Summary
Background
Both Th1 and Th17 cells specific for neuroantigen are described as encephalitogenic in the experimental autoimmune encephalomyelitis (EAE) model.
Aim
The proposal of this study was to investigate how carbon nanotubes internalized by antigen‐presenting cells (APCs) affect the development of encephalitogenic CD4+ T cells.
Methods
Therefore, we stimulated encephalitogenic T cells in the presence or not of multiwalled carbon nanotube (MWCNT). After the incubation, we analyzed the expression profile of the encephalitogenic T cells and their capacity to induce EAE.
Results
Encephalitogenic CD4+ T cells cultured with APCs that were previously incubated with MWCNTs do not express IL‐17. The adoptive transfer of these cells causes less severe EAE than the transfer of both Th1 and Th17 cells that are not incubated with MWCNTs. These results suggest that the increased IL‐27 level produced by the APCs incubated with the carbon nanotubes inhibits the development of Th17 cells. This observation is confirmed by the concomitant reduction in the level of RORγt, which is a transcription factor essential for the development of Th17 cells. Moreover, the incubation of encephalitogenic T cells devoid of Th17 cells with neutralizing anti‐IL‐27 antibodies restored the production of IL‐17.
Conclusion
This finding confirms the suppressive effect of IL‐27 on encephalitogenic Th17 cells. The results presented suggest that the stimulation of APCs with carbon nanoparticles prior to neuroantigen presentation affects the development of the Th17 subset of encephalitogenic CD4+ T lymphocytes and results in less severe EAE.
Keywords: Antigen presentation, Autoimmunity, Demyelination, Multiwalled carbon nanotube
Introduction
Carbon nanoparticles are currently under scrutiny as new tools for biomedical applications 1. In fact, various observations have demonstrated the ability of nonfunctionalized carbon nanotubes to stimulate the inflammatory response, particularly when these particles are inhaled 2.
The activation of the innate immune response may modify the subsequent acquired immune response. The cytokines produced by the cells (macrophages, dendritic, or NK cells) of the innate immune response, such as IL‐12, are responsible for the differentiation of CD4+ T helper 1 and Th17 lymphocytes, whereas the IL‐4 produced by NK cells promotes the differentiation of CD4+ T helper 2 lymphocytes 3, 4, 5.
We recently demonstrated that multiwalled carbon nanotubes (MWCNTs), which were prepared in our facilities, are internalized by macrophages and cause the activation of the cells of the innate immune response and the secretion of various cytokines. The activation of these macrophages leads to an increased stimulation of CD4+ T lymphocytes by either specific antigens or nonspecific mitogens. An increase in the production of the antibody against ovalbumin was also observed after the administration of carbon nanotubes 6. These results suggest that the inflammatory response initiated by carbon nanoparticles may evolve and subsequently modify the lymphocyte response.
The exposure of individuals to particles, such as silica and asbestos, causes chronic inflammation. Recent studies have associated this inflammatory response with the development of autoimmune diseases, particularly those caused by antibodies, such as lupus and rheumatoid arthritis 7, 8. However, the influence of carbon nanotubes on the activation of autoimmune diseases has not yet been studied. Therefore, we analyzed the role of carbon nanoparticles in the development of encephalitogenic CD4+ T lymphocytes in an experimental autoimmune encephalomyelitis (EAE) model.
Experimental autoimmune encephalomyelitis, which is a model of multiple sclerosis, is a CD4+ T‐cell‐mediated autoimmune disease that can be induced in susceptible animals either directly through their immunization with constituent proteins of myelin, such as the myelin basic protein (MBP), or passively by the adoptive transfer of sensitized CD4+ T lymphocytes into naïve animals. EAE was long considered the prototypic Th1‐mediated autoimmune disease until recent findings suggested that Th17 cells play a primary role in this model 9, 10.
Th17 cells with specificity for neuroantigen are highly pathogenic and lead to the development of inflammation and severe EAE. A combination of TGF‐β, IL‐6, and the transcription factors STAT3 and RORγt was recently found to be essential for the initial differentiation of Th17 cells; moreover, IL‐23 is required for the later stabilization of the Th17 cell subset 11, 12, 13, 14.
This study was conducted to understand how carbon nanotubes internalized by APCs affect the development of encephalitogenic CD4+ T‐cell lines prior to their adoptive transfer and the induction of EAE.
Materials and Methods
Preparation and Characterization of Carbon Nanotubes
The MWCNTs used in this research were prepared in our laboratory. The procedure involved the HFCVD of carbon atoms from carbon sources on a polished copper foil substrate as previously described 6, 15.
Animals
Six‐ to eight‐week‐old female Lewis rats were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). These animals are currently established as a colony at the University of Campinas Breeding Center and are housed and maintained in a pathogen‐free environment in the university animal facility. The experimental animals were allowed access to standard rodent chow and water ad libitum and were exposed to a temperature between 21°C and 23°C and a 12‐h light/12‐h dark cycle. The animals were age matched for individual experiments and randomly distributed into treatment or control groups. All of the procedures were conducted in accordance with the guidelines proposed by the Brazilian Council on Animal Care and approved by the Committee for Ethical Animal Experimentation (CEEA/UNICAMP #2038‐1).
Antigens and Induction of EAE
Each animal received a subcutaneous injection of 50 μg of gpMBP, which was purified from guinea pig brains in accordance with the protocol described by Deibler et al. 16 and emulsified in complete Freund's adjuvant containing 2 mg/mL Mycobacterium tuberculosis H37RA (Difco, Detroit, MI, USA). The clinical expression of the disease was graded on the clinical index scale of 0–5 17.
Generation of Encephalitogenic T‐Cell Lines (TMBP)
Ten days after the subcutaneous immunization with the MBP protein in CFA with 2 mg/mL inactivated Mycobacterium tuberculosis H37Ra (Difco Laboratories), the cells were collected from the draining lymph nodes and cultured in the presence of 10 μg/mL MBP. After 2 days of culture, the T lymphocyte blasts were expanded in IL‐2‐containing growth medium for 5 days and then restimulated in the presence of irradiated thymocytes pulsed with the specific antigen. After 2.5 days of culture, the cells were stained with Trypan blue and counted in a TC10 automated cell counter (BioRad, Berkeley, CA, USA). The TMBP cells (2 × 106 cells/rat) were injected i.v. 2.5 days after the restimulation.
MWCNT Preparation for In Vitro Assays
For the in vitro assay, the sonicated MWCNTs were heat‐treated at 250°C for 2 h in an electric furnace to remove all of the possible contaminating endotoxins. After sterilization, the MWCNTs (1 mg) were suspended in 1 mL of PBS containing 0.01% endotoxin‐free Pluronic 68 (F68; Sigma‐Aldrich, St. Louis, MO, USA) because the Pluronic surfactant reduces the hydrophobic interactions and improves the solubility of the nanotube 18. The suspension of the MWCNTs was added to the cultures (100 μg/mL).
MWCNT Labeling
The sonicated MWCNTs were tagged with a nontoxic, hydrophobic red fluorescent dye (PKH26; Sigma‐Aldrich). The PKH26 stock solution was diluted to 2 × 10−6 mol/L and added to the MWCNT stock solution; the mixture was then incubated at room temperature for 5 min. The stained nanotubes were washed three times, centrifuged at 120,000 × g for 4 h at 4°C, and suspended to a concentration of 1 mg/mL using 1% F68. This preparation of MWCNTs was used within 24 h of labeling 18.
Confocal Microscopy
A Zeiss LSM 510 Meta inverted 2‐photon confocal microscope was used for the fluorescent imaging studies. The cells were plated in 33‐mm dishes in 2 mL of culture medium. An Argon 488‐nm laser was used to excite the PKH26 dye.
Quantitative RT‐PCR
The mRNA of the spleen cells was extracted using Tryzol and reversed to obtain the corresponding cDNA. TaqMan analysis was performed with an ABI Prism 7500 TaqMan Sequence Detector (PE Applied Biosystems, Darmstadt, Germany). The primers for β‐actin, IFNγ, IL‐17A, IL‐17F, IL‐23, IL‐27, RoRa, RoRc and TGFβ were obtained from the inventoried list of applied bioscience manufacturers. The expression of each gene of interest was determined relative to the expression of the housekeeping gene (β‐actin). The data were obtained by independent duplicate measurements (five mice per group). The threshold cycle value of the individual measurements did not exceed 0.5 amplification cycles.
Statistical Analysis
The statistical significance of the results was determined using a Kruskal–Wallis nonparametric analysis of the variance or a Mann–Whitney U‐test. Differences with a P‐value less than 0.05 were considered significant.
Results
Raman, FESEM Morphological Characterization of the Carbon Nanoparticles
Figure 1A shows an atypical FESEM image of the as‐deposited nanotube particles. The scale bars range from 100 nm to 1000 nm. The images clearly illustrate that the deposited samples consist of uniform nonaligned multiwalled tubes that cover the substrate surface with an entangled agglomeration. Figure 1B shows a typical Raman spectrum of the as‐deposited samples. This spectrum can be divided into regions of first‐ and second‐order frequencies. In the first‐order region, two intense peaks are observed at 1334 and 1593 cm−1; these peaks correspond to the disorder‐induced sp2 peak (D‐line) and the graphite‐oriented E2 g mode of the sp2 peak (G‐line). In the second‐order region, the following peaks were found: a high peak at 2666 cm−1, which corresponds to the second harmonic of the D‐line (2 × D), a small peak at approximately 2927 cm−1, which corresponds to the sum of the D‐ and G‐line frequencies (D + G), and a small peak at approximately 3202 cm−1, which corresponds to the second harmonic of the G‐line (2 × G). The intensity of the D‐peak was greater than that of the corresponding G‐peak. This difference in the intensities indicates that the C‐C sp2 order is not of a high order, which implies that the Raman spectrum corresponds to a set of disordered multiwalled carbon nanotubes.
Figure 1.
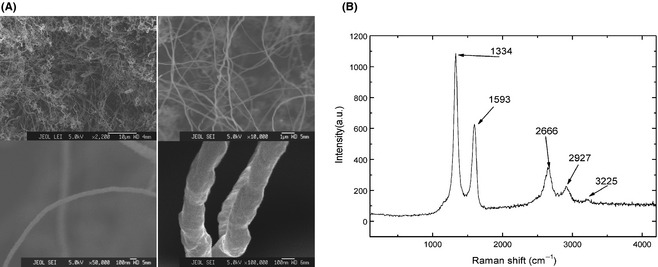
RAMAN and FESEM morphological characterization of the carbon nanoparticles. (A) FESEM images illustrate that the deposited samples consist of uniform nonaligned multiwalled tubes that cover the substrate surface with an entangled agglomeration in 2200× (upper left), 10,000× (upper right), 50,000× (lower left), and 100,000× (lower right). (B) A typical Raman spectrum of the as‐deposited samples.
The Adoptive Transfer of Auto‐Reactive T Lymphocytes Stimulated by APC‐MWCNTs Significantly Reduced the Severity of EAE
Encephalitogenic CD4+ T lymphocytes were stimulated with the MBP processed by APCs incubated with our preparation of MWCNTs. After 96 h, the cells were adoptively transferred into normal naïve rats (Figure 2A). After 6 days, the control rats and the rats that received MBP‐specific T‐cell lines plus MWCNTs in the injection developed very severe EAE (4.2 ± 0.4), whereas the rats that received encephalitogenic lymphocytes stimulated by APCs that were previously incubated with MWCNTs developed mild EAE (1.2 ± 0.3) 5 days after immunization (Figure 2B).
Figure 2.
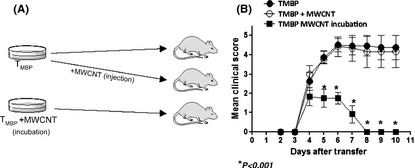
Experimental design and TMBP transfer. (A) The experimental design and the three groups: TMBP (Control group), TMBP plus multiwalled carbon nanotube (MWCNT) injection (TMBP + MWCNT), and TMBP incubated in the presence of APCs and MWCNT (TMBP MWCNT incubation). (B) The clinical course of experimental autoimmune encephalomyelitis (EAE) after transfer of TMBP, TMBP + MWCNT, or TMBP MWCNT incubation.
Analysis of the mRNA Expression Levels of Cytokines in Encephalitogenic CD4 T Lymphocytes Stimulated with the MBP Presented by APCs that Internalized Carbon Nanoparticles
The expression levels of the cytokines produced by Th1 (IFNγ) and Th17 (IL‐17a and IL‐17f) cells in the culture of CD4+ T lymphocytes stimulated by APCs that internalized the prepared carbon nanotubes were determined.
Figure 3 demonstrates that there is no significant difference in the expression of IFNγ between the CD4+ T‐cell lines expanded in the presence of APCs that were previously incubated with or without carbon nanotubes. However, a significant decrease in the IL‐17 expression was observed when the encephalitogenic T‐cell lines were expanded in the presence of APCs previously incubated with MWCNTs. These results suggest that the carbon nanoparticles affect the development of Th17 cells. To prove this hypothesis, the expression of the RORc and RORa genes, which encode RORγt and RORa, respectively, was determined. We present evidence that the expression of RORc was significantly reduced when the stimulating APCs were incubated with MWCNTs, which results in a reduction in the RORγt level. Because RORγt is a Th17‐specific transcription factor, this result confirmed the hypothesis that carbon nanotubes impair the development of Th17 cells.
Figure 3.
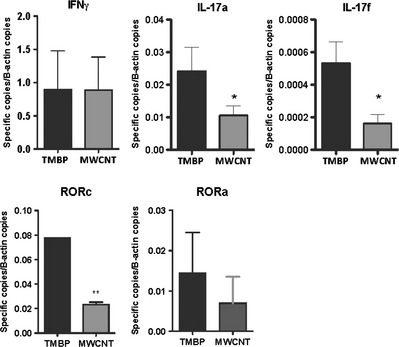
Th1 and Th17 response. Figure presents the expression levels of IFNγ, IL‐17a, IL‐17f, RORc, and RORa in TMBP control cells (black bars), and TBMP incubated in the presence of APCs and multiwalled carbon nanotubes (MWCNT; gray bars).
Analysis of the Cytokines Expressed by APCs that Internalized MWCNTs
Figure 4A demonstrates that MWCNTs stained with a fluorescent red dye were internalized by dendritic cells (APCs). To understand the effect of these carbon nanoparticles on APCs, the mRNA levels of the cytokines produced by these cells were analyzed. The results presented in Figure 4B demonstrate that APC incubated in the presence of MWCNTs (60 Hs) exhibit increased expression levels of IL‐12 and IL‐27, both of which belong to the IL‐12 family. No difference was observed in the expression levels of IL‐23 and TGFβ. The expression of IL‐17 was significantly reduced when the encephalitogenic T cells were stimulated with APCs that were previously incubated with MWCNTs. Figure 4C shows that the neutralization of IL‐27 by a polyclonal antibody to IL‐27 results in the recovery of the ability of T cells to produce IL‐17a and partial recovery of IL‐17f. These results confirm that IL‐27 is responsible for the impaired development of Th17 encephalitogenic T lymphocytes and thus leads to less severe EAE.
Figure 4.
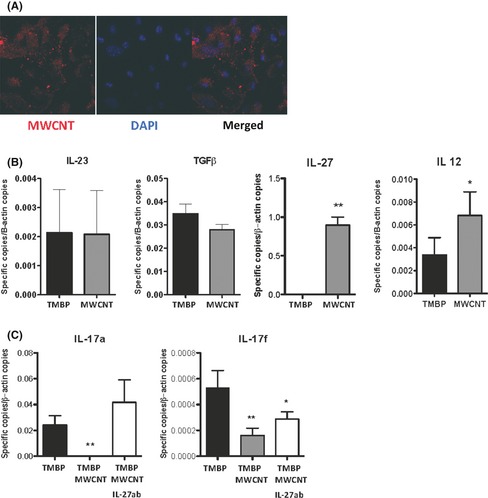
APCs incubated with multiwalled carbon nanotubes (MWCNT). (A) A confocal image of MWCNT labeled with PKH26 (red) and dendritic cells nucleus evidenced by DAPI labeling (blue). The merged image suggests the capacity of DCs to internalize the MWCNT particles. (B) The expression levels of IL‐23, TGFβ, IL‐27, and IL‐12 cytokines by APCs incubated only with TMBP cells (black bars) or with TMBP and MWCNT (gray bars). The blockage of IL‐27 protein, using anti‐IL‐27 antibody (Santa Cruz, USA), restores both IL17a and IL‐17f expression. (C) The expression profile of TMBP incubated with normal APCs (black bars), TMBP incubated in the presence of APCs and MWCNT (gray bar), and TMBP incubated in the presence of APCs and MWCNT, plus anti‐IL‐27 antibody (white bars).
Discussion
This study aimed to understand how carbon nanotubes internalized by APCs affect the development of encephalitogenic CD4+ T cells in the EAE model.
We demonstrated that the adoptive transfer of encephalitogenic CD4+ T lymphocytes (containing the Th1 and Th17 subsets) into naïve Lewis rats induced severe EAE. However, when the encephalitogenic T‐cell lines were cocultured with APCs that were previously incubated with MWCNTs, the disease induced by the adoptive transfer was significantly less severe. This result suggests that the internalization of carbon nanoparticles by the APCs prior to the presentation of MBP to the CD4+ T lymphocytes affects the development of encephalitogenic CD4+ T cells. The expression of cytokines by encephalitogenic T‐cell lines was determined, and the results revealed a significant reduction in the expression of IL‐17, which suggests that the development of Th17 cells was impaired.
The less severe disease in the absence of Th17 cells is in agreement with previous observations that have demonstrated that the adoptive transfer of encephalitogenic Th17 cells causes more severe EAE compared with the adoptive transfer of Th1 cells 19. The cytokines produced by the APCs that internalized the carbon nanoparticles were determined. We found a significant increase in the IL‐12 and IL‐27 levels in the APCs that internalized the carbon nanotubes compared with the APCs that were not incubated with the carbon nanoparticles. The expression levels of IL‐23, IL‐6, and TGFβ in the APCs did not change significantly after the incubation of these cells with the carbon nanotubes. IL‐27 and IL‐23, which are members of the IL‐12 family, are secreted by APCs in response to inflammatory stimuli and exert both a proinflammatory Th1‐enhancing activity and significant antiinflammatory functions 20, 21. The increase in the IL‐27 level observed may explain the decreased IL‐17 level because IL‐27 is hypothesized to inhibit Th17 development and consequently the development of EAE 21, 22. The inhibitory role of IL‐27 in EAE development was demonstrated in studies using IL‐27 knockout mice or anti‐IL‐27 neutralizing antibody 23. It was also recently demonstrated that IL‐27 blocks the expression of the RORc gene, which encodes RORγt; this blockage results in the inhibition of the development of the Th17 population 24. Our data are in agreement with these observations because we also observed a significant reduction in the expression level of RORc, which confirms that the IL‐27 produced by the APCs stimulated with the carbon nanotubes inhibits the development of encephalitogenic Th17 cells.
To confirm the role of IL‐27 in the reduction in the Th17 population, an IL‐27‐neutralizing antibody was added to a culture of encephalitogenic CD4+ T cells devoid of Th17 cells. The results demonstrated a reversal of the IL‐17a and partial reversal of IL‐17f levels expressed by the encephalitogenic T cells. Thus, this finding emphasizes the inhibitory effect of IL‐27.
The results presented in this manuscript confirm the hypothesis that particles that stimulate the innate immune response may also affect the subsequent adaptive immune response. Because APCs affect the adaptive immune response, the activation of these cells may modify the evolution of autoimmune diseases, such as EAE. However, the use of carbon nanoparticles for therapeutic purposes requires additional studies because these particles are hydrophobic, and the clearance of MWCNTs needs to be elucidated.
In conclusion, we presented evidence that carbon nanotubes are internalized by APCs and stimulate the production of IL‐27 by these cells and that this cytokine affects the development of encephalitogenic Th17 lymphocytes. The adoptive transfer of encephalitogenic T cells devoid of Th17 cells resulted in less severe EAE.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by grants from FAPESP (#2011/18728‐5, #2012/04565‐0) and CAPES Nanobiotec network 2008. ASF was supported by FAPESP young investigator grant #2012/01408‐0, and ASM, FP, and MPAS were supported by FAPESP scholarship grants (#2012/09879‐2, #2011/15175‐5, #2011/15639‐1).
The first two authors contributed equally to this work
References
- 1. Vardharajula S, Ali SZ, Tiwari PM, et al. Functionalized carbon nanotubes: biomedical applications. Int J Nanomed 2012;7:5361–5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boczkowski J, Lanone S. Respiratory toxicities of nanomaterials – a focus on carbon nanotubes. Adv Drug Deliv Rev 2012;64:1694–1699. [DOI] [PubMed] [Google Scholar]
- 3. Mosmann TR, Coffman RL. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 1989;7:145–173. [DOI] [PubMed] [Google Scholar]
- 4. Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006;441:235–238. [DOI] [PubMed] [Google Scholar]
- 5. Korn T, Bettelli E, Oukka M, Kuchroo VK. IL‐17 and Th17 Cells. Annu Rev Immunol 2009;27:485–517. [DOI] [PubMed] [Google Scholar]
- 6. Grecco AC, Paula RF, Mizutani E, et al. Up‐regulation of T lymphocyte and antibody production by inflammatory cytokines released by macrophage exposure to multi‐walled carbon nanotubes. Nanotechnology 2011;22:265103. [DOI] [PubMed] [Google Scholar]
- 7. Lee S, Hayashi H, Maeda M, et al. Environmental factors producing autoimmune dysregulation – chronic activation of T cells caused by silica exposure. Immunobiology 2012;217:743–748. [DOI] [PubMed] [Google Scholar]
- 8. Matsuzaki H, Maeda M, Lee S, et al. Asbestos‐induced cellular and molecular alteration of immunocompetent cells and their relationship with chronic inflammation and carcinogenesis. J Biomed Biotechnol 2012;2012:492608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuchroo VK, Martin CA, Greer JM, Ju ST, Sobel RA, Dorf ME. Cytokines and adhesion molecules contribute to the ability of myelin proteolipid protein‐specific T cell clones to mediate experimental allergic encephalomyelitis. J Immunol 1993;151:4371–4382. [PubMed] [Google Scholar]
- 10. Komiyama Y, Nakae S, Matsuki T, et al. IL‐17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol 2006;177:566–573. [DOI] [PubMed] [Google Scholar]
- 11. Bettelli E, Korn T, Kuchroo VK. Th17: The third member of the effector T cell trilogy. Curr Opin Immunol 2007;19:652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL‐17+ T helper cells. Cell 2006;126:1121–1133. [DOI] [PubMed] [Google Scholar]
- 13. McGeachy MJ, Bak‐Jensen KS, Chen Y, et al. TGF‐beta and IL‐6 drive the production of IL‐17 and IL‐10 by T cells and restrain T(H)‐17 cell‐mediated pathology. Nat Immunol 2007;8:1390–1397. [DOI] [PubMed] [Google Scholar]
- 14. Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med 2008;14:337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ceragioli HJ, Peterlevitz AC, Quispe JCR, Larena A, Pasquetto MP, Sampaio MA, Baranauskas V. Synthesis and characterization of boron‐doped carbon nanotubes. J Phys: Conf Ser 2008;100:052029. [Google Scholar]
- 16. Deibler GE, Martenson RE, Kies MW. Large scale preparation of myelin basic protein from central nervous tissue of several mammalian species. Prep Biochem 1972;2:139–165. [DOI] [PubMed] [Google Scholar]
- 17. Farias AS, Martins‐de‐Souza D, Guimaraes L, et al. Proteome analysis of spinal cord during the clinical course of monophasic experimental autoimmune encephalomyelitis. Proteomics 2012;12:2656–2662. [DOI] [PubMed] [Google Scholar]
- 18. Kateb B, Yamamoto V, Alizadeh D, et al. Multi‐walled carbon nanotube (MWCNT) synthesis, preparation, labeling, and functionalization. Methods Mol Biol 2010;651:307–317. [DOI] [PubMed] [Google Scholar]
- 19. Langrish CL, Chen Y, Blumenschein WM, et al. IL‐23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 2005;201:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pflanz S, Timans JC, Cheung J, et al. IL‐27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity 2002;16:779–790. [DOI] [PubMed] [Google Scholar]
- 21. Pot C, Apetoh L, Awasthi A, Kuchroo VK. Induction of regulatory Tr1 cells and inhibition of T(H)17 cells by IL‐27. Semin Immunol 2011;23:438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stumhofer JS, Laurence A, Wilson EH, et al. Interleukin 27 negatively regulates the development of interleukin 17‐producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol 2006;7:937–945. [DOI] [PubMed] [Google Scholar]
- 23. Batten M, Li J, Yi S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17‐producing T cells. Nat Immunol 2006;7:929–936. [DOI] [PubMed] [Google Scholar]
- 24. Diveu C, McGeachy MJ, Boniface K, et al. IL‐27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol 2009;182:5748–5756. [DOI] [PubMed] [Google Scholar]


