Summary
Neurodegenerative diseases are devastating because they cause increasing loss of cognitive and physical functions and affect an estimated 1 billion individuals worldwide. Unfortunately, no drugs are currently available to halt their progression, except a few that are largely inadequate. This mandates the search of new treatments for these progressively degenerative diseases. Neural stem cells (NSCs) have been successfully isolated, propagated, and characterized from the adult brains of mammals, including humans. The confirmation that neurogenesis occurs in the adult brain via NSCs opens up fresh avenues for treating neurological problems. The proof‐of‐concept studies demonstrating the neural differentiation capacity of stem cells both in vitro and in vivo have raised widespread enthusiasm toward cell‐based interventions. It is anticipated that cell‐based neurogenic drugs may reverse or compensate for deficits associated with neurological diseases. The increasing interest of the private sector in using human stem cells in therapeutics is evidenced by launching of several collaborative clinical research activities between Pharma giants and research institutions or small start‐up companies. In this review, we discuss the major developments that have taken place in this field to position stem cells as a prospective candidate drug for the treatment of neurological disorders.
Keywords: Cell transplantation and clinical trials, Neural differentiation, Neurorepair, Stem cells
Introduction
New drugs for various neurological disorders have become a crying need. Clinicians, patients, and their families all await the development of effective drugs against many neurological disorders that are today basically untreatable. Major drug companies have little incentive to develop drugs for obscure neurological diseases as the drug development process is complex, expensive and most major companies have had costly failures with once promising molecules. One such was semagacestat, a γ‐secretase inhibitor that was being tested in Phase 3 trials for Alzheimer's disease (AD). An interim analysis suggested that it did not slow progression of disease and seemed to worsen some of the symptoms. This subsequent scrapping of the drug development led to large losses 1. Developments of central nervous system (CNS) drugs have a high failure rate. It has been estimated that just 9% of compounds that entered Phase 1 trials finally made it to the market 2. The cost of drug development has been variously calculated, but most agree that it costs as much as US $800 million to develop a new drug. This has inhibited large companies that usually hope to recoup their research costs through a blockbuster drug, to explore drugs for what is probably a much smaller market. GlaxoSmithKline, for instance, pulled out of drug discovery in some areas of neuroscience including psychiatric disorders and pain, in February 2011 1. Similarly, AstraZeneca announced in March of the same year that it too was pulling out of the psychiatric drug area, a field in which it had a leadership position.
The need for such drugs, however, is evident. If one counts the total numbers of patients with neurological disorders, the number worldwide would be over a billion 3. Not all of them, of course, will be treated with the same drugs, but there remains a huge unmet need. WHO has estimated the disability‐adjusted life years from neurological disease to be about 92 million in 2005 and has projected an increase to 103 million by 2030 3. The mortality rate from neurological disorders as a proportion of total is expected to remain the same over this period, but in absolute numbers, the figure is alarmingly large. Under these circumstances, the advent of cell therapy is regarded by many thoughtful observers as a potential savior. The basis of using cell therapy for neurological disorders is the hypothesis that these therapies will mimic the normal cell repair and development process in the neurological system to eliminate the cause of the disorder. Stem cells are immature cells that have the potential to develop into multiple types of cells of the body. The delivery of stem cells or their derivatives and the mobilization of endogenous stem cells have been proposed as a mechanism for the treatment of neurodegenerative disease 4. While the complexity of the human neurological system is likely to be a tough hindrance to achieving this dream, animal studies as well as some clinical studies have suggested the feasibility of this approach.
Cell‐Based Therapy for Neurological Diseases
Neural cell types can be generated from various cell types by using different approaches.
Neural stem cells (NSCs)
Pluripotent stem cells (PSCs)
Direct conversion of differentiated cells into induced neural stem cells (iNSCs), induced neuronal cells (iNCs) from different cell types
Mesenchymal stromal stem cells (MSCs) from various tissues
These diverse kinds of stem cells can be useful source material for (1) understanding the basic biology of differentiation, dedifferentiation, and trans‐differentiation, (2) modeling of various diseases, including neurodegenerative diseases in vitro and screening of various drugs, and (3) cell therapy for the treatment of various diseases, including neurological diseases (Figures 1 and 2).
Figure 1.
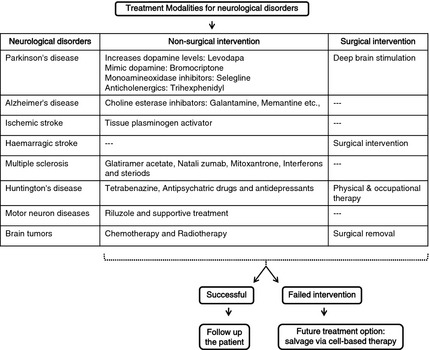
Various nonsurgical and surgical interventions for the treatment of neurological disorders with possible emerging solution using cell therapy to overcome the unsuccessful treatment options.
Figure 2.
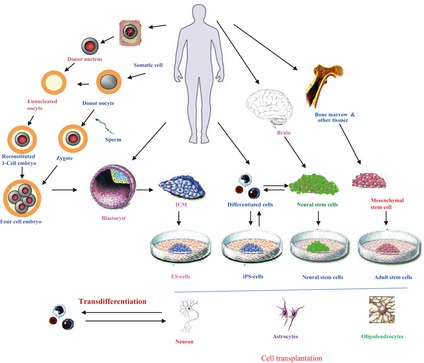
Different strategies such as direct isolation, therapeutic cloning, cellular reprogramming, and trans‐differentiation to obtain pluripotent stem cells (ESCs, iPSCs) as well as other relevant tissue‐specific stem cell types (MSCs, NSCs) to treat various neurological disorders.
Neural Stem Cells
Human (h) NSCs have been successfully grown in vitro and induced to differentiate into specific neuronal phenotypes. Isolation and propagation of NSCs in vitro can be achieved by selective growth and culture conditions. Several lines of evidence have revealed that ex vivo expanded NSCs, and their progeny may be transplanted in damaged areas of brain where they can survive, proliferate, migrate, and differentiate into glial tissues in vivo 5. Researchers have generated immortalized hNSC by infecting fetal human brain cells with a retroviral vector carrying v‐myc oncogenes and were able to differentiate into neurons and glial cells both in vitro and in vivo 6.
NSC Transplantation in Neurological Diseases
Here, we briefly discuss the transplantation of NSCs in certain neurological diseases and CNS injury. Parkinson's disease (PD) is characterized by selective loss of dopaminergic neurons in the nigro‐striatal region of the midbrain. The proof of principle that cell replacement can work in patients with PD was provided by several studies. NSCs are thus considered to be one of the cell sources for this disease condition. It has been shown that dopaminergic neurons can be generated from mouse NSCs after treatment with developmental morphogens fibroblast growth factor 8 and sonic hedgehog and by over expression of Nurr1 7, 8, 9, 10. Transplantation of hNSCs derived from fetal brain showed behavioral improvement in 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine‐lesioned monkeys following intrastriatal injection 11, 12.
Amyotrophic lateral sclerosis (ALS) is a progressive adult onset neurodegenerative disease. Glial cell line‐derived neurotrophic factor (GDNF)‐secreting hNSCs have not only shown survival and migration of graft cells in the degenerative area of spinal cord in a rat model of ALS, but also a protective effect over host motor neurons 13. This study suggests that regimens using hNSCs may help in the treatment of patients with ALS. A Phase I clinical trial conducted by the US company Neural Stem Inc after FDA approval (NCT01348451) is under way to determine the safety and feasibility of intraspinal injection of human spinal cord stem cells in ALS. Traumatic spinal cord injury results in severe and permanent neurological deficits. Studies suggest that transplantation of hNSCs into injured mouse spinal cord could generate neurons and oligodendrocytes and bring about locomotor recovery 14. AD is characterized by degeneration and loss of neurons and synapses throughout the brain particularly in the cortex, amygdala, and hippocampus. Studies have shown that NSCs along with brain‐derived neurotrophic factor (BDNF) can ameliorate complex behavioral abnormalities associated with AD pathology in a transgenic mouse AD model 15. Cerebral stroke is characterized by the loss of multiple neuron types due to occlusion of a cerebral artery. Transplanted fetal hNSCs have not only shown migration toward the ischemic lesion in rat stroke model, but also generated mature neuronal cells into stroke‐damaged rat striatum 16, 17. In addition to these studies, ReNeuron, UK‐based company, is conducting a clinical trial with immortalized hNSCs in stroke patients (NCT01151124).
Pluripotent Stem Cells
Human PSCs, including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), provide a potential tool for neurological drug development. PSCs have been effectively differentiated to region‐ and/or transmitter‐specific neuronal and glial types 18, 19, 20. By offering these unlimited disease‐relevant neuronal and glial cells, hPSC‐based disease models hold enormous promise for modeling pathological processes, identification of disease mechanisms, and development of neural phenotypic screens for drug development.
ESC‐Derived Neural Cell Types and their Applications in Neurological Drug Development
Human ESC‐derived neural precursors could be propagated and expanded long term in adherent or substrate‐free conditions under defined conditions supplemented with mitogens such as endothelial growth factor (EGF)/basic fibroblast growth factor (bFGF) 21. Because all such populations, although enriched, remain heterogeneous, there is a need for additional selection methods to further purify neuronal subtype lineages. Daadi et al. isolated an expandable and homogenous population of human ESC‐derived human NSCs (named SD56) from human ESCs using a defined medium supplemented with EGF, bFGF, and leukemia inhibitory growth factor (LIF) 22. The same group used bioluminescence and magnetic resonance imaging (MRI) to visualize the fate of grafted human ESCs in stroke‐damaged rat brain. Grafted human NSCs differentiated into neurons, into oligodendrocytes in stroke regions undergoing remyelination, and into astrocytes extending processes toward stroke‐damaged vasculatures 22, 23. Transplantation with ESC overexpressing Bcl‐2 has been shown to further increase the survival, neuronal differentiation, and functional outcome after transient cerebral ischemia 24.
iPSC‐Derived Neural Cell Types
A very recent study by Chen et al. showed that direct injection of iPSCs into damaged areas of rat cortex significantly decreased infarct size, improved motor function, attenuated inflammatory cytokines, and mediated neuroprotection after middle cerebral artery occlusion (MCAo) 25. In another study, undifferentiated iPSCs were transplanted into ipsilateral striatum and cortex at 24 h after 30 min of transient MCAo. Behavioral and histological analyses were performed 28 days after cell transplantation. iPSCs formed tridermal teratoma, but could supply a great number of Dcx‐positive neuroblasts and a few mature neurons in the ischemic lesion, reconfirming the fact that iPSCs have a promising potential to provide neural cells after ischemic brain injury, if tumorigenesis is properly controlled 26. Experimental interventions and a long‐term in vivo environment may be necessary to facilitate phenotypic presentation.
Direct Conversion of Terminally Differentiated Cells into iNSCs, iNCs
Earlier studies showed that proneural bHLH genes could induce expression of neuronal markers in non‐neural cells in vitro 27. To determine whether somatic cells can be converted directly to neurons, Werning's group transduced the fibroblast with a pool of 19 genes. They found that transduction of Ascl1 was sufficient to induce expression of pan neuronal proteins and immature active membrane properties 28. Finally, they identified a pool of three genes (Ascl1, Brn2, and Myt1l) that were sufficient to directly convert the embryonic and postnatal fibroblasts into functional neurons called iNCs. Importantly, these iNCs showed active action potentials and formed functional synapses with mouse cortical neurons and with each other in vitro 28, 29. During generation of iNCs, first neuronal markers could be detected as early as three days after the induction of the viral transgenes; in this study, most of the iNCs showed glutamatergic phenotype. Further, the same group has shown that even endodermal‐derived hepatocytes can convert to ectodermal‐derived neurons hence there seems to be no boundary between different germ layer–derived cells 30.
Very recently, many laboratories have shown that iNCs can also be generated from human fibroblasts using these same three genes 31, 32. Other studies reported that the addition of transcription factors generate dopaminergic iNCs from mouse and human fibroblasts 32, 33. They used a distinct group of transcription factors for conversion (Ascl1, Brn2, Myt1l, Lmx1a and Foxa2 vs. Ascl1, Nurr1, and Lmx1a). All these studies showed that it is possible to make different types of neurons directly from mouse and human somatic cells even from distinctly related germ layers cells. But the human iNCs appear to be less functionally mature, take long time, and the efficiency of generation is 3‐ to 5‐fold lower than mouse. But a very recent study has shown that efficiency of neuronal conversion can be highly enhanced by optimizing the culture conditions 34. They used small molecule inhibition approach for glycogen synthase kinase‐3β and SMAD, mothers against DPP homologs signaling, which leads to very high yield of human iNCs from fibroblasts.
The studies discussed above have demonstrated that defined sets of transcription factors can directly reprogram differentiated somatic cells to another differentiated cell type but proliferative potential of the resulting cells is low, hence limiting their scope for potential clinical applications where large number of cells is required. So it will be very interesting and useful if the differentiated cells can be directly converted to progenitors instead of directly going to differentiated phenotypes, as these progenitor cells can proliferate well and can give rise to large number of cells for further differentiation to particular cell types. Recent progress in this regard has led to the conversion of fibroblast cells to stably expandable iNSCs by three separate groups by using different strategies. The Wernig group used Brn2, Sox2, and FoxG1 for converting mouse embryonic fibroblast to neural progenitor cells 35, whereas the Brustle group used same transcription factors that are used for iPSC generation but they constitutively induced Sox2, Klf4, and c‐Myc expression while strictly limiting Oct4 activity to the initial phase of reprogramming. They generated neurosphere‐like colonies that are very expandable to many passages and capable of differentiation to neuron, astrocytes, and oligodendrocytes 36. Another group from Germany (Scholers group) directly converted mouse fibroblasts to iNS cells by using Brn4, Sox2, Klf4, c‐Myc, and Tcf3 transcription factors 37. Finally, now it has been shown that mouse and human differentiated cells can be converted to iNS cells using single factor (Sox2) in appropriate culture conditions 38. Collectively, these studies suggest that it is possible to convert differentiated cell directly to highly expandable iNS cells, which can be further differentiated to various types of neuronal, astrocyte, and oligodendrocyte lineages and can be used to treat various neurological diseases.
MSCs from Various Tissue Sources
Mesenchymal stromal stem cells can easily be obtained from various tissues such as bone marrow (BM), adipose tissue (ADT), Wharton's jelly, placenta (PL) and cord blood (CB), cartilage, synovium, periosteum, muscle, foreskin, and palatine tonsil and expanded vigorously until the tissues differentiate into specific cell lineages 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49. They are immunocompatible by nature, and there are no ethical issues related to their use. Over the last few years, various studies have shown that MSCs have the capacity to differentiate into ectodermal cell lineage apart from mesenchymal lineage in vitro 50. This process of trans‐differentiation shows that MSC is also capable to overcome the boundary of germ layer commitment and transform into cells of different tissues. Apart from the regenerative and trans‐differentiation ability of MSCs, it now well established that they can exert therapeutic effect by other possibilities, such as MSCs may attract resident stem cells or immature cells to the damaged area either directly by producing chemoattractants or indirectly through the well‐known immunomodulatory effects.
It has been shown that the neuronal differentiation of human and rat BM‐MSCs were induced by the addition of growth factors such as EGF and BDNF with all‐trans retinoic acid (ATRA) 51. Studies showed that the adipose‐derived stem cell can be differentiated into neuronal cells using BDNF, ATRA, bFGF, and forskolin 39, 52. Krampera et al. examined the neural potential of MSC from BM, fat, spleen, and thymus and demonstrated that the MSCs of different origin have similar neural differentiation potential 48. The first chorionic villous stromal cells (villous MSCs) from the placenta have been isolated and induced for neurogenesis by the addition of RA 53. Differentiation of PL‐MSCs into dopaminergic neurons was also reported by the addition of 3‐isobutyl‐1‐methylxanthine (IBMX) in the neurogenesis cocktail 54. It has been shown that the addition of human recombinant erythropoietin in the neuronal induction media was shown to induce the neurogenesis of CB‐MSCs by increasing the expression of neurotropic factors 55. The UC‐MSCs were shown to induce dopaminergic neuron by step‐wise culturing in neuron‐conditioned medium expressing dopaminergic phenotypes and TH 56. These studies suggest that the MSCs have amazing ability of neuronal plasticity, which would definitely require functional studies to exploit their full potential.
BM‐MSCs, when injected at a dose of 1 millions cells intravenously (IV), after 3 weeks, of PD‐induced mice displayed high increment of dopamine level in striatum 57. Studies have confirmed that committed neural progenitor cells derived from genetically modified BM stromal cells ameliorate deficits in a rat model of stroke 58. Another stem cell engineering strategy that has been used in preclinical settings was performed by adenoviral infection of vascular endothelial growth factor in human UC‐MSC, transplanted into rotenone‐induced Parkinsonian rats via infusion at corpus striatum 59. Further, researchers have demonstrated that human amniotic MSCs could aid in the nerve regeneration of injured sciatic nerve of rats, where 0.1 million cells of MSCs were settled at the nerve gap and the treatment group displayed improved nerve regeneration as compared with poor regeneration in control group 60. Results displayed that the combination greatly enhanced the nerve regeneration as compared with their earlier study 61. The impact of intravenously transplanted placenta‐derived MSCs on post‐stroke recovery has also been studied. Double infusion of placental MSCs was superior to single transplantation in the functional tests, and the findings suggest that placental tissue constitutes a promising source for experimental stroke therapies 62. Research showed the therapeutic effect of human PL‐MSCs in experimental autoimmune encephalomyelitis, the murine model of multiple sclerosis 63. They believe that the effect is caused by the reduction in the anti‐inflammatory protein such as tumor necrosis factor alpha‐stimulated gene/protein‐6 (TSG‐6). Velpula et al. showed that human UC‐MSC showed a stronger migration capacity toward glioma stem cells in vitro and exhibited enhanced migration to glioma stem cells in an intracranial human malignant glioma xenograft model 64. However, these data need to be evaluated for their translational relevance to clinical applications.
Clinical Translation of Cell‐Based Drugs Targeting Neurological Disorders
Development of a cell‐based drug will, of course, have to follow regulatory guidelines. While such guidelines are still evolving, some issues will have to be addressed by the potential manufacturer of a cell‐based drug. As Ahrlund‐Richter et al. have pointed out, three main models are likely to emerge 65. One is the personalized medicine model in which cells obtained from the patient will be injected back with minimal manipulation. A second model will be a banking model similar to the umbilical cord banking model and the third will be the manufacturing model where the cells will be produced in central facilities under strict regulatory control, and a single batch will be used for a large number of patients. This model is similar to the small molecule production method and will be further discussed here.
Several critical quality attributes have been proposed for cell‐based products and these include identity of the product, its potency, purity, and safety 66. Identity testing verifies that the cell population is indeed what is advertised. The development of flow cytometry has enabled easier characterization of the cells that make up the product or drug. Gene expression profiling may also prove to be an important tool to identify a cell‐based product. The measurement of potency, that is, the biological activity is more difficult. It will necessitate the development of quantitative measurement methods of cytokines that are essential to the product function. In most cases, the potency tests are likely to be a multiple matrix of tests, which will be indicative of several functions of the cells. The purity testing is essential to ensure that the final product does not contain residual proteins, peptides, toxins, or unwanted cell types. Finally, the safety tests ensure that the product is safe for clinical use, noninfectious and does not contain any product that can cause an adverse reaction.
The areas in which stem cells have been used for the development of a drug for neurological disorders are legion. They have been advocated for PD, cerebral palsy, traumatic encephalopathy, spinal cord trauma, and several others. There are major reviews that have examined the latest status of such cell‐based therapies; the following discussion will only touch on some major studies.
Small‐scale clinical studies have examined the use of different types of adult stem cells (ASCs), mainly MSCs for PD. Our company (Stempeutics Research) participated in a trial in which patients with advanced PD underwent transplantation with autologous BM–derived MSCs into the subventricular zone. There was modest clinical improvement and no adverse events. Thus, the feasibility of such an approach is now evident, although the clinical efficacy still remains to be proven 67.
Perinatal asphyxia is a major cause of neurological mortality and morbidity in children. A recent Korean study looked at patients with cerebral palsy using intravenous umbilical cord–derived MSCs 68. Their study also proved the feasibility of this approach, and they were able to demonstrate improvement in the neurological condition of the children both clinically and by radiology.
Stroke is another area that has received the attention of several investigators. One major study has been ongoing in South Korea, which has used autologous MSCs for the treatment of stroke 69, 70. In the initial study, they had compared five patients who underwent autologous MSC intravenously. The cells were delivered in two doses of 50 million cells at 4–5 and 7–9 weeks after the onset of stroke. The study looked at the Modified Rankin's score and the Barthel's index and showed an improved outcome in the treated patients, although the difference failed to show significance. The NIHSS scores also improved, albeit less prominently. The five‐year follow‐up, this time, of 16 patients who underwent MSC therapy was compared with 36 controls. This study conclusively established the long‐term safety of using MSC; however, the efficacy data, while promising, were not entirely convincing. Also, one important limitation of this study was that it was not double blinded. It is hoped that the presently continuing studies will be able to provide data that will reinforce the encouraging results of this study. Further, a search of the Cochrane stroke group trials register revealed three small RCTs using any form of stem cell 28. There have been no large scale trials that might be useful to direct clinical practice. The need of the moment is to have well‐conducted trials, which will form the basis of future medical practice.
Most delivery strategies have tried to utilize the window of opportunity that becomes available in the first few days after stroke. Our group has also initiated a trial (NCT0191701; clinicaltrials.gov) that will deliver cells in the first 10 days after the onset of stroke. Most trials that utilize the intravenous route have the same strategy. Another approach, of course, is to deliver the cells intracranially, an approach we used for the PD trial.
Conclusion and Future Perspective
The development of a cell‐based drug to treat neurological conditions is still a distant dream. However, as we have shown, considerable progress has been made in this direction. Advances made in the laboratory and in the preclinical field are now slowly being translated into small‐scale clinical studies and formal clinical trials. It is now clear that it is possible to use multiple approaches to deliver cell‐based drugs, which are reasonably safe. However, it is yet to be proven conclusively that these cell‐based therapies are efficacious. The principal thrust now is on well‐conducted and properly powered clinical trials that will establish the efficacy of these cell‐based therapies beyond doubt. In‐depth research also needs to be conducted on the actual logistics of cell therapy. The manufacture, quality control, transport, and clinical delivery of these cells still need much refining before they can be employed in routine clinical practice. In the foreseeable future, it is likely that these aspects will engage the scientists and clinicians working in this field. It does, however, appear that with a concerted effort from both the public and private sector, it will be possible, in the near future, to have a cell‐based drug in the market.
Conflict of Interest
The authors have no conflict of interest.
Acknowledgments
The authors are grateful to Stempeutics Research Malaysia for the funding to carry out research on stem cells and regenerative medicine.
References
- 1. Craven R. The Risky business of drug development. Lancet Neurol 2011;10:116–117. [DOI] [PubMed] [Google Scholar]
- 2. Hurko O, Ryan JL. Translational research in central nervous system drug discovery. Neuro Rx 2005;2:671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anon . Global burden of neurological disorders: estimates and projections. Chapter 2 in WHO: Neurological disorders: public health challenges. WHO, Switzerland, 2006. [Google Scholar]
- 4. Lindvall O, Kokaia Z, Martinez‐Serrano A. Stem cell therapy for human neurodegenerative disorders‐how to make it work. Nat Med 2004;10:42–50. [DOI] [PubMed] [Google Scholar]
- 5. Le Douarin NM, Calloni GW, Dupin E. The stem cells of the neural crest. Cell Cycle 2008;7:1013–1019. [DOI] [PubMed] [Google Scholar]
- 6. Kim SU. Human neural stem cells genetically modified for brain repair in neurological disorders. Neuropathology 2004;24:159–174. [DOI] [PubMed] [Google Scholar]
- 7. Kim SU, Park IH, Kim TH, et al. Brain transplantation of human neural stem cells transduced with tyrosine hydroxylase and GTP cyclohydrolase 1 provides functional improvement in animal models of Parkinson disease. Neuropathology 2006;26:129–140. [DOI] [PubMed] [Google Scholar]
- 8. Hagell P, Brundin P. Cell survival and clinical outcome following intrastriatal transplantation in Parkinson's disease. J Neuropathol Exp Neurol 2002;60:741–752. [DOI] [PubMed] [Google Scholar]
- 9. Wagner J, Akerud P, Castro DS, et al. Induction of a midbrain dopaminergic phenotype in Nurr1‐overexpressing neural stem cells by type 1 astrocytes. Nat Biotechnol 1999;17:653–659. [DOI] [PubMed] [Google Scholar]
- 10. Chung S, Sonntag KC, Andersson T, et al. Genetic engineering of mouse embryonic stem cells by Nurr‐1 enhances differentiation and maturation into dopaminergic neurons. Eur J Neurosci 2002;16:1829–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takagi Y, Takahashi J, Saiki H, et al. Dopaminergic neurons generated from monkey ES cells function in a Parkinson primate model. J Clin Invest 2005;115:102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Redmond DE Jr, Bjugstad KB, Teng YD, et al. Behavioral improvement in a primate Parkinson's model is associated with multiple homeostatic effects of human neural stem cells. Proc Natl Acad Sci USA 2007;104:12175–12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klein SM, Behrstock S, McHugh J, et al. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum Gene Ther 2005;16:509–521. [DOI] [PubMed] [Google Scholar]
- 14. Cummings BJ, Uchida N, Tamaki SJ, et al. Human neural stem cells differentiate and promote locomotor recovery in spinal cord‐injured mice. Proc Natl Acad Sci USA 2005;102:14069–14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blurton‐Jones M, Kitazawa M, Martinez‐Coria H, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci USA 2009;106:13594–13599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kelly S, Bliss TM, Shah AK, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci USA 2004;101:11839–11844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Darsalia V, Kallur T, Kokaia Z. Survival, migration and neuronal differentiation of human fetal striatal and cortical neural stem cells grafted in stroke‐damaged rat striatum. Eur J Neurosci 2007;26:605–614. [DOI] [PubMed] [Google Scholar]
- 18. Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 2008;132:661–680. [DOI] [PubMed] [Google Scholar]
- 19. Hoffman LM, Carpenter MK. Characterization and culture of human embryonic stem cells. Nat Biotechnol 2005;23:699–708. [DOI] [PubMed] [Google Scholar]
- 20. Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 2009;27:275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Joannides AJ, Fiore‐Heriche C, Battersby AA, et al. A scaleable and defined system for generating neural stem cells from human embryonic stem cells. Stem Cells 2007;25:731–737. [DOI] [PubMed] [Google Scholar]
- 22. Daadi MM, Maag AL, Steinberg GK. Adherent self renewable human embryonic stem cell‐derived neural stem cell line: functional engraftment in experimental stroke model. PLoS ONE 2008;3:e1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Daadi MM, Li Z, Arac A, et al. Molecular and magnetic resonance imaging of human embryonic stem cell‐derived neural stem cell grafts in ischemic rat brain. Mol Ther 2009;17:1282–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wei L, Cui L, Snider BJ, et al. Transplantation of embryonic stem cells overexpressing Bcl‐2 promotes functional recovery after transient cerebral ischemia. Neurobiol Dis 2005;19:183–193. [DOI] [PubMed] [Google Scholar]
- 25. Chen SJ, Chang CM, Tsai SK, et al. Functional improvement of focal cerebral ischemia injury by subdural transplantation of induced pluripotent stem cells with fibrin glue. Stem Cells Dev 2010;19:1757–1767. [DOI] [PubMed] [Google Scholar]
- 26. Kawai H, Yamashita T, Ohta Y, et al. Tridermal tumorigenesis of induced pluripotent stem cells transplanted in ischemic brain. J Cereb Blood Flow Metab 2010;30:1487–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Farah MH, Olson JM, Sucic HB, Hume RI, Tapscott SJ, Turner DL. Generation of neurons by transient expression of neural bHLH proteins in mammalian cells. Development 2000;127:693–702. [DOI] [PubMed] [Google Scholar]
- 28. Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010;463:1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vierbuchen T, Wernig M. Direct lineage conversions: unnatural but useful? Nat Biotechnol 2011;29:892–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marro S, Pang ZP, Yang N, et al. Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell 2011;9:374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pang ZP, Yang N, Vierbuchen T, et al. Induction of human neuronal cells by defined transcription factors. Nature 2011;476:220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pfisterer U, Kirkeby A, Torper O, et al. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci USA 2011;108:10343–10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Caiazzo M, Dell'Anno MT, Dvoretskova E, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 2011;476:224–227. [DOI] [PubMed] [Google Scholar]
- 34. Ladewig J, Mertens J, Kesavan J, et al. Small molecules enable highly efficient neuronal conversion of human fibroblasts. Nat Methods 2012;9:575–578. [DOI] [PubMed] [Google Scholar]
- 35. Lujan E, Chanda S, Ahlenius H, Sudhof TC, Wernig M. Direct conversion of mouse fibroblasts to self‐renewing, tripotent neural precursor cells. Proc Natl Acad Sci USA 2012;109:2527–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thier M, Worsdorfer P, Lakes YB, et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell 2012;10:473–479. [DOI] [PubMed] [Google Scholar]
- 37. Han DW, Tapia N, Hermann A, et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell 2012;10:465–472. [DOI] [PubMed] [Google Scholar]
- 38. Ring KL, Tong LM, Balestra ME, et al. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell 2012;11:100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anghileri E, Marconi S, Pignatelli A, et al. Neuronal differentiation potential of human adipose‐derived mesenchymal stem cells. Stem Cells Dev 2008;17:909–916. [DOI] [PubMed] [Google Scholar]
- 40. Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002;418:41–49. [DOI] [PubMed] [Google Scholar]
- 41. Peng L, Jia Z, Yin X, et al. Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev 2008;17:761–773. [DOI] [PubMed] [Google Scholar]
- 42. Fan J, Varshney RR, Ren L, Cai D, Wang DA. Synovium‐derived mesenchymal stem cells: a new cell source for musculoskeletal regeneration. Tissue Eng Part B 2009;15:75–86. [DOI] [PubMed] [Google Scholar]
- 43. Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res 2007;327:449–462. [DOI] [PubMed] [Google Scholar]
- 44. Zhang S, Muneta T, Morito T, Mochizuki T, Sekiya I. Autologous synovial fluid enhances migration of mesenchymal stem cells from synovium of osteoarthritis patients in tissue culture system. J Orthop Res 2008;26:1413–1418. [DOI] [PubMed] [Google Scholar]
- 45. Mamidi MK, Pal R, Mori NA, et al. Co‐culture of mesenchymal‐like stromal cells derived from human foreskin permits long term propagation and differentiation of human embryonic stem cells. J Cell Biochem 2011;112:1353–1363. [DOI] [PubMed] [Google Scholar]
- 46. Janjanin S, Djouad F, Shanti RM, et al. Human palatine tonsil: a new potential tissue source of multipotent mesenchymal progenitor cells. Arthritis Res Ther 2008;10:R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jung M, Kaszap B, Redohl A, et al. Enhanced early tissue regeneration after matrix assisted autologous mesenchymal stem cell transplantation in full thickness chondral defects in a minipig model. Cell Transplant 2009;18:923–932. [DOI] [PubMed] [Google Scholar]
- 48. Krampera M, Marconi S, Pasini A, et al. Induction of neural‐like differentiation in human mesenchymal stem cells derived from bone marrow, fat, spleen and thymus. Bone 2007;40:382–390. [DOI] [PubMed] [Google Scholar]
- 49. Padovan CS, Jahn K, Birnbaum T, et al. Expression of neuronal markers in differentiated marrow stromal cells and CD133+ stem‐like cells. Cell Transplant 2003;12:839–848. [DOI] [PubMed] [Google Scholar]
- 50. Barzilay R, Melamed E, Offen D. Introducing transcription factors to multipotent mesenchymal stem cells: making trans‐differentiation possible. Stem Cells 2009;27:2509–2515. [DOI] [PubMed] [Google Scholar]
- 51. Sanchez‐Ramos J, Song S, Cardozo‐Pelaez F, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol 2000;164:247–256. [DOI] [PubMed] [Google Scholar]
- 52. Jang S, Cho HH, Cho YB, Park JS, Jeong HS. Functional neural differentiation of human adipose tissue‐derived stem cells using bFGF and forskolin. BMC Cell Biol 2010;16:11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Portmann‐Lanz CB, Schoeberlein A, Huber A, et al. Placental mesenchymal stem cells as potential autologous graft for pre‐ and perinatal neuro‐regeneration. Am J Obstet Gynecol 2006;194:664–673. [DOI] [PubMed] [Google Scholar]
- 54. Chen L, He DM, Zhang Y. The differentiation of human placenta‐derived mesenchymal stem cells into dopaminergic cells in vitro. Cell Mol Biol Lett 2009;14:528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kang JH, Lee CK, Kim JR, et al. Estrogen stimulates the neuronal differentiation of human umbilical cord blood mesenchymal stem cells (CD34‐). NeuroReport 2007;18:35–38. [DOI] [PubMed] [Google Scholar]
- 56. Fu YS, Cheng YC, Lin MY, et al. Conversion of human umbilical cord mesenchymal stem cells in Wharton's jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cells 2006;24:115–124. [DOI] [PubMed] [Google Scholar]
- 57. Park HJ, Lee PH, Bang OY, Lee G, Ahn YH. Mesenchymal stem cells therapy exerts neuroprotection in a progressive animal model of Parkinson's disease. J Neurochem 2008;107:141–151. [DOI] [PubMed] [Google Scholar]
- 58. Hayase M, Kitada M, Wakao S, et al. Committed neural progenitor cells derived from genetically modified bone marrow stromal cells ameliorate deficits in a rat model of stroke. J Cereb Blood Flow Metab 2009;29:1409–1420. [DOI] [PubMed] [Google Scholar]
- 59. Xiong N, Zhang Z, Huang J, et al. VEGF‐expressing human umbilical cord mesenchymal stem cells, an improved therapy strategy for Parkinson's disease. Gene Ther 2011;18:394–402. [DOI] [PubMed] [Google Scholar]
- 60. Pan HC, Yang DY, Chiu YT, et al. Enhanced regeneration in injured sciatic nerve by human amniotic mesenchymal stem cell. J Clin Neurosci 2006;13:570–575. [DOI] [PubMed] [Google Scholar]
- 61. Pan HC, Chen CJ, Cheng FC, et al. Combination of G‐CSF administration and human amniotic fluid mesenchymal stem cell transplantation promotes peripheral nerve regeneration. Neurochem Res 2009;34:518–527. [DOI] [PubMed] [Google Scholar]
- 62. Kranz A, Wagner DC, Kamprad M, et al. Transplantation of placenta‐derived mesenchymal stromal cells upon experimental stroke in rats. Brain Res 2010;1315:128–136. [DOI] [PubMed] [Google Scholar]
- 63. Fisher‐Shoval Y, Barhum Y, Sadan O, et al. Transplantation of placenta‐derived mesenchymal stem cells in the EAE mouse model of MS. J Mol Neurosci 2012;48:176–184. [DOI] [PubMed] [Google Scholar]
- 64. Velpula KK, Dasari VR, Rao JS. The homing of human cord blood stem cells to sites of inflammation: unfolding mysteries of a novel therapeutic paradigm for glioblastoma multiforme. Cell Cycle 2012;11:2303–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ahrlund‐Richter L, De Luca M, Marshak DR, Munsie M, Veiga A, Rao M. Isolation and production of cells suitable for human therapy: challenges ahead. Cell Stem Cell 2009;4:20–26. [DOI] [PubMed] [Google Scholar]
- 66. Carmen J, Burger SR, McCaman M, Rowley JA. Developing assays to address identity, potency, purity and safety: cell characterization in cell therapy process development. Regen Med 2012;7:85–100. [DOI] [PubMed] [Google Scholar]
- 67. Venkataramana NK, Kumar SK, Balaraju S, et al. Open‐labeled study of unilateral autologous bone‐marrow‐derived mesenchymal stem cell transplantation in Parkinson's disease. Transl Res 2010;155:62–70. [DOI] [PubMed] [Google Scholar]
- 68. Lee YH, Choi KV, Moon JH, et al. Safety and feasibility of countering neurological impairment by intravenous administration of autologous cord blood in cerebral palsy. J Transl Med 2012;10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Boncoraglio GB, Bersano A, Candelise L, Reynolds BA, Parati EA. Stem cell transplantation for ischemic stroke. Cochrane Database Syst Rev 2010;9:CD007231. [DOI] [PubMed] [Google Scholar]
- 70. Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY. STARTING collaborators. A long‐term follow‐up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 2010;28:1099–1106. [DOI] [PubMed] [Google Scholar]


