SUMMARY
Aims: Classical fear conditioning and extinction has been used to understand the neurobiology of fear learning and its inhibition. The recall of an extinction memory involves the ventromedial prefrontal cortex and the amygdala, and patients with posttraumatic stress disorder (PTSD) have been shown to exhibit deficits in this process. Furthermore, extinction forms the basis of exposure therapies commonly used to treat PTSD patients. It is possible that effective pharmacological and/or psychological treatment regimens could influence the activity of these regions, and thereby increase the ability to retain an extinction memory. However, to test this, a fear conditioning and extinction paradigm must demonstrate within‐subject reproducibility over time. We, therefore, sought to test the within‐subject reliability of a previously used 2‐day, classical fear conditioning and extinction paradigm. Methods: Eighteen healthy participants participated in a 2‐day paradigm on three occasions, each separated by at least 12 weeks. Conditioning and extinction took place on Day 1, and extinction recall and fear renewal were evaluated on Day 2 on each of the three occasions. The conditioned stimulus was a visual cue and the unconditioned stimulus was a mild electric shock to the fingers. Skin conductance was recorded throughout the experiment to measure conditioned responses. Results: We found that conditioning and extinction recall were not significantly different across time and were correlated within subjects. Conclusion: These data illustrate the reliability of this paradigm and its potential usefulness in evaluating the influence of a given treatment on the fear extinction network in longitudinal within‐subject designs.
Keywords: Anxiety disorders, Fear conditioning, Fear extinction, Test–retest
Pavlovian fear conditioning has been used extensively in rodents to examine the neural mechanisms of fear learning and fear extinction. Variations of this animal experimental paradigm have been adapted for use in humans to translate findings across species. In a typical human fear conditioning procedure, a stimulus such as a blue square (conditioned stimulus, CS) is presented to the participant and paired with an aversive stimulus (unconditioned stimulus, US) such as a puff of air to the eye or a mild electric shock to the fingertips (e.g., see [1, 2]). We have developed a variant of this task in which we condition the participants to a light (paired with a shock to the fingertips) in one context, and then extinguish the fear by presenting the light without shock in a different, that is, “safe,” context [3, 4]. Extinction recall is tested the next day by measuring the amount of extinction memory associated with the CS when presented within the safe context. Renewal of the fear memory is also tested on Day 2 by presenting the CS in conditioning context again, but without the US.
This human fear conditioning model, and the analogous model in rodents, has been used to identify the ventromedial prefrontal cortex (vmPFC), hippocampus, and amygdala as a network of brain areas that appear crucial for fear learning and fear extinction (for reviews, see [5, 6, 7, 8]). More recently, fear conditioning procedures have been used to examine the underlying neurobiology of psychiatric disorders [9, 10]. Studies using the 2‐day fear conditioning procedure demonstrated that patients diagnosed with schizophrenia and posttraumatic stress disorder (PTSD) exhibited deficits in fear extinction memory recall[11, 12]. More recently, this procedure was combined with functional magnetic resonance imaging (fMRI) and demonstrated that patients with PTSD exhibited impaired extinction retention compared to trauma‐exposed normal controls; impaired extinction was correlated with decreased functional reactivity in the vmPFC [13].
Human fear conditioning models, such as those described above, have provided significant new insights into the pathophysiology of psychiatric disorders. However, it remains to be seen if pharmacological or behavioral treatments can improve an individual's capacity to inhibit fear and/or make long‐lasting changes to the extinction network in the brain—a key question when considering the frequent relapse of patients suffering from anxiety disorders. Before addressing this issue, however, a fear conditioning and extinction paradigm must demonstrate within‐subject reproducibility (i.e., it must be shown that one's ability to condition is not reduced or that his/her ability to extinguish a fear is not enhanced simply by repetition of the task). If reproducibility is obtained, this paradigm could be used in the future as a predictor of treatment response, or to assess the effectiveness of a given treatment on the neural circuits mediating fear inhibition. For example, the fear extinction retention index—a measure that evaluates how well an individual retains an extinction memory learned the previous day [14]—could serve as a possible predictor of treatment response.
Several investigators have used neuroimaging tools to examine the effects of a given treatment on brain function. For example, Bryant and colleagues showed that reduced activation of the anterior cingulate and amygdala during a fearful faces paradigm was predictive of treatment response in patients with PTSD[14, 15]. One study reports hyperactivity in baseline measurements of the pregenual and subgenual cortices in patients with major depressive disorder who were nonresponsive to cognitive behavioral therapy [16]. While these data provide critical evidence that treatment can lead to normalization of brain function, the expense of routine, longitudinal and repeated use of fMRI or PET as predictors of treatment response limits their clinical feasibility. If reproducible behavioral results could be achieved through the measurement of skin conductance response (SCR), this approach could provide additional and inexpensive method to predict treatment response. Moreover, it could also help identify proper treatment regimens for specific patients prior to the initiation of the treatment, and assess the progress of this treatment.
This study examined the test–retest reproducibility of our 2‐day fear conditioning and extinction procedure. Given that remission of symptoms following the initiation of pharmacologic treatment often requires approximately 12 weeks for the majority of anxiety disorders, we examined the reproducibility of scores within a comparable timeframe. We were particularly concerned that the repeated use of this procedure might lead to a decrease in conditioned response magnitude over repeated testing and/or an increase in extinction memory retention. To test for these effects, we measured acquisition and extinction of conditioned fear responses in 18 healthy participants on three separate occasions.
Materials and Methods
Participants
Eighteen healthy subjects (nine males), ages 20–60 years (M = 38.0, ± 12.7 years) were recruited from the local community via advertisement. All participants were right‐handed, without medical conditions or neurologic disorders, and no participant was using psychoactive or other potentially confounding drugs or medications. Participants who met criteria for Axis I mental disorders, evaluated using the Structured Clinical Interview for DSM‐IV[17], were excluded. Written informed consent was obtained from all participants in accordance with the requirements of the Partners Healthcare Human Research Committee.
Conditioning and Extinction Procedure
A previously validated 2‐day fear conditioning and extinction paradigm was used in which subjects underwent Habituation, Conditioning, and Extinction Learning on Day 1, and Extinction Recall, and Fear Renewal on Day 2 [18, 19], see Figure 1). During the experiment, participants were seated upright in a chair and viewed images on a computer monitor 3 feet away. Digital photographs of two different rooms constituted the visual context. Within each room, an unlit lamp was shown before being “switched on” to one of three colors (blue, red, or yellow), which constituted the conditioned stimuli (CSs). Only two colors were shown in any given visit. The CS+ color (followed by shock), the CS− color (no shock), and the contexts were pseudorandomly selected and counterbalanced across participants and across visits. The US was a 500 ms electric shock previously selected by the participant to be “highly annoying but not painful”[4, 20] and delivered to electrodes attached to the second and third finger of the right hand. The shock electrodes remained attached to the fingertips throughout both days of the experiment, but the US was administered only during the Conditioning session on Day 1.
Figure 1.
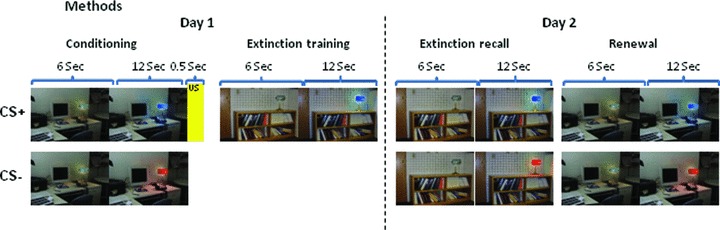
Schematic of experimental paradigm. Images shown display visual contexts used in the experiment. Two conditioned stimuli (CS) were presented: A CS+ (followed by shock) and a CS− (no shock). The CS+ light was presented in the conditioning context (office) and after a 1‐min break, was extinguished in the safe context (conference room). Extinction Recall and Fear Renewal were tested on Day 2 in the extinction and conditioning contexts, respectively. Adapted from Milad et al., 2005 and 2007.
On Day 1, the to‐be CS+ and the to‐be CS− (four trials of each) were presented within each virtual context in a counterbalanced manner with no US presentation (Habituation phase). The Conditioning phase followed with five CS+ trials that were immediately followed by the US (100% reinforcement), and five CS− trials. All conditioning trials used the same context. The Extinction phase was divided into two identical subphases separated by a 1‐min rest period. For each Extinction phase, five CS+ trials and five CS− trials were presented within the extinction context. On Day 2, the Extinction Recall phase was presented and was identical to an Extinction subphase on Day 1. The Renewal phase was similar to the Conditioning phase, but without US presentation. All subjects correctly reported the CS–US contingency.
All participants underwent the above‐described procedures on three separate visits: Tests 1, 2, and 3, separated by at least 12 weeks (Gap between Tests 1 & 2: 17.9 ± 2.1 weeks; Between Tests 2 & 3: 14.5 ± 0.7 weeks). The conditioning context and the color of the CS+ were different for each of the three test sessions and counterbalanced across visits.
Psychophysiological Measures
The context was displayed for 6 seconds with the lamp “turned off,” immediately followed by the light “turning on” for 12 seconds, with different colors representing the CS+ and CS− trials. The total stimulus presentation time was 18 seconds (6 + 12 seconds). The intertrial interval ranged from 12 to 21 seconds with an average of 16 seconds. SCR was calculated for each trial by subtracting the mean SC level (SCL) for the 2 seconds immediately preceding context onset from the highest SCL recorded during the 12‐second CS+/CS− presentation. Each SCR was square‐root transformed to reduce heteroskedasticity (for negative SCR values, the square root of the absolute value was taken and then the negative sign replaced). A differential SCR was calculated by subtracting the SCR to the CS− from the SCR to the CS+. A baseline SCL was calculated for the Habituation phase by averaging the SCL over the 5 seconds prior to the onset of each context presentation and then averaging these values across all eight trials. An unconditioned response (UCR) was calculated by subtracting the average SCL during the 1 second immediately following the shock (before onset of an SCR) from the maximum SCL during the 5 seconds after the shock.
A measure of extinction memory (“extinction retention index”[3]) during the Recall phase on Day 2 was calculated as the average SCR during the first two trials of the Extinction Recall phase divided by the largest SCR during the Day 1 Conditioning phase and then multiplying this ratio by 100, thereby yielding a percentage of the maximum conditioned response. This value was then subtracted from 100% to yield the extinction retention index. We chose the maximum SCR during the Conditioning phase as a reference to assess the extinction performance on Day 2. Animal studies typically use the last few conditioning trials for this calculation [21]; however, SCR magnitude generally declines toward the end of conditioning in humans so this was not used. Unless specified, all data are presented as means ± standard error of the mean (SE). SPSS (Version 17.0, SPSS Inc., Chicago, IL, USA 2008) was used to calculate Intraclass Correlation Coefficients (ICC) for assessing reliability of scores within subjects and one‐way analysis of variance (ANOVA) for analyzing scores across test sessions. The statistical software used was SPSS (Version 17.0, SPSS Inc., Chicago, IL, USA 2008).
Results
There were no significant differences in the selected levels of shocks across three visits (2.30 ± 0.20 mA, 2.04 ± 0.14 mA, and 2.04 ± 0.14 mA, respectively, ANOVA: F(2,51)= 0.80, P= 0.46). As expected, there was a general trend of decreasing baseline SCL across test sessions (Test 1 = 2.13 ± 0.89 μs, Test 2 = 1.86 ± 0.41 μs, Test 3 = 1.02 ± 0.17 μs; F(2,24)= 2.86, P= 0.07, Figure 2A). Similarly, there was a general decrease in the magnitude of the UCR across test sessions; however, this difference was not statistically significant (1.07 ± 0.25 μs, 0.81 ± 0.18 μs, and 0.65 ± 0.17 μs, respectively; F(2,24)= 1.11, P= 0.34, Figure 2B).
Figure 2.
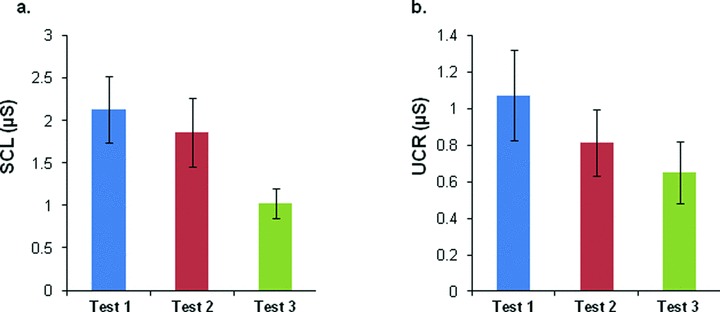
Skin conductance levels (SCL) and unconditioned responses (UCR) across three test sessions. (A) SCL values across test sessions. Baseline SCL was measured during the Habituation session by recording the 5 seconds prior to the onset of each context presentation averaged across all eight trials. (B) UCR levels across tests. Values were calculated by subtracting the average SCR during the 1 second immediately following the shock from the maximum SCR during the 5 seconds after the shock. No significant differences were found.
Reliability of SCR Data across Tests 1, 2, and 3
SCR magnitudes during Habituation, Conditioning, Extinction, Extinction Recall, and Renewal phases for Tests 1, 2, and 3 are shown in Figure 3. An ANOVA comparing the average five Habituation trials between tests revealed no differences (F(2,53)= 1.53, P= 0.23). Results of an ANOVA comparing maximum SCR (CS+) during Conditioning revealed no differences between tests (F(2,53)= 0.16, P= 0.86). An ANOVA comparing the last two trials of Extinction between tests revealed no differences (F(2,53)= 0.70, P= 0.50). In a comparison of the first two trials of Extinction Recall, an ANOVA indicated that there were no differences between tests (F(2,52)= 0.78, 0.46). Similarly, a comparison of the first two trials of Renewal revealed no differences between tests (F(2,52)= 0.77, P= 0.47). Finally, a comparison of the extinction retention index across tests revealed no significant differences across test sessions (F(2,50)= 0.87, P= 0.43). Thus, there were no statistical differences across the three different time points at any phase of the experiment, suggesting that significant fear learning and extinction were achieved across repeated training visits of the same subjects.
Figure 3.
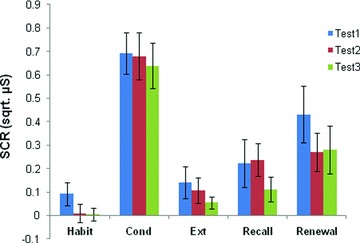
Conditioned responses (SCR, square‐root transformed) across three test sessions. Habituation (Habit) displays the mean of five trials, Conditioning (Cond) displays the mean max response, Extinction (Ext) displays the mean of the last two trials, and Recall and Renewal show the mean of the first two trials. All data display responses to the CS+. No significant differences were found.
We conducted an additional analysis to evaluate whether or not the time between tests influenced performance. Here, we conducted a median split based on time elapsed between tests (Test 1 to 2: Low = 12.3 ± 0.2 weeks, High = 22.5 ± 3.1 weeks; Test 2 to 3: Low = 12.1 ± 0.1 weeks, H = 16.9 ± 0.7 weeks), and found that there were no significant differences across any phase (data not shown) between tests. Finally, we tested whether or not a phenomenon referred to as “savings”—in which learning happens more quickly with retesting—was present here. To do this, we measured the number of trials needed to reach maximum SCR during Conditioning, and to reach minimum SCR during Extinction as a proxy for rate of learning. An ANOVA showed that there was no difference in trials to max response (F(2,53)= 0.42, P= 0.66) and no difference to minimum response (F(2,52)= 0.72, P= 0.49) during conditioning and extinction, respectively, suggesting lack of savings during the retest phases.
To specifically examine the responses within individuals across the different phases and tests, we conducted additional analysis using the intraclass correlation coefficient (ICC). This test is commonly used to assess the consistency of scores across test sessions within individuals in a given test. The ICC results across Habituation, Conditioning, Extinction Learning, and Recall phases are presented in Table 1a. As can be seen in the table, there were significant ICCs for CS+ SCR magnitude across test sessions for the Conditioning, Extinction Recall, and Renewal phases. ICCs were also calculated for differential SCRs (CS+ minus CS−) and showed a similar pattern of results as for CS+ SCR magnitude, although the ICCs tended to be somewhat smaller (Conditioning phase: ICC = 0.43, P < 0.01; Extinction Recall phase: ICC = 0.23, P= 0.07; Renewal phase: ICC = 0.50, P < 0.01) .
Table 1.
| a. Interclass correlation coefficients for Tests 1, 2, and 3 | ||
|---|---|---|
| Session | ICC | P value |
| Habituation | 0.10 | 0.23 |
| Conditioning | 0.68 | <0.01* |
| Extinction | −0.19 | 0.92 |
| Extinction recall | 0.46 | 0.01* |
| Renewal | 0.67 | <0.01* |
| Extinction Retention Index | 0.16 | 0.14 |
| b. Interclass correlation coefficients for Tests 1 and 2 | ||
| Session | ICC | P value |
| Habituation | 0.16 | 0.25 |
| Conditioning | 0.64 | <0.01* |
| Extinction | −0.24 | 0.83 |
| Extinction recall | 0.72 | <0.01* |
| Renewal | 0.66 | <0.01* |
| Extinction retention index | 0.49 | 0.02* |
Reliability of SCR Data across Tests 1 and 2 Only
We examined the reliability of our conditioning procedure across the first two test sessions only given that some treatments require only pre‐ and postassessments. The ICC values for the first two sessions are shown in Table 1b for the respective phases of the conditioning procedure. As can be seen, CS+ SCR magnitude values show overall stronger consistencies across the two sessions than across three sessions.
Discussion
We conducted longitudinal testing of a validated fear conditioning and extinction procedure across three test occasions separated by 8–12 weeks. Participants produced consistent SC scores for the Conditioning and Extinction Recall phases across the three visits, demonstrating within‐subjects reliability of this paradigm across three different visits. Reliability was strongest between Tests 1 and 2 in this paradigm.
The Extinction phase did not produce consistent SCR scores likely due to the fact that, during this phase, the CR was quickly extinguished by all subjects across test sessions. When SCR response magnitudes approach zero, they become less reliable. This interpretation is supported by the absence of a significant ICC for the Habituation phase, for which SCR magnitude would be expected to be small and differences across test sessions would be negligible. Furthermore, a significant correlation for the Extinction Recall phase was observed between Tests 1 and 2, but not when all three tests sessions were considered. This may be due to the increased extinction retention produced by the third test. In fact, SC response magnitudes to the CS+ for Conditioning, Extinction, and Extinction Recall phases were slightly smaller for the third test session compared to the two previous sessions, while they were almost identical between Tests 1 and 2.
Few investigators have sought to identify and test behavioral measures that might predict responses to treatment. Bryant and colleagues provide one such example in which they measured PTSD patients’ neural responses to fearful faces and found that magnitude of activation of the amygdala and ventral anterior cingulate cortex predicted treatment response to cognitive behavioral therapy [14]. Findings from this study help to establish the reliability of SC responses during a fear conditioning and extinction procedure after a time span of at least 12 weeks. Further studies should be conducted to test whether the same can be said within a patient population where extinction deficits have been previously reported. After this is demonstrated, studies may begin to examine whether or not treatment‐induced changes in brain function and/or structure may be correlated with and predicted by SCR measures of conditioning and extinction recall. Once this is established, obtaining SCR data from patients undergoing fear conditioning and extinction may serve as a predictor for changes in brain function, and improvements in clinical symptoms.
Funding
This work was supported by a grant from the National Institute of Mental Health (R01MH081975‐01A1) to M.R.M. The project described was also supported by Grant MH086400 to M.RM. and D.D.D. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Financial Disclosures
Darin D. Dougherty, MD has received honoraria, consultation fees, and/or royalties from Wyeth, Jazz Pharmaceuticals, Bristol Myers Squibb, Brand Ideas, and Reed Elsevier. He has also participated in research funded by Medtronic, Cyberonics, Eli Lilly, McNeil, and Northstar. Mohammed R. Milad, PhD received consultation fees from Microtranspondor. The remaining authors have no financial disclosures to report.
Conflict of Interest
The authors have no conflict of interest.
Acknowledgments
None.
References
- 1. Hettema JM, Annas P, Neale MC, Kendler KS, Fredrikson M. A twin study of the genetics of fear conditioning. Arch Gen Psychiatry 2003;60:702–708. [DOI] [PubMed] [Google Scholar]
- 2. Vansteenwegen D, Hermans D, Vervliet B, et al Return of fear in a human differential conditioning paradigm caused by a return to the original acquisition context. Behav Res Ther 2005;43:323–336. [DOI] [PubMed] [Google Scholar]
- 3. Milad MR, Zeidan MA, Contero A, et al The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience 2010;168:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology 2005;42:456–464. [DOI] [PubMed] [Google Scholar]
- 5. Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A. Neuronal circuits of fear extinction. Eur J Neurosci 2010;31:599–612. [DOI] [PubMed] [Google Scholar]
- 6. Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 2008;33:56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry 2007;12:120–150. [DOI] [PubMed] [Google Scholar]
- 8. Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: Implications for human brain imaging and anxiety disorders. Biol Psychol 2006;73:61–71. [DOI] [PubMed] [Google Scholar]
- 9. Jovanovic T, Norrholm SD, Blanding NQ, et al Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety 2010;27:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jovanovic T, Norrholm SD, Fennell JE, et al Posttraumatic stress disorder may be associated with impaired fear inhibition: Relation to symptom severity. Psychiatry Res 2009;167:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holt DJ, Lebron‐Milad K, Milad MR, et al Extinction memory is impaired in schizophrenia. Biol Psychiatry 2009;65:455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: Results of a twin study. J Psychiatr Res 2008;42:515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Milad MR, Pitman RK, Ellis CB, et al Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 2009;66:1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bryant RA, Kemp AH, Felmingham KL, et al Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: An fMRI study. Hum Brain Mapp 2008;29:517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bryant RA, Felmingham K, Kemp AH, et al Amygdala and ventral anterior cingulate activation predicts treatment response to cognitive behaviour therapy for post‐traumatic stress disorder. Psychol Med 2008;38:555–561. [DOI] [PubMed] [Google Scholar]
- 16. Konarski JZ, Kennedy SH, Segal ZV, et al Predictors of nonresponse to cognitive behavioural therapy or venlafaxine using glucose metabolism in major depressive disorder. J Psychiatry Neurosci 2009;34:175–180. [PMC free article] [PubMed] [Google Scholar]
- 17. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical interview for DSM‐IV‐TR Axis I disorders, research version, patient edition. New York : Biometrics Research, New York State Psychiatric Institute, 2002. [Google Scholar]
- 18. Milad MR, Goldstein JM, Orr SP, et al Fear conditioning and extinction: Influence of sex and menstrual cycle in healthy humans. Behav Neurosci 2006;120:1196–1203. [DOI] [PubMed] [Google Scholar]
- 19. Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci USA 2005;102:10706–10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma‐exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol 2000;109:290–298. [PubMed] [Google Scholar]
- 21. Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci 2000;20:6225–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]


