Summary
Aims
It is well known that low‐intensity exercise training (ExT) is beneficial to cardiovascular dysfunction in hypertension. The tonically active glutamatergic input to the rostral ventrolateral medulla (RVLM), a key region for control of blood pressure and sympathetic tone, has been demonstrated to be increased in hypertensive rats. The aim of this study was to determine the effect of ExT on the increased glutamatergic input to the RVLM in spontaneously hypertensive rat (SHR).
Methods
Normotensive rats Wistar‐Kyoto (WKY) and SHR were treadmill trained or remained sedentary (Sed) for 12 weeks and classed into four groups (WKY‐Sed, WKY‐ExT, SHR‐Sed, and SHR‐ExT). The release of glutamate in the RVLM and its contribution to cardiovascular activity were determined in WKY and SHR after treatment of ExT.
Results
Blood pressure and sympathetic tone were significantly reduced in SHR after treatment with ExT. Bilateral microinjection of the glutamate receptor antagonist kynurenic acid (2.7 nmol in 100 nL) into the RVLM significantly decreased resting blood pressure, heart rate, and renal sympathetic nerve activity in SHR‐Sed but not in WKY groups (WKY‐Sed and WKY‐ExT). However, the degree of reduction in these cardiovascular parameters evoked by KYN was significantly blunted in SHR‐ExT compared with SHR‐Sed group. The concentration of glutamate and the protein expression of vesicular glutamate transporter 2 in the RVLM were significantly increased in SHR‐Sed compared with WKY‐Sed, whereas they were reduced after treatment with ExT.
Conclusion
Our findings suggest that ExT attenuates the enhancement in the tonically acting glutamatergic input to the RVLM of hypertensive rats, thereby reducing the sympathetic hyperactivity and blood pressure.
Keywords: Exercise training, Glutamate, Hypertension, Rostral ventrolateral medulla, Sympathetic nerve activity
Introduction
Hypertension is characterized by elevated levels of blood pressure (BP) and sympathetic tone, which are closely related to a poor prognosis in this disease 1. It is well known that the rostral ventrolateral medulla (RVLM) is a key region for central control of sympathetic outflow and plays a crucial role in maintaining resting BP and sympathetic tone 2, 3. Abnormalities in structure and function of the RVLM have been suggested to be a major contributor to essential (neurogenic) hypertension 4, 5. It has been documented that tonically active excitatory inputs to RVLM vasomotor neurons, probably originating from commissural part of the nucleus of solitary tract (commNTS), the paraventricular nucleus (PVN), and the pontine reticular formation, play an important role in central cardiovascular regulation 6, 7, 8. It has been reported that microinjection of the excitatory glutamate receptor antagonist kynurenic acid (KYN) into the RVLM produces a significant fall in resting BP in hypertensive rats but not in normotensive rats, suggesting that tonically active glutamatergic input to the RVLM is upregulated in hypertension 9, 10. Accordingly, it is proposed that the increased tonic glutamatergic input to the RVLM is an important contributor to elevated levels of BP and sympathetic tone in hypertension.
Growing evidence has been demonstrated that exercise training (ExT) is capable of normalizing cardiovascular dysfunction in cardiovascular disorders such as hypertension and chronic heart failure 11, 12. It is reported that ExT effectively lowers BP, decreases cardiac output, and enhances the baroreflex sensitivity in patients and animals with hypertension 13, 14, 15. However, the exact mechanism(s) by which ExT improves cardiovascular dysfunction in hypertension have not been fully understood. Interestingly, it is suggested that neural plasticity in the central cardiovascular networks is an important mechanism responsible for the effects of ExT on cardiovascular activity 16, 17. It has been demonstrated that ExT is beneficial to hypertension via reducing the sympathetic activity 13, 17, 18. Moreover, ExT significantly attenuates increases in BP and sympathetic activity induced by stimulation of the RVLM 19, 20, suggesting the importance of the RVLM in mediating the effects of ExT on cardiovascular regulation. However, it is not clear if Ext alters the elevated tonically active glutamatergic input to RVLM neurons in hypertension. Therefore, this study was designed to determine the effect of ExT on tonically active glutamatergic input in the RVLM of spontaneously hypertensive rats (SHR).
Methods
Animals and General Procedures
Eight‐week‐old male normotensive Wistar‐Kyoto (WKY) rats and SHR were supplied by Sino‐British SIPPR/BK Laboratory Animal Ltd (Shanghai, China) in this study. All of the procedures in the study were approved by the Committee of Animal Care and Use, Second Military Medical University and performed in accordance with institutional animal care guidelines.
ExT Protocol and Experimental Design
Eight‐week‐old WKY and SHR were preselected for their ability to walk on a treadmill (FT‐200; Taimen Co., Chengdu, China) and only active rats were used in this study. These active rats randomly assigned either to the sedentary group (WKY‐Sed; SHR‐Sed) or to the ExT group (WKY‐ExT; SHR‐ExT). ExT groups were subjected to low‐intensity ExT on a motor‐driven treadmill continuously for a period of 12 weeks (5 days per week; 60 min per day at 15–20 m/min), as described in detail elsewhere 15, 21, 22. The treadmill speed of low‐intensity ExT was determined by 50–60% of maximal exercise capacity, which was measured by means of maximal exercise tests on week 0, 6, and 12 (Table 1). The sedentary rats were handled at least three times every week to become accustomed to the experimental procedures. Using a noninvasive computerized tail‐cuff system (ALC‐NIBP; Shanghai Alcott Biotech Inc., Shanghai, China), as described previously 23, BP was measured in conscious rats at baseline (8 weeks of age) and then every 4 weeks until the end of the study period.
Table 1.
Maximal running speeds in ExT groups
| Maximal speeds (km/h) | ||||
|---|---|---|---|---|
| n | 0 week | 6th week | 12th week | |
| WKY‐ExT | 15 | 1.31 ± 0.08 | 1.79 ± 0.04* | 2.15 ± 0.07* |
| SHR‐ExT | 15 | 1.88 ± 0.05† | 2.16 ± 0.07*, † , | 2.44 ± 0.07*, † |
Data are mean ± SE. *P < 0.05 versus 0 week. †P < 0.05 versus WKY‐ExT.
Measurement of Citrate Synthase Concentration
Citrate synthase was recognized as a maker of ExT efficacy because it is a respiratory enzyme to undergo adaptive increases due to ExT in skeletal muscle 24. Soleus muscles from the right leg of rats were collected, weighted, and stored at −80°C for measurement of citrate synthase. Measurement of citrate synthase concentration from whole muscle homogenate was measured by Rat Citrate Synthase ELISA kit (E03876; Shanghai Yueyan Biological Technology Co, China). The procedures were carried out based on the instruction of kit. Briefly, muscle tissue was homogenized in an extraction buffer and centrifuged at 4°C, an aliquot of supernatants was collected for measuring the enzyme concentration. Reaction was terminated by the addition of a sulfuric acid solution, and the color change was measured spectrophotometrically at a wavelength of 450 nm. The concentration (pg/mg) of citrate synthase in the samples was then determined by comparing the O.D. of the samples to the standard curve.
General Surgical Procedures and RVLM Microinjections
The surgical procedures, recording of renal sympathetic nerve activity (RSNA), and RVLM microinjections were carried out as described by us previously 25, 26, 27. Briefly, rats were anaesthetized with urethane (800 mg/kg i.p.) and α‐chloralose (40 mg/kg i.p.), and the trachea was cannulated to facilitate mechanical respiration. The right femoral artery was catheterized for BP measurement, and the femoral vein was cannulated for supplemental drugs. The rat was placed in a stereotaxic frame with the head fixed horizontally, and the dorsal surface of the medulla was surgically exposed. The renal sympathetic nerves were exposed and identified. The distal terminal of the renal nerve was cut to avoid the afferent activity. The renal sympathetic nerves were placed on a pair of recording electrodes. The RSNA signal was amplified, filtered, integrated, sampled, and converted to a digital signal by the PowerLab system. Baseline RSNA was taken as 100% from the absolute value after the noise level was subtracted. In each rat, maximum RSNA was measured during euthanasia at the end of the experiments, as described previously 28. Body temperature was kept at 37°C by a temperature controller.
Microinjections in the RVLM were made from a three‐barrel micropipette. The coordinates for injections into the RVLM were 2.0–2.5 mm rostral to the caudal tip of the area postrema, 2.0–2.2 mm lateral to the medline, and 3.0–3.2 mm below the dorsal surface of the brainstem. The RVLM injection (100 nL) was made over a period of 5–10 s by a pressure injector (PV820; WPI, Sarasota, FL, USA), and the RVLM was chemically identified by a pressor response to L‐glutamate (1 nmol) microinjection. Based on previous studies 9, 10, 29, 2.7 nmol dose of KYN (Sigma‐Aldrich, St. Louis, MO, USA) was chosen to effectively block ionotropic glutamate receptors at least 30 min after functional pressor site in the RVLM was identified. The interval of bilateral injections was within 2 min. The continuous recordings of cardiovascular parameters (BP, HR, and RSNA) were at least 60 min after bilateral injection of KYN into the RVLM. At the end of each experiment, the injection sites marked by 2% pontamine sky blue solution were confirmed to be located within RVLM area, which are similar to described by us previously 25, 26, 27.
Western Blot Analysis
After the rat was euthanized with an overdose of pentobarbital sodium (200 mg/kg, i.p.), brain tissues including RVLM, NTS, and PVN were punched on coronal sections of brainstem according to the rat atlas 30. The procedures of Western blot were described previously 25. The protein concentration was measured and loaded onto a SDS‐PAGE gel and then transferred to a polyvinylidene fluoride membrane. The membrane was probed with primary antibody (vesicular glutamate transporter 2, vGLUT2, MAB5504, EMD Millipore; glutaminase2 (GLS2), AV43562; Sigma‐Aldrich) and secondary antibody. The protein bands were visually detected and analyzed. The levels of target proteins were normalized to β‐actin (Sigma‐Aldrich), which served as a loading control.
Measurement of Glutamate Concentration by High‐Performance Liquid Chromatography (HPLC)
The concentration of glutamate in the brain was measured by HPLC, as described previously 25, 31. After RVLM tissue was punched and weighed from the rat which was euthanized (pentobarbital sodium, 200 mg/kg, i.p.), 0.05 mM HClO4 was added into the tube and tissue was homogenated and centrifuged for 10 min. Supernatant was filtered and collected for analysis by HPLC‐electrochemical detection (ESA; Coulochem III, Chelmsford, MA, USA) under 4°C. We used the o‐phthalaldehyde (OPA)/2‐mercaptoethanol (β‐ME) to derivatization for amino acid analysis. The autosampler vial was initially filled with 20 μL from the samples provided. The 542 autosampler was added 50 μL OPA/βME solution to remaining 20 μL of sample, mixed the reagent for four times and then injected 50 μL of the derivative sample for subsequent amino acid analysis. HPLC analysis was carried out on a reverse phase C18 column (Shiseido Capcell Pak 75 × 3 mm, 3 μm C18, P/N 88‐90816, Shiseido Co. Ltd., Tokyo, Japan). The mobile phase was composed by 100 mM di‐sodium hydrogen phosphate anhydrous, 22% methanol, and 3.5% acetonitrile at the pH of 6.75, and the flow rate was 0.60 mL/min. The detect channel potential were set at +150 mV and +550 mV. The HPLC system was controlled, and data were acquired, processed, and analyzed using Coulochem software. Analytes were identified according to the authentic standards based on their retention time and peak‐height ratios. The content of glutamate was quantified by a comparison of the area with those of known amounts of standards.
Data Analysis
All values are expressed as mean ± SE. The significance of differences between groups (WKY and SHR) and conditions (Sed and ExT) was made by a two‐way ANOVA followed by post hoc Student‐Newman–Keuls. Differences were considered to be significant at P < 0.05.
Results
Assessment of ExT Efficacy
As shown in Table 2, several values were measured for efficacy of ExT at the end of Sed or ExT protocol (at age 20 weeks). Body weight in ExT groups was significantly (P < 0.05, n = 15) reduced compared with Sed groups. Soleus muscle weight was significantly increased in ExT groups than in Sed groups. The concentration of citrate synthase, a marker of ExT efficacy, in soleus muscle was significantly higher in ExT than in Sed rats. Excretion of NE in 24‐h urine was higher in SHR‐Sed than in WKY‐Sed (1.19 ± 0.09 vs. 0.59 ± 0.07 μg, P < 0.05, n = 15), whereas it was significantly reduced by 44% (0.67 ± 0.07 μg, P < 0.05) following ExT treatment. At the beginning of ExT (at age 8 weeks), systolic BP and mean arterial pressure (MAP) in conscious rats were significantly higher in SHR‐Sed than in WKY‐Sed and remained elevated for the duration of the study (Figure 1). After 12‐week period of ExT, MAP was significantly (P < 0.05, n = 15) lower in SHR‐ExT (170 ± 2.9 mmHg) than in SHR‐Sed (182 ± 2.5 mmHg). However, ExT did not change BP in WKY rats (128 ± 1.8 mmHg in WKY‐Sed; 125 ± 1.7 mmHg in WKY‐ExT, P > 0.05).
Table 2.
Measurements of parameters for determining efficacy of ExT
| Parameters | n | WKY‐Sed | WKY‐ExT | SHR‐Sed | SHR‐ExT |
|---|---|---|---|---|---|
| Body weight (BW, g) | 15 | 311 ± 8 | 281 ± 5* | 301 ± 5 | 277 ± 4† |
| SMW (mg) | 15 | 106 ± 4 | 130 ± 3* | 101 ± 4 | 135 ± 4† |
| SMW/BW (mg/g) | 15 | 0.34 ± 0.01 | 0.46 ± 0.01* | 0.34 ± 0.01 | 0.49 ± 0.02† |
| Content of CS in SMW (pg/mg) | 5 | 653 ± 35 | 788 ± 38* | 695 ± 15 | 765 ± 32† |
| MAP (mmHg) | 5 | 120 ± 2 | 115 ± 7 | 171 ± 7* | 145 ± 4*, † |
| HR (beats/min) | 5 | 390 ± 14 | 342 ± 9* | 443 ± 6* | 382 ± 5† |
| Baseline RSNA (% of Max) | 5 | 30.2 ± 3.0 | 27.1 ± 2.6 | 53.7 ± 4.5* | 41.2 ± 3.6*, † |
n, number of animals; MAP, mean arterial pressure; SMW, Soleus muscle wet weight; CS, citrate synthase; MAP, mean arterial pressure; RSNA, renal sympathetic nerve activity; WKY‐Sed, Wistar‐Kyoto‐sedentary; SHR‐Sed, spontaneously hypertensive rat‐sedentary; WKY‐ExT, Wistar‐Kyoto‐exercise training; SHR‐ExT, spontaneously hypertensive rat‐ exercise training. Data are mean ± SE. Values for MAP, HR, and inte‐RSNA were obtained in anaesthetized rats. MAP was measured by catheterizing femoral artery. *P < 0.05 versus WKY‐Sed. †P < 0.05 versus SHR‐Sed.
Figure 1.
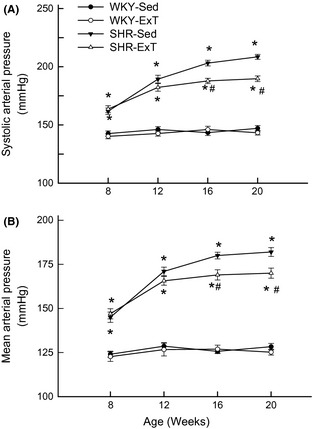
Time course of systolic arterial pressure (A) and mean arterial pressure (B) in sedentary or exercised Wistar‐Kyoto (WKY) and spontaneously hypertensive rat (SHR) groups. The values for blood pressure in conscious rats were measured by tail‐cuff method. Blood pressure has already lowered in SHR‐ExT compared with SHR‐Sed rats from 8 week of exercise training (at 16 weeks of age). Values are mean ± SE; n = 15 in each group. *P < 0.05 versus WKY‐Sed; # P < 0.05 versus SHR‐Sed.
ExT Attenuates the Decrease in BP and HR Evoked by Blockade of Glutamate Receptors in the RVLM of SHR
The baseline BP, HR, and RSNA in four groups are shown in Table 2. Baseline BP was significantly lower in SHR‐ExT compared with SHR‐Sed, but HR was decreased in ExT rats compared with Sed rats. Baseline RSNA (% maximum) in SHR‐Sed was significantly increased compared with WKY‐Sed (53.7 ± 4.5 vs. 30.2 ± 3.0%, P < 0.05, n = 5), which was significantly (P < 0.05) reduced following ExT treatment (41.2 ± 3.6%). In WKY‐Sed or WKY‐ExT group, bilateral injections of KYN (2.7 nmol for each side) into the RVLM had little effect on resting BP, HR, and RSNA compared with pre‐injection levels. However, bilateral microinjections of KYN into the RVLM elicited a profound decrease in resting BP (−51.1 ± 5.3 mmHg), HR (−62 ± 6 beats/min), and RSNA (−25.2 ± 2.2%) in SHR‐Sed compared with pre‐injection levels (Figure 2). ExT significantly (P < 0.05, n = 5) attenuated the KYN‐induced decrease in BP (−20.2 ± 2.4 mmHg), HR (−24 ± 2 beats/min), and RSNA (−8.5 ± 1.2%) in SHR. The percent peak changes in BP, HR, and RSNA before and after injections of KYN in four groups are shown in Figure 3.
Figure 2.
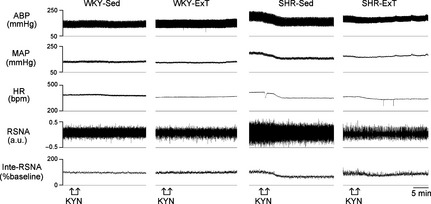
Representative original recordings of effects of kynurenic acid (KYN, 2.7 nmol in 100 nL) bilaterally injected into the rostral ventrolateral medulla (RVLM) on blood pressure, HR, and renal sympathetic nerve activity (RSNA) in sedentary or exercised Wistar‐Kyoto and spontaneously hypertensive rat groups. ABP, arterial blood pressure; bpm, beats/min; inte‐RSNA, integrated RSNA; a.u, arbitrary unit.
Figure 3.
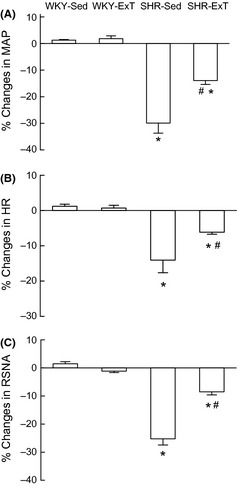
Percent changes in mean arterial pressure (A), HR (B), and renal sympathetic nerve activity (C) induced by bilateral microinjection of kynurenic acid into the rostral ventrolateral medulla of sedentary or exercised Wistar‐Kyoto (WKY) and spontaneously hypertensive rat (SHR) groups. Values are mean ± SE; n = 5 in each group. *P < 0.05 versus WKY‐Sed; # P < 0.05 versus SHR‐Sed.
ExT Decreases the Concentration of Glutamate and Expression of vGLUT2 in the RVLM of SHR
As indicated in Figure 4, the content of glutamate in the RVLM was significantly increased [459 ± 15 vs. 354 ± 13 ng/mg (wet weight), P < 0.05, n = 5] in SHR‐Sed than in WKY‐Sed. However, ExT‐treated SHR showed a significant attenuation in glutamate content (385 ± 13 ng/mg, P < 0.05 vs. SHR‐Sed). There is no significant difference of glutamate concentration in the RVLM between WKY‐Sed and WKY‐ExT.
Figure 4.
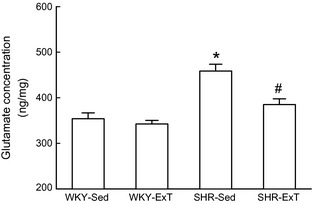
The concentrations of glutamate (ng/mg wet weight) in the rostral ventrolateral medulla of sedentary or exercised Wistar‐Kyoto (WKY) and spontaneously hypertensive rat (SHR) groups. Values are mean ± SE; n = 5 in each group. *P < 0.05 versus WKY‐Sed; # P < 0.05 versus SHR‐Sed.
As indicated in Figure 5, protein expression of vGLUT2 in the RVLM was significantly increased by an average of 82% in SHR‐Sed compared with WKY‐Sed. However, a significant decrease in vGLUT2 expression in the RVLM was observed in SHR‐ExT compared with SHR‐Sed. No significant difference of vGLUT2 expression was observed between WKY‐Sed and WKY‐ExT.
Figure 5.
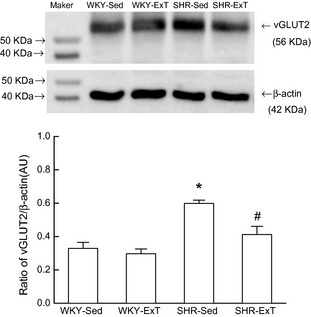
Representative Western blot (top) and densitometric analysis (bottom) of vesicular glutamate transporter 2 (vGLUT2) in the rostral ventrolateral medulla of sedentary or exercised Wistar‐Kyoto (WKY) and spontaneously hypertensive rat (SHR) groups. AU, arbitrary unit. Values are mean ± SE; n = 5 in each group. *P < 0.05 versus WKY‐Sed; # P < 0.05 versus SHR‐Sed.
ExT Downregulates the Protein Expression of Glutaminase2 (GLS2) in the NTS and PVN of SHR
A total of 10 SHR was subjected to determine the changes of glutamate synthesis in sources of glutamatergic inputs to the RVLM in response to Sed or ExT treatment (five rats for each group). Levels of GLS2 protein expression in the NTS and PVN were significantly decreased in SHR‐ExT compared with SHR‐Sed (Figure 6). No significant difference of GLS2 expression in the pontine reticular formation was found between SHR‐Sed and SHR‐ExT.
Figure 6.
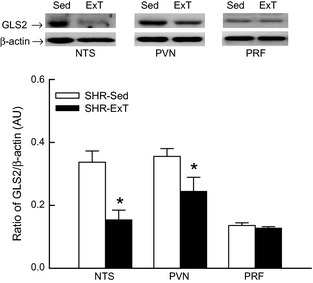
Representative Western blot (top) and densitometric analysis (bottom) of glutaminase2 in the nucleus of solitary tract (NTS), paraventricular nucleus (PVN), and pontine reticular formation (PRF) in the Sed or ExT‐treated spontaneously hypertensive rat (SHR). AU, arbitrary unit. Data are mean ± SE; n = 5 in each group. *P < 0.05 versus SHR‐Sed.
Discussion
The major observations of this study are that (1) Low‐intensity ExT effectively reduced BP and sympathetic activity in SHR; (2) The decrease in BP, HR, and RSNA evoked by blockade of glutamate receptors in the RVLM of SHR was blunted following ExT treatment, and (3) ExT significantly reduced the concentration of glutamate and expression of vGLUT2 in the RVLM of SHR. On basis of these results, we conclude that ExT is capable of lowering the enhanced tonically active glutamatergic input in the RVLM of SHR, which may contribute to elucidate the mechanism responsible for the beneficial effect of ExT on sympathetic overactivity in hypertension.
Exercise training, a part of lifestyle modification, has been widely recommended as a therapeutic strategy for hypertension12, 14. In this work, we determined the maximum ExT capacity (velocity) at the beginning of the protocol and week 6 and 12 to establish and correct the ExT intensity. The low‐intensity ExT corresponded to 50–60% of maximal excise capacity according to previous study 22. We found that soleus muscle weight and citrate synthase concentration were significantly increased in ExT groups than in Sed groups, whereas body weight was reduced in ExT group than in Sed groups. We also repeated that ExT significantly reduced BP in SHR but not in WKY, as described previously 15, 22, 23, 32. We noted that the level of BP obtained by tail cuff in conscious rats was somewhat higher compared with BP level measured directly from femoral artery in anesthetized rats. Because the tail‐cuff method is a form of restraint, it is possible that some factors such as stress result in a rise in BP. However, it was observed that BP levels measured by tail cuff in awaked SHR or by femoral artery in anaesthetized SHR were significantly reduced following ExT protocols. We also confirmed that ExT treatment significantly reduced baseline sympathetic nerve activity in SHR (Table 2). These data confirmed the efficacy of ExT to reduce BP and sympathetic activity in SHR.
Although previous studies report that ExT is capable of adjusting dysfunctions of heart and vessels in hypertension, the neuronal plasticity is suggested to be more important in the cardiovascular regulation 17. Upregulated glutamatergic input in the RVLM contributes to increase in BP and sympathetic outflow in hypertensive rats 9, 10. In this work, the changes in cardiovascular activity in response to microinjection of KYN into the RVLM were detected in four groups. Similar to previous studies 9, 10, bilateral injection of KYN into the RVLM significantly reduced basal BP, HR, and RSNA in SHR‐Sed, but not in WKY‐Sed. Interestingly, we found that the degree of reduction in BP, HR, and RSNA evoked by KYN injected into the RVLM was significantly blunted in SHR‐ExT compared with SHR‐Sed. We further confirmed that the concentration of the neurotransmitter glutamate in the RVLM was significantly reduced in SHR following ExT protocol. On the basis of the above evidence, it is indicated that ExT is capable of attenuating the increased tonically active glutamatergic input in the RVLM. Therefore, it is suggested that, in hypertensive rats, downregulation in tonically active glutamatergic input to RVLM neurons induced by ExT protocol leads to reduction in the basal activity of RVLM neurons, thereby producing a fall in sympathetic outflow and resting BP.
Clearly, a limitation is that the effect of ExT on glutamatergic mechanism is not further determined at the level of the vasomotor (presympathetic) neurons in the RVLM by in vivo electrophysiological (e.g., single‐unit extracellular recording) technique. We previously reported that blockade of glutamatergic input to the RVLM by KYN significantly inhibited the ongoing spontaneous discharge of RVLM presympathetic neurons in rats with chronic heart failure, a model of sympathetic hyperactivity 26. Sympathetic tone is mainly dependent on basal activity of RVLM presympathetic neurons. Reduction in RSNA by KYN injection was blunted following ExT protocol, suggesting that ExT may inhibit the glutamate‐mediating excitation of RVLM presympathetic neurons. Another limitation in the present study is that concentration of glutamate was measured in punched RVLM tissue but not in extracellular fluid by microdialysis. Its concentration might not completely reflect the release of glutamate in the RVLM. However, we observed that the protein expression of vGLUT2 in the RVLM was significantly decreased in SHR following ExT treatment. Because vGLUT2 packages the glutamate into presynaptic vesicles so that they can be released into the synapse 33, 34, downregulation of this transporter by ExT reflects, to some degree, a decrease in the release of glutamate in presynaptic terminals in the RVLM. In addition, it is not clear whether ExT alters inhibitory inputs to the RVLM in hypertension. It is suggested that decrease in inhibitory inputs to the RVLM also is an important contributor to hypertension 2, 35. In a previous study 36, glutamic acid decarboxylase, a major GABA synthesizing enzyme, in the caudal hypothalamus was found to be upregulated in the ExT‐treated SHR. Therefore, we do not role out the possibility that ExT upregulates the decreased GABAergic inputs to the RVLM.
The exact mechanism(s) by which ExT lowers the enhanced glutamatergic input to the RVLM of hypertension was not addressed in the present study. However, there are several explanations for the effect of ExT on glutmatergic input in hypertension. One possibility is the ExT‐induced reduction in oxidative stress in the RVLM. It has been demonstrated that increased oxidative stress in the RVLM contributes to sympathetic overactivity in hypertension 37, 38, 39. Interestingly, previous studies show that ExT is capable of effectively reducing oxidative stress in the RVLM in animal models of hypertension and chronic heart failure 40, 41. It is possible that ExT reduces the glutamatergic input to the RVLM in hypertension via reduction in oxidative stress. Another possibility is the ExT‐induced changes in sources of excitatory to the RVLM. We observed that SHR treated ExT leads to a significant reduction in expression level of glutaminase 2, a key enzyme for glutamate synthesis, in the commNTS and PVN, but not in the pontine reticular formation. Glutamatergic inputs to the RVLM may originate from multiple sources including the above three regions within the brainstem and forebrain 6, 7, 8. It has been reported that ExT inhibits activity of autonomic‐related PVN neurons in sympathoexcitatory disorders such as hypertension and heart failure 42, 43, 44. Accordingly, ExT produces a decrease in both glutamate synthesis and the pulse‐mediated activity of the RVLM‐projecting PVN neurons, which, in turn, reduces the release of glutamate in the RVLM. The NTS, the primary site in the central nervous system that receives the afferents arising from arterial baro‐ and chemoreceptors 2, has been demonstrated to be involved in mediating the effect of ExT on central cardiovascular regulation 45, 46. The commNTS also receives inputs from arterial chemoreceptors and directly sends fibers projecting to the RVLM and seems to be highly sensitive to carotid chemoreceptor stimulation 8, 47, 48. Importantly, EXT normalizes the carotid body chemoreflex by preventing an increase in afferent carotid body chemoreceptor activity in rabbit with chronic heart failure 49. A recent study also shows that swimming exercise enhances the GABAergic inhibition in the commNTS neurons of SHR 50. The above evidence indicates that ExT leads to a reduction in afferent activity as well as neuronal activity in the commNTS. Taken together with reduction in glutamate synthesis, therefore, it is possible that ExT is capable of attenuating the enhanced glutamatergic inputs to the RVLM originated from the commNTS in hypertension. With respect to pontine reticular formation, it is not clear why ExT does not affect its glutamatergic inputs to the RVLM. It has been documented that the pontine reticular formation is involved in central cardiovascular regulation during hypoxia 51. BP is increased during ExT, whereas it is reduced at post‐ExT 52. Elevated BP during ExT may be partly resulted from the enhanced glutamatergic inputs originated from pone reticular formation induced by ExT‐induced hypoxia. At post‐ExT, however, enhanced glutamatergic input from pontine reticular formation may be restored when hypoxia is disappeared. Further confirmations of these synaptic network changes during ExT would be helpful to our understanding of mechanism underlying the effects of ExT on neural control of cardiovascular activity in hypertension.
In conclusion, our data demonstrate that low‐intensity ExT downregulates the increased tonically active glutamatergic input to the RVLM in SHR. It is indicated that a reduction in enhanced excitatory input to RVLM neurons is an important mechanism responsible for the beneficial effects of ExT on high BP and sympathetic hyperactivity in hypertension. The present work provides new information to expand our knowledge of the ExT‐induced adjustment of central autonomic networks for cardiovascular activity.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
We gratefully acknowledge the statistical assistance of Dr Jian Lu (from the Department of Statistical Analysis, SMMU). This work was supported by the National Natural Science Foundation of China (30971061, 81070214, and 81170240), Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry [2010(1561)03], and the Major State Basic Research Development Program of China (2009CB521901).
The first two authors contribute equally to this work.
References
- 1. Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev 2010;90:513–557. [DOI] [PubMed] [Google Scholar]
- 2. Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 1994;74:323–364. [DOI] [PubMed] [Google Scholar]
- 3. Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 2006;7:335–346. [DOI] [PubMed] [Google Scholar]
- 4. Colombari E, Sato MA, Cravo SL, Bergamaschi CT, Campos RR Jr. Lopes OU: Role of the medulla oblongata in hypertension. Hypertension 2001;38:549–554. [DOI] [PubMed] [Google Scholar]
- 5. Sved AF, Ito S, Sved JC. Brainstem mechanisms of hypertension: Role of the rostral ventrolateral medulla. Curr Hypertens Rep 2003;5:262–268. [DOI] [PubMed] [Google Scholar]
- 6. Yang Z, Bertram D, Coote JH. The role of glutamate and vasopressin in the excitation of RVL neurones by paraventricular neurones. Brain Res 2001;908:99–103. [DOI] [PubMed] [Google Scholar]
- 7. Hayes K, Calaresu FR, Weaver LC. Pontine reticular neurons provide tonic excitation to neurons in rostral ventrolateral medulla in rats. Am J Physiol 1994;266:R237–R244. [DOI] [PubMed] [Google Scholar]
- 8. Aicher SA, Saravay RH, Cravo S, et al. Monosynaptic projections from the nucleus tractus solitarii to C1 adrenergic neurons in the rostral ventrolateral medulla: Comparison with input from the caudal ventrolateral medulla. J Comp Neurol 1996;373:62–75. [DOI] [PubMed] [Google Scholar]
- 9. Ito S, Komatsu K, Tsukamoto K, Sved AF. Excitatory amino acids in the rostral ventrolateral medulla support blood pressure in spontaneously hypertensive rats. Hypertension 2000;35:413–417. [DOI] [PubMed] [Google Scholar]
- 10. Ito S, Komatsu K, Tsukamoto K, Sved AF. Tonic excitatory input to the rostral ventrolateral medulla in Dahl salt‐sensitive rats. Hypertension 2001;37:687–691. [PubMed] [Google Scholar]
- 11. Hagberg JM, Park JJ, Brown MD. The role of exercise training in the treatment of hypertension: An update. Sports Med 2000;30:193–206. [DOI] [PubMed] [Google Scholar]
- 12. Joyner MJ, Green DJ. Exercise protects the cardiovascular system: Effects beyond traditional risk factors. J Physiol 2009;587:5551–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duncan JJ, Farr JE, Upton SJ, Hagan RD, Oglesby ME, Blair SN. The effects of aerobic exercise on plasma catecholamines and blood pressure in patients with mild essential hypertension. JAMA 1985;254:2609–2613. [PubMed] [Google Scholar]
- 14. Laterza MC, de Matos LD, Trombetta IC, et al. Exercise training restores baroreflex sensitivity in never‐treated hypertensive patients. Hypertension 2007;49:1298–1306. [DOI] [PubMed] [Google Scholar]
- 15. Veras‐Silva AS, Mattos KC, Gava NS, Brum PC, Negrao CE, Krieger EM. Low‐intensity exercise training decreases cardiac output and hypertension in spontaneously hypertensive rats. Am J Physiol 1997;273:H2627–H2631. [DOI] [PubMed] [Google Scholar]
- 16. Mitchell JH. Neural control of the circulation during exercise. Med Sci Sports Exerc 1990;22:141–154. [PubMed] [Google Scholar]
- 17. Michelini LC, Stern JE. Exercise‐induced neuronal plasticity in central autonomic networks: Role in cardiovascular control. Exp Physiol 2009;94:947–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mueller PJ. Exercise training and sympathetic nervous system activity: Evidence for physical activity dependent neural plasticity. Clin Exp Pharmacol Physiol 2007;34:377–384. [DOI] [PubMed] [Google Scholar]
- 19. Martins‐Pinge MC, Becker LK, Garcia MR, et al. Attenuated pressor responses to amino acids in the rostral ventrolateral medulla after swimming training in conscious rats. Auton Neurosci 2005;122:21–28. [DOI] [PubMed] [Google Scholar]
- 20. Mueller PJ. Exercise training attenuates increases in lumbar sympathetic nerve activity produced by stimulation of the rostral ventrolateral medulla. J Appl Physiol 2007;102:803–813. [DOI] [PubMed] [Google Scholar]
- 21. Higa‐Taniguchi KT, Silva FC, Silva HM, Michelini LC, Stern JE. Exercise training‐induced remodeling of paraventricular nucleus (nor) adrenergic innervation in normotensive and hypertensive rats. Am J Physiol Regul Integr Comp Physiol 2007;292:R1717–R1727. [DOI] [PubMed] [Google Scholar]
- 22. Melo RM, Martinho E Jr, Michelini LC. Training‐induced, pressure‐lowering effect in SHR: Wide effects on circulatory profile of exercised and nonexercised muscles. Hypertension 2003;42:851–857. [DOI] [PubMed] [Google Scholar]
- 23. Agarwal D, Haque M, Sriramula S, Mariappan N, Pariaut R, Francis J. Role of proinflammatory cytokines and redox homeostasis in exercise‐induced delayed progression of hypertension in spontaneously hypertensive rats. Hypertension 2009;54:1393–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kainulainen H, Ahomaki E, Vihko V. Selected enzyme activities in mouse cardiac muscle during training and terminated training. Basic Res Cardiol 1984;79:110–123. [DOI] [PubMed] [Google Scholar]
- 25. Peng JF, Wu ZT, Wang YK, et al. GABAergic mechanism in the rostral ventrolateral medulla contributes to the hypotension of moxonidine. Cardiovasc Res 2011;89:473–481. [DOI] [PubMed] [Google Scholar]
- 26. Wang WZ, Gao L, Wang HJ, Zucker IH, Wang W. Tonic glutamatergic input in the rostral ventrolateral medulla is increased in rats with chronic heart failure. Hypertension 2009;53:370–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang WZ, Wang LG, Gao L, Wang W. Contribution of AMPA/kainate receptors in the rostral ventrolateral medulla to the hypotensive and sympathoinhibitory effects of clonidine. Am J Physiol Regul Integr Comp Physiol 2007;293:R1232–R1238. [DOI] [PubMed] [Google Scholar]
- 28. Wang HJ, Pan YX, Wang WZ, et al. Exercise training prevents the exaggerated exercise pressor reflex in rats with chronic heart failure. J Appl Physiol 2010;108:1365–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ito S, Sved AF. Tonic glutamate‐mediated control of rostral ventrolateral medulla and sympathetic vasomotor tone. Am J Physiol 1997;273:R487–R494. [DOI] [PubMed] [Google Scholar]
- 30. Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 3rd edn New York: Academic Press, 1998. [Google Scholar]
- 31. Monge‐Acuna AA, Fornaguera‐Trias J. A high performance liquid chromatography method with electrochemical detection of gamma‐aminobutyric acid, glutamate and glutamine in rat brain homogenates. J Neurosci Methods 2009;183:176–181. [DOI] [PubMed] [Google Scholar]
- 32. Gava NS, Veras‐Silva AS, Negrao CE, Krieger EM. Low‐intensity exercise training attenuates cardiac beta‐adrenergic tone during exercise in spontaneously hypertensive rats. Hypertension 1995;26:1129–1133. [DOI] [PubMed] [Google Scholar]
- 33. Herzog E, Bellenchi GC, Gras C, et al. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci 2001;21:RC181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature 2000;407:189–194. [DOI] [PubMed] [Google Scholar]
- 35. Smith JK, Barron KW. GABAergic responses in ventrolateral medulla in spontaneously hypertensive rats. Am J Physiol 1990;258:R450–R456. [DOI] [PubMed] [Google Scholar]
- 36. Little HR, Kramer JM, Beatty JA, Waldrop TG. Chronic exercise increases GAD gene expression in the caudal hypothalamus of spontaneously hypertensive rats. Mol Brain Res 2001;95:48–54. [DOI] [PubMed] [Google Scholar]
- 37. Hirooka Y, Kishi T, Sakai K, Takeshita A, Sunagawa K. Imbalance of central nitric oxide and reactive oxygen species in the regulation of sympathetic activity and neural mechanisms of hypertension. Am J Physiol Regul Integr Comp Physiol 2011;300:R818–R826. [DOI] [PubMed] [Google Scholar]
- 38. Peterson JR, Sharma RV, Davisson RL. Reactive oxygen species in the neuropathogenesis of hypertension. Curr Hypertens Rep 2006;8:232–241. [DOI] [PubMed] [Google Scholar]
- 39. Kishi T, Hirooka Y, Kimura Y, Ito K, Shimokawa H, Takeshita A. Increased reactive oxygen species in rostral ventrolateral medulla contribute to neural mechanisms of hypertension in stroke‐prone spontaneously hypertensive rats. Circulation 2004;109:2357–2362. [DOI] [PubMed] [Google Scholar]
- 40. Agarwal D, Welsch MA, Keller JN, Francis J. Chronic exercise modulates RAS components and improves balance between pro‐ and anti‐inflammatory cytokines in the brain of SHR. Basic Res Cardiol 2011;106:1069–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gao L, Wang W, Liu D, Zucker IH. Exercise training normalizes sympathetic outflow by central antioxidant mechanisms in rabbits with pacing‐induced chronic heart failure. Circulation 2007;115:3095–3102. [DOI] [PubMed] [Google Scholar]
- 42. DiCarlo SE, Zheng H, Collins HL, Rodenbaugh DW, Patel KP. Daily exercise normalizes the number of diaphorase (NOS) positive neurons in the hypothalamus of hypertensive rats. Brain Res 2002;955:153–160. [DOI] [PubMed] [Google Scholar]
- 43. Zheng H, Li YF, Cornish KG, Zucker IH, Patel KP. Exercise training improves endogenous nitric oxide mechanisms within the paraventricular nucleus in rats with heart failure. Am J Physiol Heart Circ Physiol 2005;288:H2332–H2341. [DOI] [PubMed] [Google Scholar]
- 44. Kleiber AC, Zheng H, Schultz HD, Peuler JD, Patel KP. Exercise training normalizes enhanced glutamate‐mediated sympathetic activation from the PVN in heart failure. Am J Physiol Regul Integr Comp Physiol 2008;294:R1863–R1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Potts JT. Inhibitory neurotransmission in the nucleus tractus solitarii: Implications for baroreflex resetting during exercise. Exp Physiol 2006;91:59–72. [DOI] [PubMed] [Google Scholar]
- 46. Michelini LC. The NTS and integration of cardiovascular control during exercise in normotensive and hypertensive individuals. Curr Hypertens Rep 2007;9:214–221. [DOI] [PubMed] [Google Scholar]
- 47. Chitravanshi VC, Sapru HN. Chemoreceptor‐sensitive neurons in commissural subnucleus of nucleus tractus solitarius of the rat. Am J Physiol 1995;268:R851–R858. [DOI] [PubMed] [Google Scholar]
- 48. Colombari E, Menani JV, Talman WT. Commissural NTS contributes to pressor responses to glutamate injected into the medial NTS of awake rats. Am J Physiol 1996;270:R1220–R1225. [DOI] [PubMed] [Google Scholar]
- 49. Li YL, Ding Y, Agnew C, Schultz HD. Exercise training improves peripheral chemoreflex function in heart failure rabbits. J Appl Physiol 2008;105:782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ogihara CA, Schoorlemmer GH, Levada AC, et al. Exercise changes regional vascular control by commissural NTS in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 2010;299:R291–R297. [DOI] [PubMed] [Google Scholar]
- 51. Guyenet PG. Neural structures that mediate sympathoexcitation during hypoxia. Respir Physiol 2000;121:147–162. [DOI] [PubMed] [Google Scholar]
- 52. O'Sullivan SE, Bell C. The effects of exercise and training on human cardiovascular reflex control. J Auton Nerv Syst 2000;81:16–24. [DOI] [PubMed] [Google Scholar]


