Summary
Aims
A systematic literature review comparing the efficacy of ephedrine and phenylephrine for the management of spinal anesthesia–induced hypotension during Cesarean sections (C‐sections) was published in 2002. A number of well‐designed trials with controversial results have been published afterward. Therefore, an updated meta‐analysis was necessary.
Methods
The MEDLINE, EMBASE, and the Cochrane Library databases were searched (last search performed on September 26, 2011). Pooled risk ratio (RR) or standard mean difference (SMD) and their 95% confidence intervals (95% CI) were calculated for the incidence of intra‐operative hypotension or umbilical blood pH values.
Results
A total number of 15 trials and 742 parturients under elective C‐sections were analyzed. When used to prevent hypotension, patients receiving ephedrine and phenylephrine did not differ significantly in the incidence of hypotension (RR = 1.22; 95% CI, 0.83–1.80), umbilical arterial pH values (SMD = −0.38; 95% CI, −1.67 to 0.92) or venous pH values (SMD = −0.18; 95% CI, −0.44 to 0.07). And administration routes did not affect the incidence of hypotension and umbilical blood pH values. When used to treat hypotension, patients given ephedrine and phenylephrine had comparable incidence of intra‐operative hypotension (RR = 0.79; 95% CI, 0.40–1.56), while parturients receiving phenylephrine had neonates with higher umbilical arterial pH values (SMD = −1.32; 95% CI, −2.35 to −0.30) and venous pH values (SMD = −0.79; 95% CI, −1.09 to −0.49) than those given ephedrine.
Conclusion
Prophylactic use of ephedrine and phenylephrine were both effective in preventing maternal hypotension during C‐section under spinal anesthesia; phenylephrine was superior to ephedrine in treating hypotension, evidenced by higher umbilical blood pH values.
Keywords: Cesarean section, Ephedrine, Hypotension, Phenylephrine, Spinal anesthesia
Introduction
Spinal anesthesia–induced intra‐operative hypotension in C‐section, a long‐time topic of study, still challenges anesthetists. Maternal intra‐operative hypotension can lead to a number of severe complications for both neonate (decreased uteroplacental blood flow, fetal acidosis) and parturients (nausea, vomiting, dizziness, and decreased consciousness) 1. Many interventions, such as prehydration, vasopressor drugs (ephedrine, phenylephrine), and lower leg compression, have been used to prevent hypotension. However, none of the above interventions eliminate the need to treat hypotension 2.
Therefore, vasopressor drugs (ephedrine, phenylephrine) are often required. Ephedrine has been accepted as the vasopressor of choice in obstetric anesthesia for many years 1, 3. However, clinical trials found that ephedrine was associated with less satisfying umbilical pH values compared with phenylephrine 4, 5, 6. Anna Lee et al. 7 performed a systematic literature review comparing ephedrine and phenylephrine in 2002. They found that ephedrine and phenylephrine were both effective for the management of hypotension, but phenylephrine was associated with higher neonatal umbilical arterial pH values. Additionally, similar results were reported by other researchers 8, 9and they suggested that phenylephrine was the preferred vasopressor drug over ephedrine in managing maternal hypotension.
Recent trials, however, showed that ephedrine was more effective in the prevention of hypotension 10 and equally effective in controlling maternal hypotension 11. Furthermore, prophylactic use of vasopressor drugs is a more reasonable way to prevent hypotension, considering its high incidence and severe complications. The previous systematic review did not perform a specific analysis of trials according to vasopressor drug regimens. Thus, we attempt to perform an updated meta‐analysis on the efficacy of ephedrine and phenylephrine in the treatment and prevention of spinal‐induced hypotension during C‐sections. In this meta‐analysis, we performed detailed comparisons according to the aim of vasopressor drugs administration and administration routes.
Methods
Searching Strategy
The MEDLINE, EMBASE, and the Cochrane Library electronic databases were searched using the medical subheading terms or key words “ephedrine,” “phenylephrine,” “Cesarean section,” “spinal anesthesia,” “hypotension,” “combined spinal‐epidural anesthesia,” and “randomized controlled trial.” Alternative spellings were considered when searching. Date of publication and languages were not limited, and the last search was performed on September 26, 2011. An expanded references search of including trials and relative review articles was also performed.
Inclusion Criteria
Randomized controlled trials (RCTs) that compared the efficacy of ephedrine and phenylephrine for the treatment or prevention of spinal anesthesia–induced maternal hypotension during elective C‐sections were included. Anesthesia method was limited to spinal and combined spinal‐epidural anesthesia, because epidural anesthesia was associated with less parturients with necessary to treat hypotension (RR = 1.23; 95% confidence intervals [95% CI], 1.00–1.51) 12. The dose, timing, and other details of vasopressor drugs administration were not limited. Only elective Cesarean section was allowed, and trials with nonelective C‐section or including parturients with pregnancy complications or other severe diseases were excluded.
Data Extraction
Two reviewers (Fuqing Lin and Mantang Qiu) selected eligible trials independently and extracted data with a standard data collection form. Disagreement was resolved through discussion. The following data were collected: first author name, journal, publishing date, number of parturients, baseline data (age, height, weight, baseline blood pressure, and heart rate), anesthetic method, anesthetics, administration regimen of vasopressor (timing, method, route and dose), hypotension, and umbilical vein and artery pH values. All data collected were defined according to the definition chosen by individual trial and the data not standardized. Vasopressors were given by infusion after a bolus injection in the trials by Hall 5 and Alahuhta 13, in which the administration method was classified as infusion. The trials by Ayorinde 14 and Hall 5 included multiple arms, and we chosen the arm with a comparable dose to other trials, namely the arm of phenylephrine intramuscular 4 mg 14 and the arm of ephedrine 1 mg/mL infusion 5.
Assessment of Bias Risk
The quality of eligible trials was assessed using the “risk of bias” tool according to the Cochrane Handbook V5.0.2. Sequence generation, allocation concealment, blinding, incomplete data, and selective reporting were assessed, and based on trial method, each of them was graded as “yes,” “no,” or “unclear,” which equates to “high risk of bias,” “low risk of bias,” and “uncertain of bias,” respectively. Accordingly, risk of bias of each trial was assessed by two reviewers (Fuqing Lin and Mantang Qiu) independently, while discrepancies were discussed with the third reviewer (Quan Li) until consensus was achieved.
Statistical Method
Incidence of maternal hypotension was the primary outcome of this meta‐analysis, and secondary outcomes were umbilical arterial and venous pH values. Pooled RR with 95% CI for the incidence of hypotension and SMD with 95% CI for umbilical blood pH values were calculated, respectively. A 95% CI was used for statistical significance test, and a 95% CI without 1 for RR or a 95% CI without 0 for SMD shows significant statistical difference. A RR < 1 indicates that ephedrine is associated with less intra‐operative hypotension, and an SMD < 0 indicates that ephedrine is associated with lower umbilical blood pH values. The random‐effects model was used in all analyses, because the included studies were expected to have some heterogeneity. Heterogeneity across trials was analyzed using the Q‐statistic (with a P ≤ 0.10 considered significant heterogeneity) and the I 2 statistic (with an I 2 > 50% regarded as significant heterogeneity). Sensitivity analyses were performed to indentify the effect of individual trial and test the reliability of results. Publication bias was assessed by means of visual inspection of a funnel plot and quantitative Begg's and Egger's tests (P value < 0.05 considered significant). Trials, in which vasopressor drugs were used to treat spinal‐induced hypotension, were analyzed separately from those with prophylactic use of vasopressor drugs, namely to prevent hypotension. We also performed subgroup analysis to explore the effects of confounding variables: intramuscular injection, intravenous bolus injection, and intravenous infusion. The data were analyzed using meta‐analysis software Review Manager (RevMan 5.0.2, Cochrane Collaboration). Begg's and Egger's tests were carried out using STATA 11.1 (College Station, TX, USA).
Results
Characteristics of Eligible Trials
Fifteen trials 4, 5, 6, 10, 11, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 and 742 parturients under elective Cesarean section were analyzed: eight of them were newly identified 6, 10, 11, 15, 16, 17, 18, 22 since the previous meta‐analysis 7. Twenty‐two trials with full‐text were further checked for validity, and seven trials 23, 24, 25, 26, 27, 28, 29 were excluded because they included nonelective C‐section cases 23, 24, did not have available data 25, contained inappropriate comparison 26, 27, 28 (did not compare the efficacy of ephedrine and phenylephrine on the management of spinal anesthesia–induced hypotension), or were conference articles 29 (Figure 1). Parturient selection was addressed in most trials, and parturients with hypertension, cardiovascular diseases, and other complications were excluded by all trials. Spinal anesthesia was performed in 14 trials 4, 5, 6, 10, 11, 13, 15, 16, 17, 18, 19, 20, 21, 22, and combined epidural spinal anesthesia was used in Ayorinde BT's trial 14. Bupivacaine was the local anesthetic for spinal anesthesia in all 15 trials. Intravenous prehydration was adopted in 12 trials 4, 5, 6, 11, 13, 14, 15, 16, 19, 20, 21, 22. No intravenous prehydration was given in the trials of Ngan Kee 18, Dyer 17, and Magalhães 10. They started rapid intravenous infusion at intrathecal injection 10, 18 or after the appearance of cerebrospinal fluid 17 (details shown in Table S1).
Figure 1.
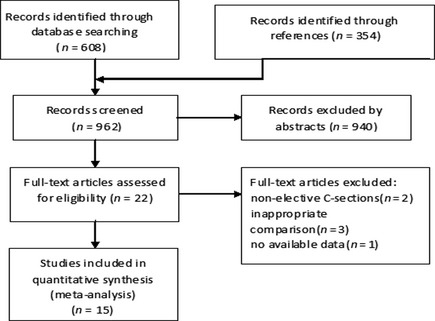
Flow diagram.
Risk of Bias
The risk of bias assessment in the included trials showed that most of trials had low risk of bias (shown in Table S1). Because of publication date, some trials did not clearly address their sequence generation and concealment clearly. With no access to each trial's original protocol, all analyzed clinical trials were considered free from “selective reporting” bias.
A funnel plot of 9 trials 4, 5, 6, 10, 13, 14, 15, 18, 22 which reported the number of parturients with hypotension was generated using Review Manager. The funnel plot was visually asymmetrical, suggesting the presence of publication bias. Then, quantitative Egger's and Begg's tests were performed to assess the degree of asymmetry. However, neither Egger's test (P = 0.059) nor Begg's test (P = 0.118) showed the evidence of publication bias. The asymmetry of funnel plot may be due to insufficient number of trials and different dosages of ephedrine and phenylephrine across trials. Different bupivacaine regimens may also lead to the asymmetry of funnel plot, because bupivacaine's dosage was associated with the incidence of hypotension 30, 31.
Prophylactic Use of Ephedrine and Phenylephrine
A number of eight trials 5, 6, 10, 11, 13, 14, 18, 22 (Table 1) compared prophylactic use of ephedrine and phenylephrine for the prevention of hypotension. Vasopressor drugs were administrated by intravenous injection or infusion in 6 trials 5, 6, 10, 11, 13, 18 and the other two trials 14, 22 adopted intramuscular injections.
Table 1.
Characteristics of trials analyzed
Prophylactic ephedrine and phenylephrine were equally effective for the prevention of hypotension 5, 6, 10, 13, 14, 18, 22 (RR = 1.09; 95% CI, 0.74–1.60) (Figure 2). In this comparison, heterogeneity was significant across trials (P = 0.005 and I 2 = 67%). Sensitivity analyses indicated that the trial reported by Magalhães 10 accounted for the source of heterogeneity, in which a relatively high dose of ephedrine was administrated. When this trial was excluded, heterogeneity was acceptable (P = 0.11 and I 2 = 45%) and the results did not change much (RR = 1.22; 95% CI, 0.83–1.80), which meant that our results were stable. A subgroup analysis was performed to explore the effects of different administration route (intravenous and intramuscular) on the incidence of hypotension. The results showed that incidence of hypotension did not differ significantly with intravenous administration 5, 6, 10, 13, 18 (RR = 1.08; 95% CI, 0.66–1.75) and intramuscular administration 14, 22(RR = 1.24; 95% CI, 0.71–2.18).
Figure 2.
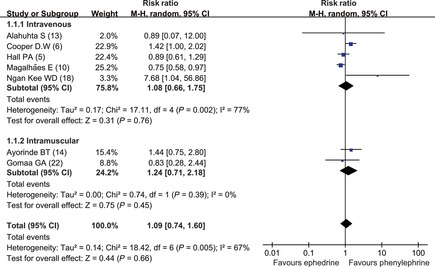
Comparison of the incidence of hypotension (prophylactic use of vasopressor drugs). Phe, phenylephrine; E, ephedrine.
Opposing Anna Lee's meta‐analysis 7, prophylactic use of phenylephrine did not result in a higher umbilical arterial pH values 10, 11 (SMD = −0.38; 95% CI, −1.67 to 0.92) (Figure 3) or venous pH values 10, 11, 14, 22 (SMD = −0.14; 95% CI, −0.50 to 0.21) (Figure 4) than ephedrine. Heterogeneity existed in the comparison of arterial pH values (P < 0.01 and I 2 = 92%), because Magalhães 10 used a high dose of ephedrine. In terms of administration route, ephedrine and phenylephrine had similar venous pH values when given intravenously (SMD = −0.14; 95% CI, −0.50 to 0.21) and intramuscularly (SMD = −0.23; 95% CI, −0.59 to 0.14).
Figure 3.

Comparison of umbilical arterial pH values (intravenous prophylactic use of vasopressor drugs). Phe, phenylephrine; E, ephedrine.
Figure 4.
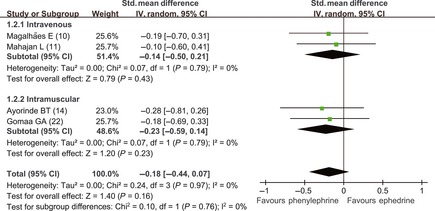
Comparison of umbilical venous pH values (intravenous prophylactic use of vasopressor drugs). Phe, phenylephrine; E, ephedrine.
Ephedrine and Phenylephrine for the Treatment of Hypotension
For the treatment of intra‐operative hypotension, intravenous administration of ephedrine and phenylephrine was used in the seven analyzed trials 4, 14, 15, 16, 19, 20, 21. Our meta‐analysis results showed that ephedrine and phenylephrine were associated with similar incidence of maternal hypotension 4, 15 (RR = 0.79; 95% CI, 0.40–1.56), but parturients receiving phenylephrine had neonates with higher umbilical arterial pH values 16, 17, 19, 20, 21 (SMD = −1.32; 95% CI, −2.35 to −0.30) (Figure 5) and venous pH values 16, 19, 20, 21 (SMD = −0.79; 95% CI, −1.09 to −0.49) (Figure 6) than those given ephedrine. In the comparison of arterial pH values, heterogeneity was significant (P < 0.01 and I 2 = 91%). Sensitivity analyses showed that Moran' trial 19 was responsible for heterogeneity. When Moran' trial 19 was excluded, there was no significant heterogeneity (P = 0.94 and I 2 = 0%), and the result was still statistically significant (SMD = −0.72; 95% CI, −1.03 to −0.40).
Figure 5.
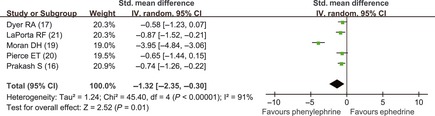
Comparison of umbilical arterial pH values (vasopressor drugs used to treat hypotension). Phe, phenylephrine; E, ephedrine.
Figure 6.
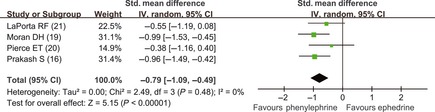
Comparison of umbilical venous pH values (vasopressor drugs used to treat hypotension). Phe, phenylephrine; E, ephedrine.
Conclusion
This updated meta‐analysis showed no significant differences between ephedrine and phenylephrine in the incidence of hypotension when used to treat spinal‐induced intra‐operative hypotension during C‐sections, although parturients treated with phenylephrine had neonates with higher umbilical pH value than those treated with ephedrine. These results were consistent with the systematic review performed by Anna Lee 7. However, we also found that prophylactic use of ephedrine (intravenously or intramuscularly) was comparable to phenylephrine in the incidence of hypotension and umbilical pH values.
Administration of vasopressor drugs is the main prevention and treatment strategy of spinal anesthesia–induced hypotension. Ephedrine, a mixed agonist of α and β adrenoreceptor, maintains blood pressure predominantly by activating β1 adrenoreceptor and increasing cardiac output and heart rate 32. However, ephedrine is able to cross the placental barrier and causes an increase in fetal heart rate and an increase in fetal catecholamine levels, which may mediate an increase in oxygen consumption and increase in glucose and lactic acid concentrations 5. Phenylephrine is a pure α1 adrenergic agonist, which may counteract the decrease in systemic vascular resistance induced by spinal anesthesia 4. Whether or not ephedrine is superior to phenylephrine or not has been argued for years. On the basis of the above pharmacological mechanism, many trials and reviews have concluded that phenylephrine had the advantage, resulting in higher umbilical pH values 4, 5, 6, 7, 8, 9. They suggested phenylephrine should replace ephedrine in maintaining maternal hypotension; hence, ephedrine fell out of favor as the vasopressor drug of choice for spinal anesthesia for C‐section 4, 5, 6, 7, 8, 9.
Pooled results of our meta‐analysis showed that prophylactic use of ephedrine and phenylephrine did not result in significant differences in the incidence of maternal hypotension. Additionally, we also noted that both intramuscular and intravenous use of prophylactic ephedrine result in similar umbilical blood pH values as did phenylephrine. Ephedrine affects umbilical blood pH values mainly because that it crosses the placenta 21 and has a direct effect on the fetus. Therefore, prophylactic use of ephedrine may allow for a long duration for the fetus to adapt to it. This may explain why the umbilical pH values were equal when ephedrine and phenylephrine were administrated prophylactically, while phenylephrine was associated with higher pH values when it came to treatment.
In most trials comparing ephedrine and phenylephrine, the vasopressor drug was given when hypotension occurred. Considering the high incidence of maternal hypotension and its severe complications, prophylactic use of vasopressor drugs should be a more effective and logical approach to maintaining maternal blood pressure 14. In addition, prophylactic use of ephedrine has been proved more effective than control for preventing hypotension but did not improve neonatal outcome 33. In the trial of Magalhães 10, prophylactic intravenous bolus of ephedrine 10 mg or phenylephrine 80 μg was administrated immediately after the subarachnoid block. This trial showed that ephedrine had a significant advantage over phenylephrine in the incidence of hypotension (P < 0.05). On the basis of the above reports and our findings, we can conclude that prophylactic use of ephedrine is at least as effective as phenylephrine during spinal anesthesia for C‐section. Additionally, both intravenous and intramuscular prophylactic administration of vasopressor drugs is effective.
Compared to the previous meta‐analysis 7, we performed more specific analyses according to the purpose of vasopressor administration regimen (prevention or treatment) and administration route (intravenous or intramuscular). Trials with different routes (intravenous and intramuscular) of administration were analyzed in subgroup to minimize heterogeneity and to explore the effect of administration route. A number of trials were published long ago without e‐mail, so details of trial design, such as sequence generation and allocation concealment, were not described and it was unable to obtain original data. Thus, we did not compare reactive hypertension and bradycardia, and Apgar scores were not analyzed either.
Our meta‐analysis has some limitations. First, all of the 15 trials were small in size, which may lead to a small‐study effect, in which reported effects are larger 34. Furthermore, Anna Lee estimated that a large RCT of 4638 women would be able to detect a small risk of fetal acidosis associated with phenylephrine. Thus, the application of findings of this meta‐analysis based on small studies should be cautious. Secondly, various definitions of hypotension were involved in this meta‐analysis. Klöhr 35 found 15 different definitions and there was not a widely accept definition of hypotension in spinal anesthesia for C‐section. Thus, the results of incidence of hypotension may be influenced by bias. As it is hard to obtain original data from all trials, most of the articles collected data based on the specific definition in each trial and did not attempt to perform standardization 7, 36, 37.
In summary, this meta‐analysis showed that, during C‐section under spinal anesthesia, phenylephrine was superior to ephedrine, resulting in higher umbilical blood pH values when used to treat hypotension. However, prophylactic use of ephedrine and phenylephrine was equally effective for the prevention of maternal hypotension when administrated intravenously and intramuscularly.
Conflict of Interest
The authors declare no conflict of interest.
Authors' Contribution
Fu‐Qing Lin has designed the study, viewed the original study data, reviewed the analysis of the data, and approved the final manuscript. Man‐Tang Qiu has designed the study, viewed the original study data, reviewed the analysis of the data, approved the final manuscript, and responsible for archiving the study files. Xiang‐Xiang Ding has viewed the original study data, reviewed the analysis of the data, and approved the final manuscript. Shu‐Kun Fu has designed the study, viewed the original study data, and approved the final manuscript. Quan Li has designed the study, viewed the original study data, reviewed the analysis of the data, and approved the final manuscript. Fu‐Qing Lin and Man‐Tang Qiu contributed equally to this work.
Supporting information
Table S1 Detailed characteristics of eligible trials.
Acknowledgments
This research was partly supported by Shanghai Natural Science Foundation by Grants 10411951400 to Shukun Fu and 11ZR1428100 to Quan Li.
References
- 1. Rout CC, Rocke DA. Prevention of hypotension following spinal anesthesia for cesarean section. Int Anesthesiol Clin 1994;32: 117–135. [PubMed] [Google Scholar]
- 2. Cyna AM, Andrew M, Emmett RS, Middleton P, Simmons SW. Techniques for preventing hypotension during spinal anaesthesia for caesarean section. Cochrane Database Syst Rev 2006;18: CD002251. [DOI] [PubMed] [Google Scholar]
- 3. Burns SM, Cowan CM, Wilkes RG. Prevention and management of hypotension during spinal anaesthesia for elective caesarean section: A survey of practice. Anaesthesia 2001;56: 777–798. [DOI] [PubMed] [Google Scholar]
- 4. Thomas DG, Robson SC, Redfern N, Hughes D, Boys RJ. Trial of bolus phenylephrine or ephedrine for maintenance of arterial pressure during spinal anesthesia for Caesarean section. Br J Anaesth 1996;76: 61–65. [DOI] [PubMed] [Google Scholar]
- 5. Hall PA, Bennet MP, Wilkes MP, Lewis M. Spinal anesthesia for Caesarean section: Comparison of phenylephrine and ephedrine. Br J Anaesth 1994;73: 471–474. [DOI] [PubMed] [Google Scholar]
- 6. Cooper DW, Carpenter M, Mowbray P, Desira WR, Ryall DM, Kokri MS. Fetal and maternal effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology 2002;97: 1582–1590. [DOI] [PubMed] [Google Scholar]
- 7. Lee A, Ngan Kee WD, Gin T. A quantitative, systematic review of randomized controlled trials of ephedrine versus phenylephrine for the management of hypotension during spinal anesthesia for cesarean delivery. Anesth Analg 2002;94: 920–926. [DOI] [PubMed] [Google Scholar]
- 8. Vercauteren M. Obstetric spinal analgesia and anesthesia. Curr Opin Anaesthesiol 2003;16: 503–507. [DOI] [PubMed] [Google Scholar]
- 9. Macarthur A, Riley ET. Obstetric anesthesia controversies: Vasopressor choice for postspinal hypotension during cesarean delivery. Int Anesthesiol Clin 2007;45: 115–132. [DOI] [PubMed] [Google Scholar]
- 10. Magalhães E, Govêia CS, de Araújo Ladeira LC, Nascimento BG, Kluthcouski SM. Ephedrine versus phenylephrine: Prevention of hypotension during spinal block for cesarean section and effects on the fetus. Rev Bras Anestesiol 2009;59: 11–20. [DOI] [PubMed] [Google Scholar]
- 11. Mahajan L, Anand LK, Gombar KK. A randomized double‐blinded comparison of ephedrine, phenylephrine and mephentermine infusions to maintain blood pressure during spinal anaesthesia for cesarean delivery: The effects on fetal acid‐base status and haemodynamic control. J Anaesth Clin Pharmacol 2009;25: 427–432. [Google Scholar]
- 12. Ng K, Parsons J, Cyna AM, Middleton P. Spinal versus epidural anaesthesia for caesarean section. Cochrane Database Syst Rev 2004;2: CD003765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alahuhta S, Räsänen J, Jouppila P, Jouppila R, Hollmén AI. Ephedrine and phenylephrine for avoiding maternal hypotension due to spinal anaesthesia for caesarean section. Effects on uteroplacental and fetal haemodynamics. Int J Obstet Anesth 1992;1: 129–134. [DOI] [PubMed] [Google Scholar]
- 14. Ayorinde BT, Buczkowski P, Brown J, Shah J, Buggy DJ. Evaluation of pre‐emptive intramuscular phenylephrine and ephedrine for reduction of spinal anaesthesia‐induced hypotension during Caesarean section. Br J Anaesth 2001;86: 372–376. [DOI] [PubMed] [Google Scholar]
- 15. Adigun TA, Amanor‐Boadu SD, Soyannwo OA. Comparison of intravenous ephedrine with phenylephrine for the maintenance of arterial blood pressure during elective caesarean section under spinal anaesthesia. Afr J Med Med Sci 2010;39: 13–20. [PubMed] [Google Scholar]
- 16. Prakash S, Pramanik V, Chellani H, Salhan S, Gogia AR. Maternal and neonatal effects of bolus administration of ephedrine and phenylephrine during spinal anaesthesia for caesarean delivery: A randomized study. Int J Obstet Anesth 2010;19: 24–30. [DOI] [PubMed] [Google Scholar]
- 17. Dyer RA, Reed AR, van Dyk D, Arcache MJ, Hodges O, Lombard CJ, et al. Hemodynamic effects of ephedrine, phenylephrine, and the coadministration of phenylephrine with oxytocin during spinal anesthesia for elective cesarean delivery. Anesthesiology 2009;111: 753–765. [DOI] [PubMed] [Google Scholar]
- 18. Ngan Kee WD, Lee A, Khaw KS, Ng FF, Karmakar MK, Gin T. A randomized double‐blinded comparison of phenylephrine and ephedrine infusion combinations to maintain blood pressure during spinal anesthesia for cesarean delivery: The effects on fetal acid‐base status and hemodynamic control. Anesth Analg 2008;107: 1295–1302. [DOI] [PubMed] [Google Scholar]
- 19. Moran DH, Perillo M, LaPorta RF, Bader AM, Datta S. Phenylephrine in the prevention of hypotension following spinal anesthesia for cesarean delivery. J Clin Anesth 1991;3: 301–305. [DOI] [PubMed] [Google Scholar]
- 20. Pierce ET, Carr DB, Datta S. Effects of ephedrine and phenylephrine on maternal and fetal atrial natriuretic peptide levels during elective cesarean section. Acta Anaesthesiol Scand 1994;38: 48–51. [DOI] [PubMed] [Google Scholar]
- 21. LaPorta RF, Arthur GR, Datta S. Phenylephrine in treating maternal hypotension due to spinal anaesthesia for caesarean delivery: Effects on neonatal catecholamine concentrations, acid base status and Apgar scores. Acta Anaesthesiol Scand 1995;39: 901–905. [DOI] [PubMed] [Google Scholar]
- 22. Gomaa GA, Elewa SA. Prophylactic use of vasopressors for reduction of spinal anaesthesia‐induced hypotension during caesarean section. Eg J Anaesth 2003;19: 45–50. [Google Scholar]
- 23. Bhattarai B, Bhat SY, Upadya M. Comparison of bolus phenylephrine, ephedrine and mephentermine for maintenance of arterial pressure during spinal anesthesia in cesarean section. JNMA J Nepal Med Assoc 2010;49: 23–28. [PubMed] [Google Scholar]
- 24. Ngan Kee WD, Khaw KS, Lau TK, Ng FF, Chui K, Ng KL. Randomised double‐blinded comparison of phenylephrine vs ephedrine for maintaining blood pressure during spinal anaesthesia for non‐elective Caesarean section. Anaesthesia 2008;63: 1319–1326. [DOI] [PubMed] [Google Scholar]
- 25. Gunda CP, Malinowski J, Tegginmath A, Suryanarayana VG, Chandra SBC. Vasopressor choice for hypotension in elective Cesarean section: Ephedrine or phenylephrine? Arch Med Sci 2010;6: 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hennebry MC, Stocks GM, Belavadi P, Barnes J, Wray S, Columb MO, Lyons G. Effect of i.v. phenylephrine or ephedrine on the ED50 of intrathecal bupivacaine with fentanyl for caesarean section. Br J Anaesth 2009;102: 806–811. [DOI] [PubMed] [Google Scholar]
- 27. Saravanan S, Kocarev M, Wilson RC, Watkins E, Columb MO, Lyons G. Equivalent dose of ephedrine and phenylephrine in the prevention of post‐spinal hypotension in Caesarean section. Br J Anaesth 2006;96: 95–99. [DOI] [PubMed] [Google Scholar]
- 28. Cooper DW, Jeyaraj L, Hynd R, Thompson R, Meek T, Ryall DM, Kokri MS. Evidence that intravenous vasopressors can affect rostral spread of spinal anesthesia in pregnancy. Anesthesiology 2004;101: 28–33. [DOI] [PubMed] [Google Scholar]
- 29. Van Elsen K, Van Den Bossche H, Lauwers M, Poelaert J. Prophylactic phenylephrine or ephedrine to prevent hypotension during spinal anesthesia for cesarean delivery. Acta Anaesthesiol Belg 2009;60: 2 (142). [Google Scholar]
- 30. Yu SC, Ngan Kee WD, Kwan AS. Addition of meperidine to bupivacaine for spinal anesthesia for Cesarean section. Br J Anaesth 2002;33: 379–383. [DOI] [PubMed] [Google Scholar]
- 31. Kang FC, Tsai YC, Chang PJ, Chen TS. Subarachnoid fentanyl with diluted small‐dose bupivacaine for cesarean section delivery. Acta Anaesthesiol Sin 1998;36: 207–214. [PubMed] [Google Scholar]
- 32. Critchley LAH, Stuart JC, Conway F, Short TG. Hypotension during subarachnoid anesthesia: Haemodynamic effects of ephedrine. Br J Anaesth 1995;74: 373–378. [DOI] [PubMed] [Google Scholar]
- 33. Lee A, Ngan Kee WD, Gin T. Prophylactic ephedrine prevents hypotension during spinal anesthesia for Cesarean delivery but does not improve neonatal outcome: A quantitative systematic review. Can J Anaesth 2002;49: 588–599. [DOI] [PubMed] [Google Scholar]
- 34. Sterne JA, Egger M. Funnel plots for detecting bias in meta‐analysis: Guidelines on choice of axis. J Clin Epidemiol 2001;54: 1046–1055. [DOI] [PubMed] [Google Scholar]
- 35. Klöhr S, Roth R, Hofmann T, Rossaint R, Heesen M. Definitions of hypotension after spinal anesthesia for caesarean section: Literature search and application to parturients. Acta Anaesthesiol Scand 2010;54: 909–921. [DOI] [PubMed] [Google Scholar]
- 36. Banerjee A, Stocche RM, Angle P, Halpern SH. Preload or coload for spinal anesthesia for elective Cesarean delivery: A meta‐analysis. Can J Anaesth 2010;57: 24–31. [DOI] [PubMed] [Google Scholar]
- 37. Arzola C, Wieczorek PM. Efficacy of low‐dose bupivacaine in spinal anaesthesia for Caesarean delivery: Systematic review and meta‐analysis. Br J Anaesth 2011;107: 308–318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Detailed characteristics of eligible trials.


