Summary
Background
Parkinson's disease (PD) is a degenerative brain disorder that is caused by neural defects in the substantia nigra. Numerous studies have reported that acupuncture treatment on GB34 (Yanglingquan) leads to significant improvements in patients with PD and in PD animal models. Studies using functional magnetic resonance imaging (fMRI) have shown that patients with PD, compared to healthy participants, have lower neural responses in extensive brain regions including the putamen, thalamus, and the supplementary motor area.
Objective
This study investigated the reported association between acupuncture point GB34 and PD.
Methods
Using fMRI, neural responses of 12 patients with PD and 12 healthy participants were examined before and after acupuncture stimulation.
Results
Acupuncture stimulation increased neural responses in regions including the substantia nigra, caudate, thalamus, and putamen, which are impaired caused by PD.
Conclusions
Areas associated with PD were activated by the acupuncture stimulation on GB34. This shows that acupuncture treatment on GB34 may be effective in improving the symptoms of PD. Although more randomized controlled trials on the topic will be needed, this study shows that acupuncture may be helpful in the treatment of symptoms involving PD.
Keywords: Acupuncture, Functional magnetic resonance imaging, Parkinson's disease, Regional homogeneity, Resting state
Introduction
Acupuncture, used in Eastern Medicine, is increasingly gaining focus as a form of complementary treatment 1, 2, 3. According to survey, it was found that 61% 4 of patients with PD in Singapore and 76% 5 of patients with PD in Korea reported using complementary treatment, with acupuncture being the most frequent method used. In Washington State, a recent survey revealed that 32% of the hospices offer acupuncture as complementary treatment to patients with PD 6. Accordingly, many studies have been performed to prove the efficacy of this treatment 7, 8, 9, 10. Several studies have reported that acupuncture treatment leads to significant improvements in patients with PD 1, 11, 12 and significant neuroprotective effects in PD animal models 10, 13, 14, 15. Acupuncture prevents 6‐hydroxydopamine‐induced neuronal death in the nigrostriatal dopaminergic system in the rat PD model 10, 13 and inhibits the microglial activation and inflammatory events in the MPTP‐induced PD mouse model 15. Proteomic analysis of the neuroprotective mechanisms of acupuncture treatment suggests that acupoint GB34‐specific acupuncture changes protein expression profiles in the substantia nigra in favor of dopamine neuronal survival 14. In addition, research using neuroimaging demonstrated that acupuncture stimulations can activate neural responses 1, 16.
Previous functional neuroimaging experiments have studied event‐related neural responses induced by acupuncture stimulations 17, 18. However, recent studies suggest that neural responses in event‐related performances also rely on the integrity of the resting‐state network 19, 20. With fMRI, the functional changes in neural responses after removal of the acupuncture needle can be evaluated. The results of these studies demonstrate that the resting‐state network could be altered after acupuncture stimulation and removal of the needle 19, 21, 22.
In fMRI studies on healthy participants, the medial prefrontal cortex, posterior cingulate cortex, precuneus, lateral parietal, and medial temporal cortices were reported to exhibit a default mode network 23. However, patients with PD were shown to have different default mode networks compared to the healthy participants 24. In these studies, patients with PD not only showed less deactivation of the posterior cingulate cortex and the precuneus, but even demonstrated a reversed pattern of activation and deactivation 24.
Recently, a new method called regional homogeneity (ReHo) has been used to investigate functional modulations during the resting state in patients with Alzheimer's disease 25, schizophrenia 26, and PD 27. ReHo reflects the temporal homogeneity of the regional BOLD signal. As the BOLD signal of fMRI may reflect neural activity 28, abnormal ReHo is possibly relevant to the changes of the temporal aspects of neural activity in the regional brain, and then, ReHo may detect the brain regions with abnormal activity. In the patients with PD, ReHo was lower in certain brain regions including the putamen, thalamus, and supplementary motor area, and higher in other areas including the cerebellum, primary sensorimotor cortex, and premotor area 27. In the current study, ReHo was used to investigate whether acupuncture modulates the resting‐state network associated with PD.
In this fMRI study on acupuncture, the following hypothesis arose from a review of the scientific literature: acupuncture stimulation modulates the resting‐state networks not only in healthy participants but also in patients with PD. Neural responses between healthy participants and patients with PD were expected to differ. Moreover, it was predicted that acupuncture would modulate the areas associated with PD, which could help clarify the efficacy of acupuncture.
Methods
Participants Allowed
Twenty‐four volunteers participated in this study following written informed consent procedures according to the institutional guidelines of the Human Research Committee. Twelve participants were idiopathic patients with PD (mean age: 53.5 years [range: 38–72], six men), whereas 12 volunteers were healthy participants, matched for age (mean age: 55.9, [range: 35–71]) and gender (six men). Participants with PD were diagnosed with clinically definite idiopathic PD by a neurologist, and participants with medical histories of other neurological illnesses were excluded. All were studied in the “off” condition; 12 h after all anti‐parkinsonian drugs had been withheld. Disability was assessed immediately after the patients were scanned. All had Hoehn and Yahr stage 29 1, 2, or 2.5. The mean Unified Parkinson's Disease Rating Scale (UPDRS) (Fahn and Elton, 1987) motor score was 7.8 (SD = 3.9 points). All patients with PD were right‐handed as verified by Edinburg Handedness Inventory 30; their mean score was 99.58% (SD = 1.44%). The average duration of the disease was 2.7 years. All patients with PD were responsive to either levodopa or dopamine agonists. The healthy participants were without any neurological or psychiatric history and were all right‐handed as verified by Edinburgh Handedness Inventory 30; their mean score was 100% (SD = 0%). Initial dyskinesia was left‐sided in six patients, right‐sided in three, and left‐ and right‐sided in three (Table 1).
Table 1.
Demographic characteristics of patients with Parkinson's disease (PD) compared to healthy participants
| HP (n = 12) | PDa (n = 12) | |
|---|---|---|
| Sex (male:female) | 6:6 | 6:6 |
| Age (years) | 55.9 ± 9.8 | 53.5 ± 10.9 |
| Disease duration (years) | – | 2.67 ± 2.3 |
| Medication duration (years) | – | 2.67 ± 2.3 |
| Side of initial dyskinesia (L:R:LR) | – | 6:2:3 |
| Hoen and Yahr stage | – | 1.5 ± 0.6 |
| UPDRS motor score | – | 7.8 ± 3.9 |
| K‐MMSE | – | 27.8 ± 0.4 |
| BDI II | – | 15.36 ± 7.9 |
| EHI (right:left) | 12:0 | 12:0 |
HP, healthy participants; UPDRS, Unified Parkinson's Disease Rating Scale; K‐MMSE, Korean Mini‐Mental State Examination; BDI II, Beck Depression Inventory II; EHI, Edinburgh Handedness Inventory.
Patients with PD.
Acupuncture
An experienced Eastern medical doctor conducted acupuncture on patients with PD and healthy participants at right GB34 (Yanglingquan). For acupuncture stimulation (ACUP), the needle (0.25 × 40 mm, Dong Bang Acupuncture Inc. Sungnam, Korea) was manually inserted into the right GB34 to a depth of approximately 1.0 cm. The needle remained in the skin for 1 min and was then rotated bidirectionally for 1 min. After that, needle remained in the skin without rotation for 1 min and then the pattern of 1 min rotation and 1‐min rest was repeated. For sham acupuncture stimulation (SHAM), the blunt type needle was used to poke the skin at the right GB34. In contrast to ACUP, the blunt type needle was not inserted into the skin, but only came in contact with the skin. All other aspects followed the same paradigm as for ACUP, and the blunt type needle was also rotated bidirectionally at 1Hz.
MRI Data Acquisition
A Philips 3.0 T MRI system equipped for echo planar imaging (EPI) was used for data acquisition. The fMRI paradigm started with a “REST” condition of 4 min. After both SHAM and ACUP, another “REST” condition of 4 min followed. The structural images were acquired between SHAM and ACUP for 10 min. Although the participants were told that the order was randomized, the actual order was SHAM first and ACUP second.
One hundred and twenty contiguous EPI functional volumes for “REST” and 150 for “ACUP” or “SHAM” (time repetition [TR] = 2000 ms, time echo [TE] = 35 ms, flip angle = 90°, slice thickness = 4.5, number of slices = 30, matrix = 96 × 128, field of view [FOV] = 230 × 182 × 135 mm, acquisition voxel size = 2.4 × 2.4 × 4.5 mm) were collected. During the scanning, participants remained in the supine position with their heads immobilized by cushioned supports and wore ear plugs throughout the experiment to attenuate MRI gradient noise. In addition, they were instructed to rest with their eyes closed and not to move. For spatial normalization and localization, a high‐resolution T1‐weighted anatomical image was acquired using a magnetization‐prepared gradient echo sequence (time repetition [TR] = 9.9 ms, time echo [TE] = 4.6 ms, flip angle = 90°, slice thickness = 1 mm, number of slices = 196, matrix = 236 × 240, field of view [FOV] = 235 × 235 × 196 mm, acquisition voxel size = 1 × 1 × 1 mm).
MRI Data Analysis
The fMRI data were analyzed using SPM5 (Welcome Department of Cognitive Neurology, London, UK). The functional EPI‐BOLD images were realigned, and the subject‐mean functional MR images were co‐registered with the corresponding structure MR images. These images were spatially normalized and transformed into a common space, as defined by the SPM Montreal Neurological Institute (MNI) T1 template.
The Kendal coefficient of concordance (KCC) was used to measure the similarity of the time series within a functional cluster based on the regional homogeneity hypothesis 31, 32. The 27 nearest neighboring voxels were defined as a cluster, and a KCC value (range 0–1) was given to the voxel at the center of this cluster. A custom software routine, Resting‐State fMRI Data Analysis Toolkit (REST, http://forum.restfmri.net/rest), was used for ReHo analysis in a voxel‐wise fashion. For all participants, the ReHo map was spatially smoothed with 9 mm of full width at half maximum (FWHM). In the analyses, the corrected threshold was P < 0.05 for the one‐sample t‐test and corrected cluster level was P < 0.05 for the two‐sample t‐test and the paired t‐test 33. Rex 34 and (SPSS Inc., Chicago, IL, USA) were used for neural signal change analysis.
Results
Psychophysical Responses
The intensities of sensations measured by an average score (with standard error bars) were reported on a scale from 0 denoting no sensation to 10 denoting an unbearable sensation among patients with PD and healthy participants during ACUP and SHAM. The sensations were compared between stimulation groups using a two‐sample t‐test, significant at P < 0.05 (SigmaPlot 9.0, Systat Software Inc., San Jose, CA, USA). The average stimulus intensities (mean ± SE) were approximately similar during ACUP of patients with PD (2 ± 2.4), SHAM of patients with PD (1.3 ± 2.0), ACUP of healthy participants (1.4 ± 2.1), and SHAM of healthy participants (0.9 ± 1.9). There was no significant statistical difference.
Resting‐State Results Among Healthy Participants and Patients with PD Before and After ACUP
Before acupuncture stimulations, neural responses for healthy participants and patients with PD commonly demonstrated that the posterior cingulate gyrus (BA 29/30), precuneus (BA 7/31), medial prefrontal cortex, and inferior parietal lobule (BA 40), which are reported as a default mode network in the previous study, exhibited significantly higher neural responses than other brain areas. Through these results, we could confirm our data.
After ACUP, the resting state of healthy participants and patients with PD had changed (see Table 2 for complete list of regions activated).
Table 2.
Resting state among healthy participants and patients with Parkinson's Disease (PD) before stimulations and after acupuncture stimulations
| Brain region | Hemisphere | Resting state before stimulation | Resting state after acupuncture stimulation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Statistical values | Coordinates anatomical location | Statistical values | Coordinates anatomical location | ||||||||||
| Cluster size | t‐value | x | y | z | Brodmann area | Cluster size | t‐value | x | y | z | Brodmann area | ||
| Healthy participants | |||||||||||||
| Frontal lobe | |||||||||||||
| Cingulate gyrus | L | 12 | 12.79 | −4 | 15 | 35 | 32 | – | – | – | – | – | – |
| Parietal lobe | |||||||||||||
| Angular gyrus | R | 87 | 13.5 | 34 | −62 | 37 | 39 | – | – | – | – | – | – |
| Inferior parietal lobule | R | – | 11.53 | 43 | −43 | 44 | 40 | – | – | – | – | – | – |
| L | – | – | – | – | – | – | 118 | 15.17 | −38 | −50 | 42 | 39/40 | |
| Temporal lobe | |||||||||||||
| Superior temporal gyrus | R | – | 11.51 | 49 | −57 | 16 | 22 | – | – | – | – | – | – |
| L | 55 | 18.84 | −51 | −31 | 11 | 13/22/39/41 | – | – | – | – | – | – | |
| Middle temporal gyrus | R | 15 | 13.74 | 54 | −47 | 3 | 22 | 20 | 14.42 | 54 | −30 | 0 | 21 |
| L | – | – | – | – | – | – | 148 | 17.11 | −43 | −53 | 9 | 39 | |
| Supramarginal gyrus | R | 61 | 13.31 | 48 | −50 | 33 | 40 | – | – | – | – | – | – |
| L | 136 | 13.41 | −51 | −52 | 26 | 40 | – | – | – | – | – | – | |
| Sublobar | |||||||||||||
| Insula | R | 17 | 12.85 | 51 | −23 | 19 | 13 | – | – | – | – | – | – |
| Limbic lobe | |||||||||||||
| Posterior cingulate | R | 13 | 11.35 | 4 | −53 | 10 | 29 | – | – | – | – | – | – |
| L | – | 18.85 | −10 | −62 | 14 | 30 | – | – | – | – | – | – | |
| Occipital lobe | |||||||||||||
| Cuneus | R | – | – | – | – | – | – | 795 | 18.63 | 1 | −75 | 32 | 19 |
| Precuneus | L | 546 | 24.48 | −4 | −69 | 27 | 7/31 | – | 10.67 | −27 | −57 | 50 | 7/31 |
| Cerebellum | R | – | – | – | – | – | – | – | 18.07 | 2 | −50 | 5 | – |
| Patients with PD | |||||||||||||
| Parietal lobe | |||||||||||||
| Inferior parietal lobule | L | 448 | 23.64 | −35 | −53 | 39 | 40 | 56 | 15.19 | −35 | −50 | 39 | 40 |
| Temporal lobe | |||||||||||||
| Middle temporal gyrus | R | – | 14.77 | 40 | −63 | 23 | 39 | – | – | – | – | – | – |
| L | – | 17.44 | −43 | −63 | 25 | 39 | – | – | – | – | – | – | |
| Supramarginal gyrus | R | 111 | 15.95 | 49 | −52 | 25 | 40 | – | – | – | – | – | – |
| Sublobar | |||||||||||||
| Insula | R | 11 | 11.6 | 51 | −35 | 21 | 13 | – | – | – | – | – | – |
| Thalamus | L | – | – | – | – | – | – | 57 | 15.48 | −7 | −11 | 11 | – |
| Limbic lobe | |||||||||||||
| Posterior cingulate | R | – | – | – | – | – | – | – | 14.86 | 4 | −56 | 7 | 30 |
| L | – | 15.71 | −1 | −53 | 13 | 29 | – | – | – | – | – | – | |
| Occipital lobe | |||||||||||||
| Precuneus | R | – | 12.56 | 29 | −67 | 34 | 7 | 368 | 18.29 | 10 | −66 | 23 | 31 |
| L | 385 | 16.04 | −2 | −72 | 30 | 7/31 | – | 11.86 | −27 | −67 | 35 | 7 | |
| Cerebellum | R | – | – | – | – | – | – | – | 18.03 | 4 | −41 | 0 | – |
| L | 12 | 11.95 | −31 | −57 | −34 | – | – | – | – | – | – | – | |
The table describes the location of the peak voxel and the corresponding brain regions and Brodmann areas comprised by the cluster. Results are reported if corrected P < 0.05. The cluster level is at least 10 voxels per cluster, and the voxel size is 2.4 × 2.4 × 4.5 mm.
In healthy participants, the right cuneus (BA 19), right cerebellum, left inferior parietal lobule (BA 39/40), left precuneus (BA 7/31), and left and right middle temporal gyrus (BA 21) exhibited significantly higher neural responses than other brain areas.
In patients with PD, the right cerebellum, right posterior cingulate (BA 30), left thalamus, left inferior parietal lobule (BA 40), and left and right precuneus (BA 7/31) exhibited significantly higher neural responses than other brain areas.
Resting State of Patients with PD Compared to Healthy Participants Before Stimulations
Compared to the healthy participants, patients with PD did not show significantly higher neural responses within our threshold.
Compared to patients with PD, the healthy participants showed significantly higher neural responses in the right precentral gyrus (BA 4/6), right postcentral gyrus (BA 2/3), right thalamus, and right caudate (Figure 1, Table 3).
Figure 1.
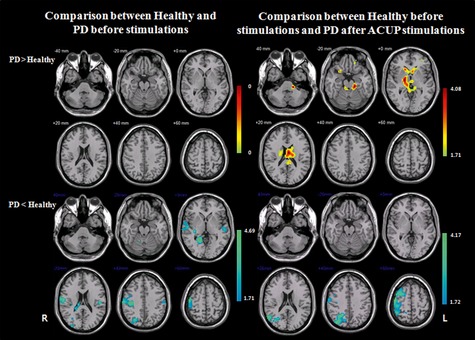
A comparison of the Kendall's coefficient of concordance (KCC) maps between patients with PD and healthy participants during the resting state. “PD > Healthy” indicates more activated neural responses of patients with PD compared to healthy participants, and “Healthy > PD” indicates more activated neural responses of healthy participants compared to patients with PD. “Before stimulations” means comparison of healthy participants and patients with PD before receiving any stimulation, and “Healthy before stimulations and PD after ACUP stimulations” means comparison of healthy participants before any stimulations and patients with PD after removal of the acupuncture needle. The bar is the t‐value. Note that R, right hemisphere; whereas L, left hemisphere.
Table 3.
Higher and lower neural responses among healthy participants before stimulations compared to Parkinson's Disease (PD) before and after acupuncture stimulations
| Brain region | Hemisphere | PD, compared to healthy participants before stimulations | PD after acupuncture stimulations, compared to healthy participants before stimulations | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| t‐value | Coordinates anatomical location | t‐value | Coordinates anatomical location | ||||||||
| x | y | z | Brodmann area | x | y | z | Brodmann area | ||||
| PD > Healthy participants | PD > Healthy participants | ||||||||||
| Frontal lobe | |||||||||||
| Superior frontal gyrus | L | – | – | – | – | – | 3.59 | −17 | 52 | −2 | 10 |
| Middle frontal gyrus | L | – | – | – | – | – | 2.08 | −31 | 43 | 5 | 10/11 |
| Inferior frontal gyrus | R | – | – | – | – | – | 2.41 | 24 | 14 | −13 | 47 |
| Cingulate gyrus | R | – | – | – | – | – | 2.73 | 1 | −41 | 27 | 31 |
| L | – | – | – | – | – | 3.67 | −4 | −9 | 22 | 24 | |
| Sublobar | |||||||||||
| Thalamus | R | – | – | – | – | – | 3.59 | 16 | −11 | 6 | – |
| L | – | – | – | – | – | 3.72 | −4 | −16 | 8 | – | |
| Caudate | R | – | – | – | – | – | 3.35 | 13 | 15 | 9 | – |
| L | – | – | – | – | – | 4.08 | −7 | 0 | 12 | – | |
| Putamen | L | – | – | – | – | – | 2.39 | −21 | −6 | 14 | – |
| Limbic lobe | |||||||||||
| Parahippocampal gyrus | L | – | – | – | – | – | 2.64 | −12 | −35 | −5 | 30 |
| Occipital lobe | |||||||||||
| Posterior cingulate | L | – | – | – | – | – | 2.55 | −7 | −37 | 20 | 23 |
| Cerebellum | R | – | – | – | – | – | 2.94 | 10 | −24 | 2 | – |
| L | – | – | – | – | – | 3.14 | −15 | −25 | −15 | – | |
| PD < Healthy participants | PD < Healthy participants | ||||||||||
| Frontal lobe | |||||||||||
| Superior frontal gyrus | R | – | – | – | – | – | 3.95 | 20 | 0 | 67 | 6 |
| Middle frontal gyrus | R | – | – | – | – | – | 3.11 | 40 | −4 | 53 | 6 |
| Precentral gyrus | R | 3.97 | 48 | −12 | 53 | 4/6 | 3.69 | 51 | −6 | 48 | 4 |
| Parietal lobe | |||||||||||
| Superior parietal lobule | R | – | – | – | – | – | 2.81 | 37 | −55 | 54 | 5/7 |
| Postcentral gyrus | R | 4.7 | 37 | −25 | 35 | 2/3 | 2.71 | 37 | −35 | 56 | 2/40 |
| Temporal lobe | |||||||||||
| Superior temporal gyrus | R | 4.06 | 55 | −13 | −1 | 22/41 | – | – | – | – | – |
| Middle temporal gyrus | R | – | – | – | – | – | 3.15 | 40 | −62 | 13 | 19 |
| Sublobar | |||||||||||
| Insula | R | 2.4 | 43 | −20 | 11 | 3 | – | – | – | – | – |
| Thalamus | R | 3 | 29 | −27 | 2 | – | – | – | – | – | – |
| Caudate | R | 2.18 | 18 | −40 | 17 | – | – | – | – | – | – |
| Occipital lobe | |||||||||||
| Lingual gyrus | R | 3.42 | 16 | −52 | −1 | 19 | – | – | – | – | – |
| Superior occipital gyrus | R | – | – | – | – | – | 4.18 | 34 | −81 | 30 | 19 |
| Posterior cingulate | R | 2.22 | 21 | −51 | 13 | 23/30 | 2.97 | 26 | −64 | 9 | 30 |
| Precuneus | R | – | – | – | – | – | 3.54 | 18 | −58 | 34 | 7/31 |
The table describes the location of the peak voxel and the corresponding brain regions and Brodmann areas comprised by the cluster. Results are reported if corrected cluster level P < 0.05. The voxel size is 2.4 × 2.4 × 4.5 mm.
Higher and Lower Neural Responses Among Patients with PD after ACUP, Compared to Healthy Participants Before ACUP
Compared to the healthy participants, patients with PD showed significantly higher neural responses in the left superior frontal gyrus (BA 10), left middle frontal gyrus (BA 10/11), right inferior frontal gyrus (BA 47), left and right thalamus, left and right caudate, left putamen, and left and right cerebellum.
Patients with PD showed significantly lower neural responses in the right superior frontal gyrus (BA 6), right middle frontal gyrus (BA 6), right precentral gyrus (BA 4), superior parietal lobule (BA 5/7), and right postcentral gyrus (BA 2/40) (Figure 1, Table 3).
Resting‐state Modulations Induced by ACUP and SHAM
In comparing the neural responses before and after ACUP, significantly increased foci of healthy participants were found in the left caudate body, left putamen, and left thalamus.
In comparing the neural responses before and after SHAM, however, significantly increased foci of healthy participants were located in the right paracentral lobule (BA 6), left superior parietal lobule (BA 7), right postcenral gyrus (BA 3), and left and right cerebellum (Figure 2, Table 4).
Figure 2.
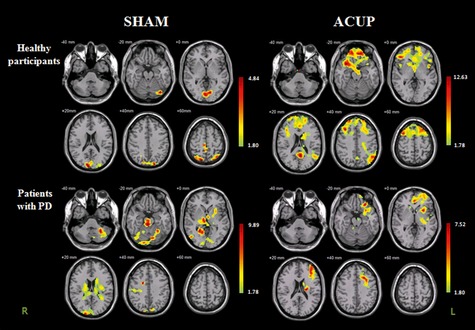
Increased neural responses after removal of the acupuncture needle and sham needle among healthy participants and patients with PD. The bar is the t‐value. Note that R, right hemisphere; whereas L, left hemisphere.
Table 4.
Increased neural responses of healthy participants and patients with Parkinson's Disease (PD), after sham acupuncture stimulation (SHAM) and acupuncture stimulation (ACUP)
| Brain region | Hemis‐ phere | Healthy participants | Patients with PD | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sham | Acupuncture | Sham | Acupuncture | ||||||||||||||||||
| t‐value | x | y | z | Brodmann area | t‐value | x | y | z | Brodmann area | t‐value | x | y | z | Brodmann area | t‐value | x | y | z | Brodmann area | ||
| Frontal lobe | |||||||||||||||||||||
| Superior frontal gyrus | R | – | – | – | – | – | 7.86 | 21 | 48 | 42 | 6/8/10/11 | – | – | – | – | – | – | – | – | – | |
| L | – | – | – | – | – | 6.85 | −24 | 15 | 62 | 6/8/9 | – | – | – | – | 4.99 | −29 | 60 | 7 | 10 | ||
| Middle frontal gyrus | R | – | – | – | – | – | 9.97 | 32 | 4 | 59 | 6/46 | – | – | – | – | – | – | – | – | – | |
| L | – | – | – | – | – | 12.63 | −29 | 11 | 48 | 6 | – | – | – | – | 5.56 | −23 | 28 | −12 | 6/9/10/11 | ||
| Medial frontal gyrus | L | – | – | – | – | – | 6.03 | −16 | 8 | 53 | 6/10 | – | – | – | – | 6.3 | −17 | 49 | 3 | ‐ | |
| Inferior frontal gyrus | R | – | – | – | – | – | 6.62 | 33 | 14 | −10 | 13/46/47 | – | – | – | – | – | – | – | – | – | |
| L | – | – | – | – | – | 4.31 | −26 | 25 | −7 | 47 | – | – | – | – | 4.01 | −26 | 11 | −12 | 47 | ||
| Paracentral lobule | R | 3.17 | 4 | −32 | 58 | 6 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Parietal lobe | |||||||||||||||||||||
| Superior parietal lobule | L | 3.09 | −32 | −66 | 54 | 7 | 5.79 | −49 | −61 | 33 | 39 | – | – | – | – | – | – | – | – | – | |
| Angular gyrus | L | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Postcentral gyrus | R | 3.67 | 12 | −36 | 69 | 3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Precuneus | R | 4.84 | 4 | −52 | 59 | 7/19 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| L | 4.52 | −21 | −84 | 39 | 7/19 | 6.56 | −4 | −60 | 26 | 31 | – | – | – | – | – | – | – | – | – | ||
| Temporal lobe | |||||||||||||||||||||
| Inferior temporal gyrus | R | – | – | – | – | – | – | – | – | – | – | 4.08 | 43 | −69 | −2 | – | – | – | – | – | – |
| Sublobar | |||||||||||||||||||||
| Caudate body | R | – | – | – | – | – | – | – | – | – | – | 3.79 | 18 | −10 | 25 | – | 3.6 | 13 | 17 | 12 | – |
| L | – | – | – | – | – | 5.78 | −18 | 5 | 18 | – | 3.68 | −15 | −1 | 23 | – | – | – | – | – | – | |
| Caudate tail | L | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 3.56 | −34 | −30 | 4 | – |
| Putamen | L | – | – | – | – | – | 4.81 | −23 | 6 | 15 | – | 3.45 | −26 | 13 | −6 | – | 5.69 | −29 | 10 | 2 | – |
| Lateral globus pallidus | L | – | – | – | – | – | – | – | – | – | – | 3.83 | −20 | −7 | −2 | – | – | – | – | – | – |
| Thalamus | R | – | – | – | – | – | – | – | – | – | – | 5.36 | 7 | −22 | 5 | – | – | – | – | – | – |
| L | – | – | – | – | – | 4.13 | −4 | −2 | 9 | – | 4.45 | −7 | −10 | −2 | – | 3.99 | −21 | −20 | 16 | – | |
| Insula | R | – | – | – | – | – | 7.9 | 44 | 7 | −5 | 13 | – | – | – | – | – | – | – | – | – | – |
| L | – | – | – | – | – | – | – | – | – | – | 3.82 | −29 | −17 | 21 | 13 | 10.34 | −32 | 20 | 14 | 13 | |
| Extranuclear | L | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 3.37 | −37 | 14 | −11 | 13 |
| Limbic lobe | |||||||||||||||||||||
| Anterior cingulate | L | – | – | – | – | – | 5.12 | −12 | 45 | −8 | 32 | – | – | – | – | – | 4.28 | −1 | 37 | 8 | 24 |
| Posterior cingulate | R | – | – | – | – | – | 5.43 | 1 | −51 | 13 | 30 | 4.23 | 15 | −67 | 6 | 23/30 | – | – | – | – | – |
| Cingulate gyrus | L | – | – | – | – | – | 3.46 | −21 | −22 | 40 | 31 | – | – | – | – | – | 5.62 | −24 | 12 | 40 | 32 |
| Parahippocampal gyrus | R | – | – | – | – | – | – | – | – | – | – | 5.23 | 21 | −52 | 0 | 19 | – | – | – | – | – |
| L | – | – | – | – | – | – | – | – | – | – | 3.75 | −21 | −39 | 6 | 30 | 3.71 | −20 | −17 | −9 | 35 | |
| Midbrain | |||||||||||||||||||||
| Substantia nigra | R | – | – | – | – | – | – | – | – | – | – | 4.77 | 5 | −15 | −14 | – | – | – | – | – | – |
| L | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 3.92 | −12 | −20 | −6 | – | |
| Red nucleus | R | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 3.29 | 2 | −26 | −12 | – |
| L | – | – | – | – | – | – | – | – | – | – | 6.99 | −1 | −23 | −11 | – | – | – | – | – | – | |
| Occipital lobe | |||||||||||||||||||||
| Cuneus | R | 3.27 | 10 | −88 | 15 | 18 | – | – | – | – | – | 9.89 | 18 | −96 | 12 | 18 | – | – | – | – | – |
| L | 3.05 | −1 | −81 | 10 | 17 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Fusiform gyrus | R | – | – | – | – | – | – | – | – | – | – | 4.14 | 43 | −68 | −10 | 19 | – | – | – | – | – |
| L | – | – | – | – | – | – | – | – | – | – | 3.98 | −21 | −85 | −12 | 19 | – | – | – | – | – | |
| Lingual gyrus | R | 3.06 | 2 | −89 | −1 | 18 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| L | 3.42 | −12 | −74 | −3 | 18 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Cerebellum | R | 3.88 | 2 | −80 | 9 | – | – | – | – | – | – | 5.5 | 10 | −55 | 2 | – | 3.02 | 2 | −40 | −13 | – |
| L | 3.46 | −34 | −72 | −19 | – | – | – | – | – | – | 5.68 | −15 | −68 | −19 | – | – | – | – | – | – | |
The table describes the location of the peak voxel and the corresponding brain regions and Brodmann areas comprised by the cluster. Results are reported if cluster level corrected was P < 0.05 and t‐value >3. The voxel size is 2.4 × 2.4 × 4.5 mm.
When the same comparison before and after ACUP was made for patients with PD, significantly increased foci were in the right caudate body, left caudate tail, left putamen, left thalamus, and left substantia nigra.
In comparing the neural responses before and after Sham, however, significantly increased foci of patients with PD were in the left and right caudate, left putamen, left lateral globus pallidus, left and right thalamus, right substantia nigra, right cuneus (BA 18), and left and right cerebellum (Figure 2, Table 4).
Compared to the healthy participants, patients with PD showed a significantly higher signal increase in the thalamus after ACUP than before (p < 0.05) (Figure 3).
Figure 3.
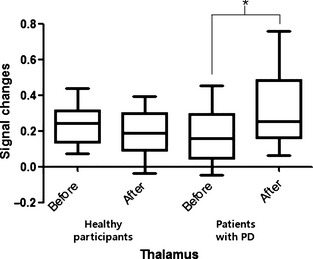
Neural signal changes after removal of the acupuncture needle among healthy participants and patients with PD in the thalamus. *P < 0.05.
Discussion
Although a relatively recent arrival in many countries, acupuncture is used more frequently as a treatment in addition to conventional medicine for patients with PD 1. Therefore, it is important to better understand its neural mechanisms. The acupoint GB34 was chosen because it has been reported to lead to significant improvements in patients with PD 1, 11, 12 and significant neuroprotective effects in PD animal models 10, 13, 14, 15. The aim of this study was to demonstrate changes to the resting state modulated by acupuncture stimulations on GB34 and to show that the areas associated with PD could be modulated by acupuncture stimulations.
The analysis of the psychophysical responses to ACUP and SHAM showed that the average stimulus intensities were similar during ACUP of patients with PD and SHAM of patients with PD, as well as during ACUP of healthy participants and SHAM of healthy participants, indicating that in each group, no differences were felt between ACUP and SHAM. These results are in line with previous studies 19, 35, in which the general finding was that the psychophysical responses to ACUP and SHAM were similar, although significant neural differences were found 19.
Before stimulations, healthy participants and patients with PD exhibited significantly higher neural responses in the posterior cingulate gyrus, precuneus, medial prefrontal cortex, and inferior parietal lobule than in other brain areas during the resting state (Table 2). These results are consistent with previous research indicating that a set of brain regions, including the posterior cingulate, precuneus, and medial prefrontal cortex, demonstrate higher cerebral blood flow than average for the brain in the resting state 23, 36. After ACUP, the resting state of healthy participants and patients with PD had changed. Especially, significantly higher brain activations of the thalamic regions were shown in patients with PD, but not in healthy participants (Table 2). It is consistently shown in patients with PD compared to healthy participants after ACUP (Table 3).
Previous research has shown that the resting state of patients with PD was different compared to that of healthy participants 24, 27. In these studies, the neural responses of patients with PD were lower in extensive brain regions including the putamen, thalamus, and supplementary motor area and were higher in other areas including the cerebellum, primary sensorimotor cortex, and premotor area 27. Our study identified somewhat different areas of initially lower neural responses in patients with PD. Before stimulations, patients with PD showed significantly lower neural responses compared to the healthy participants in the precentral gyrus, postcentral gyrus, thalamus, and caudate. It is interesting that a reduced neural response was observed in the right and not the left precentral gyrus in patients with PD (Figure 1, Table 3). For this reason, it may be worth noting that initial dyskinesia was left‐sided in six patients, right‐sided in three, and left‐ and right‐sided in three. The dominance of initial left‐sided bradykinesia could help explain these data.
Compared to the healthy participants before stimulations, patients with PD showed significantly higher neural responses after ACUP in certain areas (Figure 1, Table 3). Among these, the thalamus 37, putamen 38, and caudate 24, 39 are the areas that were reported to be associated with PD in previous studies. Moreover, the thalamus and caudate of patients with PD had lower neural responses compared to healthy participants before ACUP (Table 3). These results suggest that acupuncture stimulation may increase brain activations of regions associated with PD.
Our first hypothesis was that ACUP could modulate the resting‐state network not only in healthy participants 19, 22, 40 but also in patients with PD. When comparing the neural responses before and after ACUP, significantly increased foci of each healthy participants and patients with PD were found (Figure 2, Table 4). Moreover, different neural responses between healthy participants and patients with PD were observed (Figure 2). When the resting‐state changes caused by ACUP and SHAM were compared among healthy participants, no commonly changed areas were found except the left precuneus (Table 4). In comparing these changes in patients with PD, significantly increased foci were common in the right caudate body, left putamen, left thalamus, left insula, left parahippocampal gyrus, and right cerebellum. Additionally, the basal ganglia areas exhibited resting‐state changes in response to both ACUP and SHAM in patients with PD. In contrast, healthy participants only showed changes in these areas after ACUP stimulations. The pressure of sham stimulations on the acupoint may have evoked such responses only in patients with PD. This is consistent with previous reports which stated that the neural responses between patients and healthy participants differed 41, 42, 43, 44. These differences between patients with PD and healthy participants were thought to come from cognitive impairment. In Parkinson's disease, cognitive impairment 45, 46, which is related with the sensory nerve system 47, visuospatial and visuoperceptual problems 48, influence of vestibular loss 49, was reported. Cognitive impairment of patients with PD may also affect the brain activations on ACUP and SHAM, so the neural responses were different compared to healthy participants. In our study, the basal ganglia areas exhibited the resting‐state changes in response to SHAM only in the patients with PD. In contrast, the healthy participants did not show the changes in these areas after SHAM. These different observations may depend on the cognitive impairment in PD. The patients with PD must have had neuronal dysfunction by the nigral dopamine depletion, which might cause abnormal activations of the basal ganglia compared to the healthy participants. For this reason, studies on acupuncture efficacy should thus investigate its effects on patients with diseases as well as healthy participants.
In previous fMRI studies of patients with PD, the brain activation in patients with PD also differed from that of healthy participants during performance of a motor task 42, 50, 51, 52; patients with PD exhibited a markedly different pattern of activation characterized by a significant over‐activation in the ipsilateral cerebellar hemisphere 50 and a significant under‐activation in the supplementary motor area 50, 51, 52 and right dorsolateral prefrontal cortex 42, 51, 52. The differences found in patients with PD performing motor tasks can be explained by a functional deficit of the striato‐cortical‐motor loops 42, 50, 53. To compensate for the dopamine deficit in the striato‐cortical‐motor loops, other areas are activated in the brain that are likely to participate in the same putative attempt by the dopamine‐denervated brain to recruit parallel motor circuits. After examining the literature, we hypothesized that brain activations induced by acupuncture stimulations would be shown in the malfunctional areas to make effect. Moreover, these brain activations would differ from those of healthy participants. In a previous study, the neural responses of patients with PD compared to those of healthy participants were lower in extensive brain regions, including the putamen, thalamus, and supplementary motor area 27. Our results demonstrated that the initial neural responses were lower in extensive brain regions of patients with PD including the postcentral gyrus, superior temporal gyrus, precentral gyrus, lingual gyrus, thalamus, insula, posterior cingulate, and caudate (Table 3). After receiving ACUP, neural responses in patients with PD increased in the caudate, putamen, cingulate gyrus, thalamus, substantia nigra, anterior cingulate, prefrontal gyrus, insula, parahippocampal gyrus, and cerebellum (Table 4). In addition, the caudate, insula, putamen, and thalamus, which demonstrated lower neural responses when compared to the healthy participants before ACUP (Table 3), showed statistically significant signal increases after receiving ACUP (Table 4).
In a simple finger‐tapping task, acupuncture at GB34 showed a significant improvement in the motor function of the affected hand before and after acupuncture stimulations on GB34, 13% 12. Considering the previous motor task studies 42, 43, 44, 50, 53 and the previous acupuncture studies on PD with GB34 12, 54, it was expected that the patients with PD would have different neural responses to overcome their functional deficiencies in the striato‐cortical‐motor loops. Several studies have shown that patients with PD use different motor pathways to compensate for the functional deficiencies of the striato‐cortical‐motor loops 37, 42, 43, 50, 53, 55, one of which is the cerebello‐thalamic pathway 37, 55. The fact that neural responses increased in the thalamus and cerebellum after ACUP in patients with PD therefore supports the hypothesis that acupuncture modulates the resting state of areas associated with PD. Interestingly, the neural responses of areas shown to be associated with PD in previous studies such as the substantia nigra 56, caudate 24, 39, thalamus 37, and putamen 38 also increased after ACUP. This suggests that acupuncture stimulations have effect on the brain areas that are impaired caused by PD. These results were consistent with the previous study, which demonstrated that the putamen and primary motor cortex were activated when patients with PD received ACUP on GB34 and that acupuncture treatment might facilitate improvement in the motor functioning of patients with PD via the basal ganglia–thalamocortical circuit 12.
The limitations of this study are that acupuncture stimulations were short term, and we should have observed motor improvement instead of just referring to existing literature. Moreover, the patients’ mean disease duration of our participants was 2.67 years, which is a relatively benign state of PD, so the results were slightly differing from previous research and might only apply to relatively patients with early PD. Although future randomized controlled trials of neural response increases in these areas are needed to confirm acupuncture's role in improving symptoms of PD, the results of this study support the hypothesis that acupuncture stimulations on GB34 modulate the resting state of areas associated with PD. We believe that our study holds importance for future clinical and acupuncture studies in patients with neurodegenerative diseases, especially PD.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This study was supported by the Ministry of Health, Welfare and Family Affairs, Republic of Korea (Oriental Medicine R&D Project, B080049) and the National Research Foundation of Korea (NRF) grant funded by the Korea government [MEST] (No. 2010‐0001278).
References
- 1. Cristian A, Katz M, Cutrone E, Walker RH. Evaluation of acupuncture in the treatment of Parkinson's disease: a double‐blind pilot study. Mov Disord 2005;20: 1185–1188. [DOI] [PubMed] [Google Scholar]
- 2. Staud R. Mechanisms of acupuncture analgesia: effective therapy for musculoskeletal pain? Curr Rheumatol Rep 2007;9: 473–481. [DOI] [PubMed] [Google Scholar]
- 3. Kaptchuk TJ. Acupuncture: theory, efficacy, and practice. Ann Intern Med 2002;136: 374–383. [DOI] [PubMed] [Google Scholar]
- 4. Tan LC, Lau PN, Jamora RD, Chan ES. Use of complementary therapies in patients with Parkinson's disease in Singapore. Mov Disord 2006;21: 86–89. [DOI] [PubMed] [Google Scholar]
- 5. Kim SR, Lee TY, Kim MS, Lee MC, Chung SJ. Use of complementary and alternative medicine by Korean patients with Parkinson's disease. Clin Neurol Neurosurg 2009;111: 156–160. [DOI] [PubMed] [Google Scholar]
- 6. Kozak LE, Kayes L, McCarty R, et al. Use of complementary and alternative medicine (CAM) by Washington State hospices. Am J Hosp Palliat Care 2008;25: 463–468. [DOI] [PubMed] [Google Scholar]
- 7. Liang XB, Luo Y, Liu XY, et al. Electro‐acupuncture improves behavior and upregulates GDNF mRNA in MFB transected rats. NeuroReport 2003;14: 1177–1181. [DOI] [PubMed] [Google Scholar]
- 8. Kim SK, Moon HJ, Na HS, et al. The analgesic effects of automatically controlled rotating acupuncture in rats: mediation by endogenous opioid system. J Physiol Sci 2006;56: 259–262. [DOI] [PubMed] [Google Scholar]
- 9. Lam YC, Kum WF, Durairajan SS, et al. Efficacy and safety of acupuncture for idiopathic Parkinson's disease: a systematic review. J Altern Complement Med 2008;14: 663–671. [DOI] [PubMed] [Google Scholar]
- 10. Park HJ, Lim S, Joo WS, et al. Acupuncture prevents 6‐hydroxydopamine‐induced neuronal death in the nigrostriatal dopaminergic system in the rat Parkinson's disease model. Exp Neurol 2003;180: 93–98. [DOI] [PubMed] [Google Scholar]
- 11. Shulman LM, Wen X, Weiner WJ, et al. Acupuncture therapy for the symptoms of Parkinson's disease. Mov Disord 2002;17: 799–802. [DOI] [PubMed] [Google Scholar]
- 12. Chae Y, Lee H, Kim H, et al. Parsing brain activity associated with acupuncture treatment in Parkinson's diseases. Mov Disord 2009;24: 1794–1802. [DOI] [PubMed] [Google Scholar]
- 13. Lin Y, Lin X. Comparative study of D2 receptors and dopamine content in striatum before and after electro‐acupuncture treatment in rats. Chin Med J (Engl) 2000;113: 408–411. [PubMed] [Google Scholar]
- 14. Jeon S, Kim YJ, Kim ST, et al. Proteomic analysis of the neuroprotective mechanisms of acupuncture treatment in a Parkinson's disease mouse model. Proteomics 2008;8: 4822–4832. [DOI] [PubMed] [Google Scholar]
- 15. Kang JM, Park HJ, Choi YG, et al. Acupuncture inhibits microglial activation and inflammatory events in the MPTP‐induced mouse model. Brain Res 2007;1131: 211–219. [DOI] [PubMed] [Google Scholar]
- 16. Napadow V, Dhond RP, Kim J, et al. Brain encoding of acupuncture sensation–coupling on‐line rating with fMRI. Neuroimage 2009;47: 1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Siedentopf CM, Golaszewski SM, Mottaghy FM, Ruff CC, Felber S, Schlager A. Functional magnetic resonance imaging detects activation of the visual association cortex during laser acupuncture of the foot in humans. Neurosci Lett 2002;327: 53–56. [DOI] [PubMed] [Google Scholar]
- 18. Fang JL, Krings T, Weidemann J, Meister IG, Thron A. Functional MRI in healthy subjects during acupuncture: different effects of needle rotation in real and false acupoints. Neuroradiology 2004;46: 359–362. [DOI] [PubMed] [Google Scholar]
- 19. Liu P, Zhang Y, Zhou G, et al. Partial correlation investigation on the default mode network involved in acupuncture: an fMRI study. Neurosci Lett 2009;462: 183–187. [DOI] [PubMed] [Google Scholar]
- 20. Hui KK, Marina O, Claunch JD, et al. Acupuncture mobilizes the brain's default mode and its anti‐correlated network in healthy subjects. Brain Res 2009;1287: 84–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Y, Qin W, Liu P, et al. An fMRI study of acupuncture using independent component analysis. Neurosci Lett 2009;449: 6–9. [DOI] [PubMed] [Google Scholar]
- 22. Dhond RP, Yeh C, Park K, Kettner N, Napadow V. Acupuncture modulates resting state connectivity in default and sensorimotor brain networks. Pain 2008;136: 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A 2001;98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Eimeren T, Monchi O, Ballanger B, Strafella AP. Dysfunction of the default mode network in Parkinson disease: a functional magnetic resonance imaging study. Arch Neurol 2009;66: 877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. He Y, Wang L, Zang Y, et al. Regional coherence changes in the early stages of Alzheimer's disease: a combined structural and resting‐state functional MRI study. Neuroimage 2007;35: 488–500. [DOI] [PubMed] [Google Scholar]
- 26. Liu H, Liu Z, Liang M, et al. Decreased regional homogeneity in schizophrenia: a resting state functional magnetic resonance imaging study. NeuroReport 2006;17: 19–22. [DOI] [PubMed] [Google Scholar]
- 27. Wu T, Long X, Zang Y, et al. Regional homogeneity changes in patients with Parkinson's disease. Hum Brain Mapp 2009;30: 1502–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature 2001;412: 150–157. [DOI] [PubMed] [Google Scholar]
- 29. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17: 427–442. [DOI] [PubMed] [Google Scholar]
- 30. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9: 97–113. [DOI] [PubMed] [Google Scholar]
- 31. Xu X, Shibasaki H, Shindo K. Effects of acupuncture on somatosensory evoked potentials: a review. J Clin Neurophysiol 1993;10: 370–377. [DOI] [PubMed] [Google Scholar]
- 32. Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage 2004;22: 394–400. [DOI] [PubMed] [Google Scholar]
- 33. Mezer A, Yovel Y, Pasternak O, Gorfine T, Assaf Y. Cluster analysis of resting‐state fMRI time series. Neuroimage 2009;45: 1117–1125. [DOI] [PubMed] [Google Scholar]
- 34. Duff EP, Cunnington R, Egan GF. REX: response exploration for neuroimaging datasets. Neuroinformatics 2007;5: 223–234. [DOI] [PubMed] [Google Scholar]
- 35. Kong J, Kaptchuk TJ, Polich G, et al. Expectancy and treatment interactions: a dissociation between acupuncture analgesia and expectancy evoked placebo analgesia. Neuroimage 2009;45: 940–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zou Q, Wu CW, Stein EA, Zang Y, Yang Y. Static and dynamic characteristics of cerebral blood flow during the resting state. Neuroimage 2009;48: 515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lewis MM, Slagle CG, Smith AB, et al. Task specific influences of Parkinson's disease on the striato‐thalamo‐cortical and cerebello‐thalamo‐cortical motor circuitries. Neuroscience 2007;147: 224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson's disease. Neuroimage 2007;35: 222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Farid K, Sibon I, Guehl D, Cuny E, Burbaud P, Allard M. Brain dopaminergic modulation associated with executive function in Parkinson's disease. Mov Disord 2009;24: 1962–1969. [DOI] [PubMed] [Google Scholar]
- 40. Bai L, Qin W, Tian J, et al. Time‐varied characteristics of acupuncture effects in fMRI studies. Hum Brain Mapp 2009;30: 3445–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dhond RP, Kettner N, Napadow V. Neuroimaging acupuncture effects in the human brain. J Altern Complement Med 2007;13: 603–616. [DOI] [PubMed] [Google Scholar]
- 42. Sabatini U, Boulanouar K, Fabre N, et al. Cortical motor reorganization in akinetic patients with Parkinson's disease: a functional MRI study. Brain 2000;123: 394–403. [DOI] [PubMed] [Google Scholar]
- 43. Mallol R, Barros‐Loscertales A, Lopez M, Belloch V, Parcet MA, Avila C. Compensatory cortical mechanisms in Parkinson's disease evidenced with fMRI during the performance of pre‐learned sequential movements. Brain Res 2007;1147: 265–271. [DOI] [PubMed] [Google Scholar]
- 44. Elsinger CL, Rao SM, Zimbelman JL, Reynolds NC, Blindauer KA, Hoffmann RG. Neural basis for impaired time reproduction in Parkinson's disease: an fMRI study. J Int Neuropsychol Soc 2003;9: 1088–1098. [DOI] [PubMed] [Google Scholar]
- 45. Poletti M, Emre M, Bonuccelli U. Mild cognitive impairment and cognitive reserve in Parkinson's disease. Parkinsonism Relat Disord 2011;17: 579–586. [DOI] [PubMed] [Google Scholar]
- 46. Aarsland D, Bronnick K, Fladby T. Mild cognitive impairment in Parkinson's disease. Curr Neurol Neurosci Rep 2011;11: 371–378. [DOI] [PubMed] [Google Scholar]
- 47. Palomar FJ, Diaz‐Corrales F, Carrillo F, Fernandez‐del‐Olmo M, Koch G, Mir P. Sensory perception changes induced by transcranial magnetic stimulation over the primary somatosensory cortex in Parkinson's disease. Mov Disord 2011;26: 2058–2064. [DOI] [PubMed] [Google Scholar]
- 48. Dalrymple‐Alford JC, Livingston L, MacAskill MR, et al. Characterizing mild cognitive impairment in Parkinson's disease. Mov Disord 2011;26: 629–636. [DOI] [PubMed] [Google Scholar]
- 49. Allum JH, Tang KS, Carpenter MG, Oude Nijhuis LB, Bloem BR. Review of first trial responses in balance control: influence of vestibular loss and Parkinson's disease. Hum Mov Sci 2011;30: 279–295. [DOI] [PubMed] [Google Scholar]
- 50. Rascol O, Sabatini U, Fabre N, et al. The ipsilateral cerebellar hemisphere is overactive during hand movements in akinetic parkinsonian patients. Brain 1997;120: 103–110. [DOI] [PubMed] [Google Scholar]
- 51. Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self‐initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement‐related potentials in normal and Parkinson's disease subjects. Brain 1995;118: 913–933. [DOI] [PubMed] [Google Scholar]
- 52. Owen AM, Doyon J, Dagher A, Sadikot A, Evans AC. Abnormal basal ganglia outflow in Parkinson's disease identified with PET. Implications for higher cortical functions. Brain 1998;121: 949–965. [DOI] [PubMed] [Google Scholar]
- 53. Samuel M, Ceballos‐Baumann AO, Blin J, et al. Evidence for lateral premotor and parietal overactivity in Parkinson's disease during sequential and bimanual movements. A PET study. Brain 1997;120: 963–976. [DOI] [PubMed] [Google Scholar]
- 54. Na BJ, Jahng GH, Park SU, et al. An fMRI study of neuronal specificity of an acupoint: electroacupuncture stimulation of Yanglingquan (GB34) and its sham point. Neurosci Lett 2009;464: 1–5. [DOI] [PubMed] [Google Scholar]
- 55. Cerasa A, Hagberg GE, Peppe A, et al. Functional changes in the activity of cerebellum and frontostriatal regions during externally and internally timed movement in Parkinson's disease. Brain Res Bull 2006;71: 259–269. [DOI] [PubMed] [Google Scholar]
- 56. Foster ER, Black KJ, Antenor‐Dorsey JA, Perlmutter JS, Hershey T. Motor asymmetry and substantia nigra volume are related to spatial delayed response performance in Parkinson disease. Brain Cogn 2008;67: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


