 2 weeks after STZ injection and lasted for another 4 weeks. STZ injection significantly enhanced expression of p65 and CBS in lumbar L4‐6 DRGs when compared with age‐matched controls. EA markedly attenuated mechanical allodynia. Importantly, EA treatment remarkably inhibited p65 and CBS expression in DRGs. Additionally, intrathecal injection of the p65 antagonist pyrrolidine dithiocarbamate attenuated mechanical allodynia and markedly inhibited CBS expression in DRGs in STZ rats.
Conclusions
These data indicate that EA produced an analgesic effect, which might be mediated at least in a part by inhibition of NF‐κB signaling pathway in primary sensory neurons in rats with diabetes.
Keywords: Dorsal root ganglion, Electroacupuncture, Hydrogen sulfide, Neuropathic pain, Nuclear factor kappa B
Introduction
Diabetic mellitus (DM), a debilitating chronic disease, is one of the leading causes of death in developed and developing countries. It is reported that about 70% of diabetic patients have various forms of nerve damage (neuropathy). The most common type of diabetic neuropathy is nerve damages in the periphery 1. It generally influences hands and feet 2. Peripheral neuropathy patients often experience aberrant pain sensation and painful diabetic peripheral neuropathy (PDPN), including spontaneous pain, allodynia (pain with innocuous stimuli, e.g., light touch), and hyperalgesia (severe pain with mild painful stimuli) 3. Diabetic neuropathic pain (DNP) imposes a negative impact on all aspects of daily life of patients with diabetes. However, diabetic neuropathic pain remains an unmet clinical problem and is poorly relieved by conventional analgesics.
Both conventional acupuncture and electroacupuncture (EA) have been used for treating chronic pain and diabetes for the past several decades 4, 5, 6. It has been shown that acupuncture results in a significant improvement both in general well‐being and in symptoms of chronic pain. Although these data suggest that EA could be a promising method to treat chronic pain in patients, scientific evidence is little, fragmentary, and often contradictory. Thus, further investigations into the efficacy of EA and its underlying mechanisms are definitely warranted.
Emerging evidence has suggested that transcription factor nuclear factor kappa B (NF‐κB) played an important role in immune responses and inflammatory diseases 7. While recently, many researches confirm that it is also involved in development of neuropathic pain 8. In a painful model induced by sciatic nerve injury, activated NF‐κB is significantly increased in dorsal root ganglions (DRGs) of rats 9. The sensitization of DRGs is considered to be a major cause of aberrant pain induced by diabetes 10. Hydrogen sulfide (H2S) is synthesized by the endogenous enzymes including cystathionine‐β‐synthetase (CBS) and cystathionine‐γ‐lyase (CSE) 11. It is increasingly recognized as a biologically important signaling molecule in various tissues and processes including pain and inflammation 12. A growing body of evidence suggests a pronociceptive role for H2S 13, 14. However, roles of NF‐κB and CBS‐H2S signaling pathway in diabetic neuropathic pain remain unknown. We therefore hypothesized that CBS‐H2S signal pathway activated by NF‐κB participates in diabetic mechanical allodynia and that EA produced analgesic effects and suppressed NF‐κB expression. To test this hypothesis, we investigated roles of CBS and p65 in DRGs in streptozotocin (STZ)‐injected rats with EA or sham EA treatment. Our results indicate that STZ‐induced peripheral mechanical allodynia is likely mediated by upregulation of p65 expression in DRGs and that EA produced analgesic effects via inhibition of NF‐κB‐CBS‐H2S signaling pathway.
Materials and Methods
Induction of Diabetes
All experiments were approved by the Institutional Animal Care and Use Committee at Soochow University and were in accordance with the guidelines of the International Association for the Study of Pain. Female Sprague‐Dawley rats initially weighting 160–180 g were used in our experiments. They were housed four per cage in a 12‐h/12‐h light/dark cycle and temperature‐controlled room (25 ± 1°C). Rats were allowed access to tap water and standard laboratory chow ad libitum.
Diabetes was induced by a single injection of STZ (65 mg/kg i.p., Sigma Chemicals, St. Louis, MO, USA), which was freshly dissolved in citrate buffer (10 mmol/L, Na citrate, pH 4.3‐4.4). The control group received citrate buffer only, in an equivalent volume. Two weeks later, diabetes was confirmed by measurements of fasting blood glucose (FBG) by glucometer (Johnson & Johnson, New Brunswick, NJ, USA) in blood samples obtained from the tail vein. Only rats with FBG >16.7 mmol/L and mechanical paw withdrawal threshold (PWT) <5 g were further used in the study.
Measurement of Hindpaw Withdrawal Threshold
Experiments were performed on STZ‐treated rats and age‐matched control rats. The mechanical PWT was examined before and after injection of STZ or citrate buffer. Behavioral test was performed between 08:00 and 11:00 am to avoid possible action of the circadian cycle and female hormones in rats. Rats were allowed to acclimate for 20–30 min. A series of calibrated von Frey filaments (VFF) (ranging from 0.4 to 15.0 g) were applied perpendicularly to the plantar surface of the hindpaw with a sufficient force to bend the filaments for 60s or until paw withdrew as described previously 15, 16. Paw withdrawal, paw raising, and hissing were considered positive reactions. To avoid injury during tests, the cutoff strength of the von Frey filament was 15.0 g. The tactile stimulus producing a 50% likelihood of withdrawal was determined using the “up‐down” method. Each trial was repeated two–three times at approximately 1‐min interval with the mean value of filament forces used as the force producing a withdrawal response. All behavioral studies were performed under “blind” conditions. The experimenter, who assigned the PWT scores, was masked to the control or drug assignment and to the sham or EA treatment as well.
Electroacupuncture Treatment
Electroacupuncture was applied at ST‐36 (stomach‐36, Zusanli) acupoints 4, 6 for 1 week on rats 3 weeks after STZ or citrate buffer injection. EA was delivered by a pair of stainless steel suture hook‐shaped needles inserted bilaterally at a depth of 5 mm into the skin and underlying muscles at ST‐36. The needles inserted into the acupoints were stimulated by an EA apparatus (Model G‐6805‐2; Shanghai Medical Electronic Apparatus Company, Shanghai, China) with a constant rectangular current of alternating trains of dense‐sparse frequencies (100 Hz for 1.05 seconds and 2 Hz for 2.85 seconds alternately, pulse width, 0.1 ms). This combination has been shown to induce maximal release of met‐enkephalin and dynorphin A 17, 18, 19. Electrical stimulus intensity was set at the threshold for a detectable muscle twitch (approximately 1 mA). The stimulation was delivered for 30 min once to observe the acute effect or 30 min once every day for consecutive 7 days to observe the accumulative effect in rats. For sham EA group, the needle set was inserted into the acupoints ST‐36 without real electrical stimulation. Acupoints BL‐43 (Gao Huang), which is irrelevant to hindpaws, was used as a control EA point.
Drug Application
The NF‐κB antagonist, pyrrolidine dithiocarbamate (PDTC; Sigma‐Aldrich, St. Louis, MO, USA), freshly dissolved in 0.9% sterile isotonic saline was used in this study. To avoid possible infection caused by implantation of intrathecal catheter, intrathecal drug was applied by acute intrathecal injection as described previously 20. Briefly, a 1‐mL hypodermic syringe was temporarily inserted into rat's subarachnoid space between lumbar vertebrae L5 and L6 under ethyl ether anesthesia. PDTC or normal saline (NS) was administered in a volume of 50 μL over 1 min. After injection, the needle was removed. Rats were recovered from anesthesia within 5 min, and no abnormal motor behavior was observed after injection. Rats were randomly divided into three groups: NS, PDTC 0.1, and 1.0 μg. To observe the accumulative effect, PDTC was intrathecally injected once daily for consecutive 7 days.
Western Blot Analysis
The expressions of p65 and CBS in DRGs from control and STZ‐injected rats with EA or sham EA were measured using Western blot analyses, as described previously 9, 12. The rats were euthanized by an overdose of chloral hydrate 4 weeks after the injection of citrate buffer or STZ. Six DRGs (L4‐L6) from both sides of spinal cord were quickly dissected out and put into liquid nitrogen, and then stored at −80°C for further examination.
The amount of proteins in the cytoplasmic extracts was quantified by Protein Quantitative Analysis Kit (P0012‐BCA; Beyotime, Shanghai, China). The homogenates were electrophoresed and then transferred to nitrocellulose membranes. Membranes were blocked for 2 h at room temperature in blocking buffer, and then incubated overnight with mouse anti‐p65 antibody (1:100, Santa Cruz, CA, USA) or mouse anti‐CBS antibody (1:1000, Abnova, Walnut, CA, USA). After washing three times each for 15 min, membranes were incubated for 2 h at room temperature with horseradish peroxidase‐conjugated secondary antibodies (1:2000 for NF‐κB or 1:4000 for CBS and β‐actin). Immunoreactive proteins were detected by ECL plus (BIOIND). The densities of protein bands were analyzed using NIH image J software (Bethesda, WA, USA).
Data Analysis
All data were expressed as mean ± SEM. The two‐sample t‐test, Friedman ANOVA followed by Dunn's post hoc test, Mann–Whitney test, or Kruskal–Wallis ANOVA followed by Tukey post hoc test was performed where appropriate using commercial software OriginPro 8 (OriginLab, Northampton, MA, USA) and Matlab (MathWorks, Natick, MA, USA). Normality and variance was checked for all analyses. A P value < 0.05 was considered statistically significant.
Results
STZ Injection Induces Mechanical Allodynia in Adult Female Rats
Streptozotocin has been widely used to induce diabetes in rodents for the study of pain associated with diabetic neuropathy 15, 16, 21, 22. Following a single injection of STZ, FBG, fasting body weight (FBW), and PWT of female rats were monitored for 12 weeks. A majority of the rats (72.5%) developed hyperglycemia 2 weeks after STZ injection. These rats displayed polyuria and an increase in food and water intake (data not shown). From the week 2 after STZ injection, the FBG of STZ rats maintained at a high level (Figure 1A, n = 6 for each group, *P < 0.01, Mann–Whitney test following Friedman ANOVA). Compared with citrate‐injected rats, the growth rate of STZ rats was noticeably reduced (Figure 1B, n = 6 for each group, *P < 0.05, Mann–Whitney test following Friedman ANOVA). In parallel with elevated FBG levels, STZ‐injected rats also developed mechanical allodynia when tested with VFF (Figure 1C, n = 6 for each group, *P < 0.01, Mann–Whitney test following Friedman ANOVA). The mechanical PWT was decreased substantially 2 weeks after STZ injection and lasted for 4 weeks. PWT returned to baseline 12 weeks after STZ injection.
Figure 1.
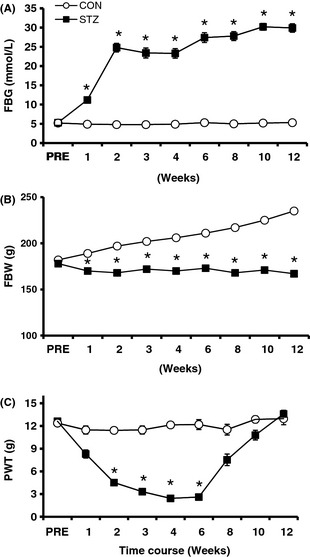
Hyperglycemia and mechanical allodynia in streptozotocin (STZ)‐induced diabetic rats. (A) Following a single i.p. injection of STZ, the fasting blood glucose level was significantly enhanced 1 week later. The enhancement persisted for at least another 11 weeks within our observation period of time. *P < 0.01 versus control, n = 6 for both groups. (B) The growth rate of STZ rats was reduced. The fasting body weight (FBW) of control rats exhibited a steady increase, whereas the FBW of STZ rats remained unchanged. *P < 0.05 versus control, n = 6 for both groups. (C) Paw withdrawal threshold (PWT) in response to von Frey filament stimulation was significantly lowered 2 weeks and reached the lowest point 4 weeks after STZ injection. The mechanical allodynia lasted for at least 4 weeks. Data points are expressed as mean ± SEM. Error bars smaller than the size of symbols are not shown. *P < 0.01 versus control, n = 6 for both groups.
Inhibition of NF‐κB Activity Suppresses STZ‐Induced Mechanical Allodynia
To determine whether NF‐κB is involved in the development of allodynia in STZ rats, PDTC was used in this study. PDTC has been shown to suppress the release of the inhibitory subunit IκB from the latent cytoplasmic form of NF‐κB, thereby indirectly inhibiting NF‐κB activation 23. There was a significant time and dose effect on PWTs after a single intrathecal injection of PDTC (n = 7 for each group, Friedman ANOVA). PDTC (0.1 μg) injection did not have significant effect on PWT (Figure 2A). But PDTC (1 μg) injection significantly increased PWTs 1 h after PDTC injection and lasted for another hour when compared with Preinjection group (Figure 2A, *P < 0.05, n = 7 for each group, Dunn's post hoc test following Friedman ANOVA), while the PWTs were also markedly increased at 30 min and lasted until 2 h after PDTC injection when compared with NS group (Figure 2A, #P < 0.05, n = 7 for each group, Tukey post hoc test following Kruskal–Wallis ANOVA). Injection of PDTC at the dose of 1 μg did not produce any effect on PWTs in age‐matched healthy control rats (n = 4, data not shown).
Figure 2.
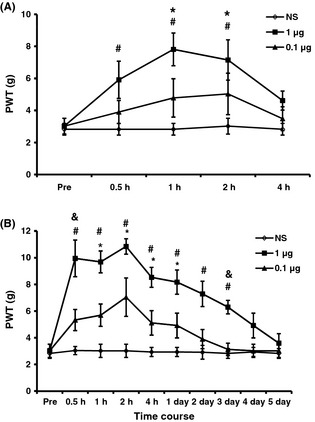
NF‐κB antagonist significantly attenuates streptozotocin‐induced mechanical allodynia. (A) Effects of a single intrathecal injection of pyrrolidine dithiocarbamate (PDTC). A single injection of PDTC (1 μg) significantly increased the paw withdrawal thresholds (PWTs), while NS injection or PDTC (0.1 μg) did not show any significant change in PWTs in diabetic rats when compared with those before injection (Pre). (B) Effects of PDTC injection once every day for consecutive 7 days. Note that the antiallodynia effect of PDTC (1 μg) lasted for 3 days. *P < 0.05 versus Pre, #P < 0.05 versus NS, & P < 0.05 versus 0.1 μg group, n = 7.
To further determine the antinociceptive effect of PDTC, the same doses of PDTC were injected intrathecally once a day for consecutive 7 days. PWTs were determined 30 min after last injection of PDTC and continued to be measured for 5 days. As expected, both doses of PDTC and test time affected PWTs significantly (Figure 2B, n = 7 for each group, Friedman ANOVA). PDTC (0.1 μg) injection did not have significant effect on PWT. But PDTC (1 μg) injection markedly increased the PWTs for about 3 days in STZ‐injected rats when compared with NS group and remarkably increased PWTs at 1 h to 1 day when compared with Preinjection group; meanwhile, PDTC (1 μg) injection markedly increased PWTs at 30 min and 3 days when compared with PDTC (0.1 μg) injection (Figure 2B, n = 7 for each group, *P < 0.05 versus Preinjection, using Dunn's post hoc test following Friedman ANOVA; #P < 0.05 1 μg compared with NS, & P < 0.05 1 μg compared with 0.1 μg, using Tukey post hoc test following Kruskal–Wallis ANOVA). NS injection did not produce any effect on PWTs.
Expressions of NF‐κB and CBS are Upregulated in STZ Rats
To determine the mechanism underlying the STZ‐induced mechanical allodynia, the expression levels of p65 and CBS in lumber DRGs were analyzed. Proteins were extracted from lumbar 4–6 DRGs of rats 4 weeks after STZ or citrate injection. As shown in figure 3A, p65 protein expression was increased by ∼3‐fold after STZ injection (**P < 0.01, n = 4 for STZ and n = 3 for control, two‐sample t‐test). In addition, STZ injection markedly increased expression of CBS when compared with controls. The CBS protein expression was increased by ~25% after STZ injection (**P < 0.01, n = 5 for each group, two‐sample t‐test, Figure 3B).
Figure 3.
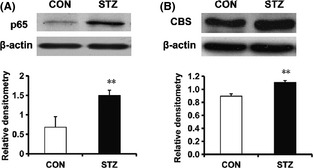
Streptozotocin (STZ) treatment enhances expression of p65 and cystathionine β synthase (CBS). Proteins were extracted from L4‐6 dorsal root ganglions in rats 4 weeks after STZ or citrate buffer injection. (A) The expressions of p65 were significantly enhanced in STZ‐injected rats (n = 4) when compared with controls (CON, n = 3). **P < 0.01 compared with those of CON. (B) CBS was significantly enhanced in STZ‐injected rats (n = 5) when compared with controls (n = 5). **P < 0.01 compared with those of CON. The protein expressions were normalized with their respective β‐actin.
Inhibition of NF‐κB Activity Reverses CBS Expression
To determine the relationship between NF‐κB and CBS, we measured CBS expression after treatment of PDTC. In association with the suppression of PWT, PDTC intrathecal injection (once every day for consecutive 7 days) remarkably reversed the expression of CBS in DRGs from STZ rats when compared with NS group (Figure 4, * P < 0.05, n = 6 for NS group and n = 5 for PDTC group, two‐sample t‐test).
Figure 4.
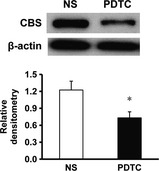
Pyrrolidine dithiocarbamate (PDTC) treatment reduces expression of cystathionine β synthase (CBS). The expression CBS in L4‐6 dorsal root ganglion was significantly reduced after 1‐week PDTC treatments (n = 5) in streptozotocin‐injected rats when compared with controls (NS, n = 6). The protein expressions were normalized with their respective β‐actin *P < 0.05.
EA Treatment Suppresses Mechanical Allodynia in Diabetic Rats
To determine whether EA suppressed mechanical allodynia in female diabetic rats, PWTs post‐EA treatment were compared with STZ rats treated with sham EA (sham). We first examined acute EA effect after 30‐min EA treatment. One‐time EA treatment markedly enhanced the PWTs at 2 and 4 h after EA when compared sham (*P < 0.05, n = 7 for each group, Mann–Whitney test following Friedman ANOVA, Figure 5A) and when compared with before EA treatment (# P < 0.05, n = 7 for each group, Dunn's post hoc test following Friedman ANOVA, Figure 5A). Sham EA treatment did not have any effect on PWTs when compared with before sham EA treatment.
Figure 5.
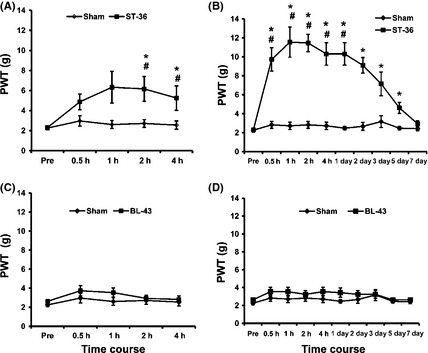
Inhibitory effect of electroacupuncture (EA) on mechanical threshold. (A) Effect of one‐time EA treatment (30 min). EA at ST‐36 produced the analgesic effect in streptozotocin (STZ) rats when compared with STZ rats with sham EA treatment (sham). Sham EA at ST‐36 and EA at BL‐43 did not produce any effect in STZ rats (BL‐43). n = 7 for each group. (B) Time course of prolonged analgesic effect of EA treatment. EA treatments were given once every day (30 min) for seven consecutive days in STZ rats. EA at ST‐36 greatly enhanced the mechanical threshold in STZ‐injected rats. This effect lasted for about 5 days. The sham EA treatment had no effect on paw withdrawal threshold (PWT). n = 7 for each group. *P < 0.05 when compared with values of sham. #P < 0.05 when compared with before EA treatment (Pre). (C) One‐time EA treatment at BL‐43 did not produce any effect on PWT. (D) EA treatment for seven consecutive days in STZ rats at BL‐43 did not produce any effect on PWT.
We then examined accumulative effect of EA on mechanical allodynia. EA treatment for seven times significantly increased the PWTs from 30 min to 1 day after accumulative EA treatment compared to those before EA treatment (# P < 0.05, n = 7 for each group, use Dunn's post hoc following Friedman ANOVA to test the effect of time, Figure 5B). EA treatment remarkably increased the PWTs from 30 min to 5 days after accumulative EA treatment compared to sham EA treatment (*P < 0.05, n = 7 for each group, Mann–Whitney test following Friedman ANOVA, Figure 5B). However, sham EA treatment did not have any effect on PWTs when compared with before sham EA treatment.
To further exclude nonspecific effect, EA treatment at Gao Huang (BL‐43) was performed. Gao Huang, an equivalent to the human acupoint BL‐43, was chosen as an irrelevant acupuncture point to the hindpaw. EA at BL‐43 for 30 min (Figure 5C, n = 7 for each group) or 30 min every day for consecutive 7 days did not produce any effect on PWTs in STZ rats (Figure 5D, n = 7 for each group). These data strongly suggested that EA treatment at ST‐36 suppressed the mechanical allodynia in rats with diabetes.
EA Treatment Suppresses p65 and CBS Expression in Diabetic Rats
To determine whether NF‐κB involved in EA‐induced analgesic effect, the effect of EA on the expression of p65 and CBS in L4‐6 DRGs from STZ‐injected rats was examined. EA treatment dramatically inhibited expression of p65 when compared with sham EA (Figure 6A, n = 6 for each group, *P < 0.05, Mann–Whitney test). In addition, EA treatment significantly reduced CBS expression in diabetic rats when compared with sham EA (Figure 6B, n = 8 for each group, *P < 0.05, two‐sample t‐test).
Figure 6.
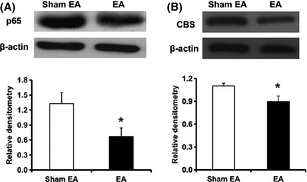
Inhibitory effect of electroacupuncture (EA) on p65 and cystathionine β synthase (CBS) expression. EA treatments at ST‐36 once every day (30 min) for seven consecutive days significantly inhibited the expression of p65 (A, n = 6 for each group, *P < 0.05) and CBS (B, n = 8 for each group, *P < 0.05) in L4‐6 dorsal root ganglion from streptozotocin rats when compared with sham EA. The protein expressions were normalized with their respective β‐actin.
Discussion
In the present study, we provide evidence for the functional involvement of the NF‐κB signaling in diabetic allodynia and in the EA‐induced analgesia in STZ‐injected female diabetic rats. In particular, the present findings identify that upregulation of p65 expression contributes to the mechanical allodynia through upregulation of CBS expression in DRGs in diabetic rats, as reflected by attenuation of mechanical allodynia and suppression of CBS expression by p65 inhibitor. Moreover, our studies demonstrate that downregulation of NF‐κB expression underlies the EA‐induced analgesic effect on the mechanical allodynia, as indicated by the inhibitory effects of EA on hindpaw withdrawal threshold and expression of p65 in lumber DRGs. To the best of our knowledge, this is the first report showing an involvement of NF‐κB‐CBS signaling in the primary sensory neurons in a painful diabetic rat model, and inhibition of this pathway underlies the EA‐induced analgesic effect in diabetic allodynia in female rats.
Streptozotocin‐induced diabetic rat model is a frequently used animal model for study of PDPN 24, 25. In this study, we successfully induced DM model in female rats by a single injection of STZ. The female rats were used in this experiment because there are relatively small researches on female animals used as diabetic model compared with male ones 26, 27, and the prevalence of PDPN is higher in female patient with diabetes than in male patients 28, 29. One week after STZ injection, these female rats showed a significant reduction in hindpaw mechanical threshold, indicating rapid onset of mechanical allodynia 30. The mechanical threshold was most conspicuous at week 4 and lasted to week 8 after STZ injection (Figure 1C). This is consistent with our previous report that mechanical threshold maintained at reduced level for 9 week within the observation period of time 16. However, we extended our observation period to 12 weeks and showed that the mechanical threshold returned to normal level 12 weeks after STZ injection. This is surprising. Although detailed mechanism for this “U” shape‐like change awaits further elucidations, hyperglycemia is an important factor leading to this change in diabetic neuropathy. It is likely that hyperglycemia might increase endogenous opioid peptide levels in spinal cord 31 or alter TRPV1 expression and function in primary sensory neurons 32, thus contributing to an increase in pain threshold or hypoalgesia in chronically diabetic rats.
In the present study, NF‐κB activation was involved in neuropathic pain in STZ‐induced diabetic female rats. This is evidenced by the following findings. Firstly, p65 inhibitor PDTC attenuated mechanical threshold in a dose‐dependent fashion. Notably, the inhibitory effect also strongly depends on the number of intrathecal injections of PDTC, as reflected by the statistically significant difference between single injection (acute effect) and multiple injections (accumulative effect). This result is consistent with previous report that NF‐κB was involved in cytokine‐mediated inflammatory pain 33. Secondly, expression of p65 in DRGs was significantly enhanced 4 weeks after STZ injection. Similarly, NF‐κB expression was increased in DRGs after peripheral nerve injury 9. Thirdly, EA produced an analgesic effect while suppressed expression of p65 in DRGs. Together, these findings suggest that activation of NF‐κB plays an important role in development of mechanical allodynia in female diabetic rats. However, the upstream factors for activation of NF‐κB remain unknown. Under inflammatory conditions, cytokine such as TNF‐α, IL‐1β, and IL‐6 might be the upstream molecular that stimulated NF‐κB activation, thus leading to inflammatory pain 33. Laughlin et al. 34 reported that NF‐κB played a role in dynorphin‐induced allodynia where cytokine is involved. Another possibility for activation of NF‐κB is advanced glycation end products (AGEs) and their receptors (RAGE). A recent study pointed that RAGE‐dependent NF‐κB activation might be an important mechanism to cause neuropathic dysfunction 35. Interaction of AGE and RAGE promotes activation of NADPH, enhances oxidative stress, and activates redox‐sensitive NF‐κB, thus leading to structural and functional changes of neurons and disorder of nerve conduction 36, 37. Therefore, it seems that there is a combination of various pathways that induced NF‐κB activation in diabetic mechanical allodynia. Further investigation is warranted to identify more upstream molecules in diabetic neuropathic pain.
NF‐κB, as a central transcriptional factor, has been thought to regulate expression of various key nociceptive genes under pathophysiological conditions 33, 38. In this study, we showed that CBS was markedly increased in STZ‐injected rats, indicating a role for CBS in diabetic neuropathic pain. Moreover, CBS expression was reduced significantly after administration of PDTC (Figure 4), indicating that upregulation of CBS protein expression was mediated by activation of NF‐κB. Although the detailed mechanism underlying the interaction of NF‐κB with CBS expression has yet to be investigated, it is tempting to speculate that activated NF‐κB enters into nuclear to combine with CBS gene and then regulates the expression of CBS at transcriptional level. As mentioned previously, H2S participated in regulations of many ion channels. H2S increased the influx of calcium ions 39. Increase in intracellular calcium concentration activates CaMKII, which promotes the trafficking of P2X3 receptor from cytoplasmic vesicles to cell membrane 40. There is an increasing body of evidence suggesting that P2X3 receptors in primary sensory neurons have a role in neuropathic pain 41, 42. Our recent article has reported that purinergic P2X3 receptor trafficking from cytosolic to cell surface membrane contributes to diabetic neuropathic pain 16. In addition, CBS and P2X3 receptors are colocalized in DRG neurons 12, indicating a possible modulation of P2X3 receptors by H2S in DRG neurons. Together, these data suggest that CBS‐H2S signaling pathway plays an important role in PDPN. Further experiments are warranted to determine the role for CBS‐H2S signaling pathway in P2X receptor‐mediated diabetic neuropathic pain and other signaling pathways involved.
Another important finding in the present study is that both acute and repetitive EA treatment produced a significant inhibitory effect on mechanical threshold in female diabetic rats. The accumulative EA‐produced effect maintained about 5 days (Figure 5). Sham EA at acupoints ST‐36 in diabetic rats failed to produce any effect on mechanical threshold, suggesting that EA‐produced analgesic effect is not a nonspecific effect. In the present study, EA treatment with same parameters at acupoint BL‐43 in diabetic rats failed to produce any effect on mechanical threshold. This may be because that acupoints at different locations may have diverse sensibility. EA treatment at BL‐43 may take effect at some other parameters. As ST‐36 is relevant to hindpaws, other acupoints relevant to hindpaws will be tested with EA treatment in future study. These findings support previous reports 4, 17 and provide additional evidence for EA treatment for diabetic pain. It appears that analgesic effect induced by EA in various conditions may be mediated by different mechanisms depending on the specific conditions 43, 44. In this study, we showed for the first time that repetitive EA treatment remarkably suppressed expression of p65 and CBS in lumber DRGs of female diabetic rats, indicating that inhibition of NF‐κB pathway might be involved in EA‐induced analgesia in diabetic pain. As inhibition of p65 by PDTC injection blocked upregulation of CBS expression (Figure 4), it is reasonable to speculate that EA‐induced reduction of CBS expression is mediated by inhibition of p65 expression.
In summary, this study is the first to demonstrate that NF‐κB‐induced upregulation of CBS expression contributes to mechanical allodynia in streptozotocin‐induced diabetic rats and provides evidence that EA treatment at ST‐36 attenuates the mechanical allodynia in association with reduced expression of NF‐κB in diabetic rats.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (81070884, 81230024) and from Jiangsu Province (SR21500111).
The first two authors contribute equally to this work.
References
- 1. Ross MA. Neuropathies associated with diabetes. Med Clin North Am 1993;77:111–124. [DOI] [PubMed] [Google Scholar]
- 2. Dyck PJ, Kratz KM, Karnes JL, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population‐based cohort: the Rochester Diabetic Neuropathy Study. Neurology 1993;43:817–824. [DOI] [PubMed] [Google Scholar]
- 3. Khan GM, Chen SR, Pan HL. Role of primary afferent nerves in allodynia caused by diabetic neuropathy in rats. Neuroscience 2002;114:291–299. [DOI] [PubMed] [Google Scholar]
- 4. Tu WZ, Cheng RD, Cheng B, et al. Analgesic effect of electroacupuncture on chronic neuropathic pain mediated by P2X3 receptors in rat dorsal root ganglion neurons. Neurochem Int 2012;60:379–386. [DOI] [PubMed] [Google Scholar]
- 5. Yin J, Chen JD. Gastrointestinal motility disorders and acupuncture. Auton Neurosci 2010;157:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol 2008;85:355–375. [DOI] [PubMed] [Google Scholar]
- 7. Niederberger E, Geisslinger G. The IKK‐NF‐kappaB pathway: a source for novel molecular drug targets in pain therapy. FASEB J 2008;22:3432–3442. [DOI] [PubMed] [Google Scholar]
- 8. Sun T, Song WG, Fu ZJ, Liu ZH, Liu YM, Yao SL. Alleviation of neuropathic pain by intrathecal injection of antisense oligonucleotides to p65 subunit of NF‐kappaB. Br J Anaesth 2006;97:553–558. [DOI] [PubMed] [Google Scholar]
- 9. Ma W, Bisby MA. Increased activation of nuclear factor kappa B in rat lumbar dorsal root ganglion neurons following partial sciatic nerve injuries. Brain Res 1998;797:243–254. [DOI] [PubMed] [Google Scholar]
- 10. Jagodic MM, Pathirathna S, Nelson MT, et al. Cell‐specific alterations of T‐type calcium current in painful diabetic neuropathy enhance excitability of sensory neurons. J Neurosci 2007;27:3305–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qu K, Lee SW, Bian JS, Low CM, Wong PT. Hydrogen sulfide: neurochemistry and neurobiology. Neurochem Int 2008;52:155–165. [DOI] [PubMed] [Google Scholar]
- 12. Xu GY, Winston JH, Shenoy M, Zhou S, Chen JD, Pasricha PJ. The endogenous hydrogen sulfide producing enzyme cystathionine‐beta synthase contributes to visceral hypersensitivity in a rat model of irritable bowel syndrome. Mol Pain 2009;5:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matsunami M, Tarui T, Mitani K, et al. Luminal hydrogen sulfide plays a pronociceptive role in mouse colon. Gut 2009;58:751–761. [DOI] [PubMed] [Google Scholar]
- 14. Lee AT, Shah JJ, Li L, Cheng Y, Moore PK, Khanna S. A nociceptive‐intensity‐dependent role for hydrogen sulphide in the formalin model of persistent inflammatory pain. Neuroscience 2008;152:89–96. [DOI] [PubMed] [Google Scholar]
- 15. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- 16. Xu GY, Li G, Liu N, Huang LY. Mechanisms underlying purinergic P2X3 receptor‐mediated mechanical allodynia induced in diabetic rats. Mol Pain 2011;7:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang C, Huang ZQ, Hu ZP, et al. Electroacupuncture effects in a rat model of complete Freund's adjuvant‐induced inflammatory pain: antinociceptive effects enhanced and tolerance development accelerated. Neurochem Res 2008;33:2107–2111. [DOI] [PubMed] [Google Scholar]
- 18. Zhang RX, Li A, Liu B, et al. Electroacupuncture attenuates bone‐cancer‐induced hyperalgesia and inhibits spinal preprodynorphin expression in a rat model. Eur J Pain 2008;12:870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Han JS, Chen XH, Sun SL, et al. Effect of low‐ and high‐frequency TENS on Met‐enkephalin‐Arg‐Phe and dynorphin A immunoreactivity in human lumbar CSF. Pain 1991;47:295–298. [DOI] [PubMed] [Google Scholar]
- 20. Gao YJ, Zhang L, Ji RR. Spinal injection of TNF‐alpha‐activated astrocytes produces persistent pain symptom mechanical allodynia by releasing monocyte chemoattractant protein‐1. Glia 2010;58:1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Messinger RB, Naik AK, Jagodic MM, et al. In vivo silencing of the Ca(V)3.2 T‐type calcium channels in sensory neurons alleviates hyperalgesia in rats with streptozocin‐induced diabetic neuropathy. Pain 2009;145:184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yusaf SP, Goodman J, Gonzalez IM, et al. Streptozocin‐induced neuropathy is associated with altered expression of voltage‐gated calcium channel subunit mRNAs in rat dorsal root ganglion neurones. Biochem Biophys Res Commun 2001;289:402–406. [DOI] [PubMed] [Google Scholar]
- 23. Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med 1992;175:1181–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoybergs YM, Biermans RL, Meert TF. The impact of bodyweight and body condition on behavioral testing for painful diabetic neuropathy in the streptozotocin rat model. Neurosci Lett 2008;436:13–18. [DOI] [PubMed] [Google Scholar]
- 25. Dobretsov M, Hastings SL, Romanovsky D, Stimers JR, Zhang JM. Mechanical hyperalgesia in rat models of systemic and local hyperglycemia. Brain Res 2003;960:174–183. [DOI] [PubMed] [Google Scholar]
- 26. DeLeo JA, Rutkowski MD. Gender differences in rat neuropathic pain sensitivity is dependent on strain. Neurosci Lett 2000;282:197–199. [DOI] [PubMed] [Google Scholar]
- 27. Tall JM, Stuesse SL, Cruce WL, Crisp T. Gender and the behavioral manifestations of neuropathic pain. Pharmacol Biochem Behav 2001;68:99–104. [DOI] [PubMed] [Google Scholar]
- 28. Ammendola A, Gemini D, Iannaccone S, et al. Gender and peripheral neuropathy in chronic alcoholism: a clinical‐electroneurographic study. Alcohol Alcohol 2000;35:368–371. [DOI] [PubMed] [Google Scholar]
- 29. Berkley KJ. Sex differences in pain. Behav Brain Sci 1997;20:371–380; discussion 435–513. [DOI] [PubMed] [Google Scholar]
- 30. Sugimoto K, Rashid IB, Shoji M, Suda T, Yasujima M. Early changes in insulin receptor signaling and pain sensation in streptozotocin‐induced diabetic neuropathy in rats. J Pain 2008;9:237–245. [DOI] [PubMed] [Google Scholar]
- 31. Kolta MG, Ngong JM, Rutledge LP, Pierzchala K, Van Loon GR. Endogenous opioid peptide mediation of hypoalgesic response in long‐term diabetic rats. Neuropeptides 1996;30:335–344. [DOI] [PubMed] [Google Scholar]
- 32. Pabbidi RM, Yu SQ, Peng S, Khardori R, Pauza ME, Premkumar LS. Influence of TRPV1 on diabetes‐induced alterations in thermal pain sensitivity. Mol Pain 2008;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ledeboer A, Gamanos M, Lai W, et al. Involvement of spinal cord nuclear factor kappaB activation in rat models of proinflammatory cytokine‐mediated pain facilitation. Eur J Neurosci 2005;22:1977–1986. [DOI] [PubMed] [Google Scholar]
- 34. Laughlin TM, Bethea JR, Yezierski RP, Wilcox GL. Cytokine involvement in dynorphin‐induced allodynia. Pain 2000;84:159–167. [DOI] [PubMed] [Google Scholar]
- 35. Haslbeck KM, Schleicher E, Bierhaus A, et al. The AGE/RAGE/NF‐(kappa)B pathway may contribute to the pathogenesis of polyneuropathy in impaired glucose tolerance (IGT). Exp Clin Endocrinol Diabetes 2005;113:288–291. [DOI] [PubMed] [Google Scholar]
- 36. Wada R, Yagihashi S. Role of advanced glycation end products and their receptors in development of diabetic neuropathy. Ann N Y Acad Sci 2005;1043:598–604. [DOI] [PubMed] [Google Scholar]
- 37. Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab 2001;280:E685–E694. [DOI] [PubMed] [Google Scholar]
- 38. Baeuerle PA, Henkel T. Function and activation of NF‐kappa B in the immune system. Annu Rev Immunol 1994;12:141–179. [DOI] [PubMed] [Google Scholar]
- 39. Nagai Y, Tsugane M, Oka J, Kimura H. Hydrogen sulfide induces calcium waves in astrocytes. FASEB J 2004;18:557–559. [DOI] [PubMed] [Google Scholar]
- 40. Xu GY, Huang LY. Ca2 + /calmodulin‐dependent protein kinase II potentiates ATP responses by promoting trafficking of P2X receptors. Proc Natl Acad Sci USA 2004;101:11868–11873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Inoue K. The function of microglia through purinergic receptors: neuropathic pain and cytokine release. Pharmacol Ther 2006;109:210–226. [DOI] [PubMed] [Google Scholar]
- 42. Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature 1995;377:428–431. [DOI] [PubMed] [Google Scholar]
- 43. Zhang Y, Meng X, Li A, et al. Electroacupuncture alleviates affective pain in an inflammatory pain rat model. Eur J Pain 2011;16:170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gim GT, Lee JH, Park E, et al. Electroacupuncture attenuates mechanical and warm allodynia through suppression of spinal glial activation in a rat model of neuropathic pain. Brain Res Bull 2011;86:403–411. [DOI] [PubMed] [Google Scholar]


