Conflict of Interest
The authors declare no conflict of interest.
Constraint‐induced movement therapy (CIMT) has been extensively used for stroke rehabilitation 1. It encourages the use of impaired limbs by constraining the use of the unaffected limbs. Recent studies suggest that CIMT can promote behavioral recovery and enhance functional and structural reorganization of the brain after stroke. However, CIMT‐induced structural plasticity and its significance remain to be studied. In the present study, we examined whether the enhanced behavioral recovery induced by CIMT after stroke in adult rats is a direct consequence of increased neurogenesis.
Male Wistar rats (200–250 g) were randomly assigned to four groups (n = 8 per group): sham, ischemia, ischemia treated with CIMT (CIMT group), irradiation followed by ischemia and then treated with CIMT (irradiation group). Sixty days before ischemia, modified low‐dose, focal brain x‐irradiation was used to suppress neurogenesis in the subventricular zone (SVZ) and hippocampus in rats with parameters based on previous studies 2, 3. The 5 Gy total irradiation dose was given at a rate of 1.25 Gy/min. Focal cerebral ischemia was induced by injection of endothelin‐1 into three coordinates of the motor cortex and striatum at a concentration of 0.5 μg/μL. The dose of ET‐1 for each injection site was 2 μL 4. CIMT beginning from post‐stroke day 7 was performed by fitting a plaster cast around the unimpaired upper limb of rats for 3 weeks as described by Muller et al. 5. All casts were removed before the behavioral tests. The motor functional recovery was assessed by the beam walking test on day 29 and spatial learning performance was tested by the Morris water maze on days 30–32 after ischemia. After the behavioral tests, the rats' brains were removed to perform infarct volume measurements and immunofluorescence staining. In the present study, we used doublecortin (DCX) as a specific marker to study neurogenesis. DCX is a microtubule‐associated protein expressed in neural progenitors and immature neurons. The DCX‐positive cells in the SVZ and dentate gyrus (DG) and their neuroblast migration ipsilateral to the injury site were counted in five 50 μm coronal sections per animal (n = 6 per group).
There was no significant difference in infarct volumes between the ischemia group, CIMT group and the irradiation group. After cerebral ischemia, immunofluorescence staining showed that neuroblasts composed of DCX‐positive cells in the SVZ were expanded and the distance of the neuroblast migration toward the infarct area was increased markedly compared with the sham group (Figure 1A,B). CIMT further increased the expansion and migration distance of DCX‐positive cells (Figure 1C). However, irradiation clearly suppressed the enhancement of the neurogenic proliferative and migratory response in the SVZ induced by CIMT (Figure 1D). It was difficult to count individual DCX‐positive cells precisely because they were densely packed in the SVZ and sometimes intertwined. Hence, we used an image processing algorithm of NIH Image J to calculate the pixel areas of DCX‐positive signals in the SVZ as previously described by Lee et al. 6. Correspondingly, this showed that the DCX‐positive signals areas in the SVZ were significantly different between groups (Figure 1E). Meanwhile, CIMT significantly increased the number of DCX‐positive cells in the DG compared with the ischemia group and compared with the sham group. This enhancement of neurogenesis induced by CIMT was significantly suppressed after x‐irradiation (Figure 1F–J).
Figure 1.
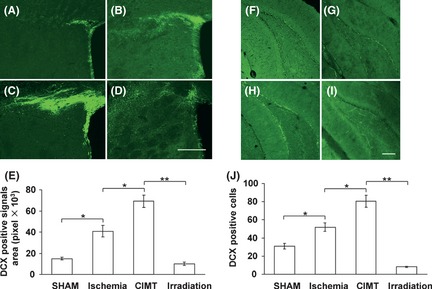
Proliferation and migration of doublecortin (DCX)‐positive cells in the subventricular zone (SVZ) and dentate gyrus (DG). Representative immunofluorescence images of the SVZ (A–D) and DG (F–I) for DCX 4 weeks after ischemia demonstrated that the DCX‐positive signals area in the SVZ or the number of DCX‐positive cells in the DG were increased significantly in the ischemia group (B, G) compared with the sham group (A, F), and were much greater in the constraint‐induced movement therapy group (C, H) but were scarce in the irradiation group (D, I). Quantification of DCX‐positive signals areas in the SVZ (E) and DCX‐postive cells in the DG (J) showed that the differences between groups were significant. (*P < 0.05, **P < 0.01, n = 6, data are presented as means ± SEM, scale bar = 200 μm).
In the beam walking test, the slip ratio increased significantly after ischemia compared with the sham group, and it was decreased after CIMT compared with the ischemia group. This suggests that motor function was impaired after ischemia and that it can be improved by CIMT. This improvement was ablated by irradiation, as the slip ratio increased significantly after irradiation compared with the CIMT group (Figure 2A). In the water maze test, escape latency data in the spatial navigation trial (Figure 2B) and data from the passes over the removed platform as well as swim paths in the probe trial (Figure 2C,D) demonstrated that the spatial learning and memory functions were impaired after ischemia and that they can be improved by CIMT. This improvement was also ablated by irradiation.
Figure 2.
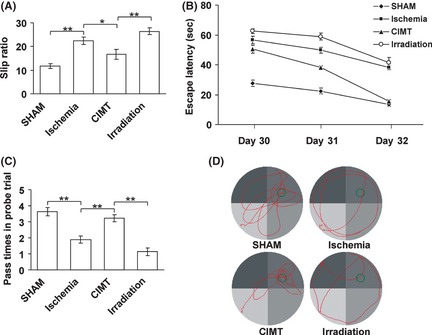
Performances in the behavioral tests 4 weeks after ischemia. (A) Slip ratio in the beam‐walking test showed significant differences of motor functional recovery between groups. (B) Data from spatial navigation trial showed significant differences in escape latencies between groups. (C) Data from the passes over the removed platform in the probe trial showed that the memory function of the ischemia and irradiated rats were clearly impaired compared with the sham and constraint‐induced movement therapy (CIMT) rats. (D) Representative swim paths in the probe trial. Sham and CIMT rats concentrated their searches in the target quadrant, while the paths of the ischemia and irradiation rats were dispersed and far from the target location. The position of the platform during acquisition is marked in the upper right quadrant. (*P < 0.05, **P < 0.01, n = 8, data are presented as means ± SEM).
Our previous study demonstrated that CIMT after focal experimental stroke significantly increased the number of BrdU‐positive cells in both the SVZ and DG, and it also promoted behavioral recovery 7. Although we observed enhanced behavioral recovery induced by CIMT in this study, the direct evidence that extra newborn neurons seen post‐CIMT contribute to functional recovery after stroke remains missing. Many studies have shown that manipulations to increase neurogenesis improve functional outcome, however, to identify whether ablating adult‐born neurons impairs stroke recovery may provide a more convincing link between neurogenesis and behavioral recovery after cerebral ischemia 8, 9.
To determine a cause‐and‐effect relationship between functional improvement and neurogenesis induced by CIMT in the present study, a focal brain irradiation paradigm was used before the induction of ischemia. We found that the number of DCX‐positive cells in the DG granule cell layer and the SVZ, as well as their migration, was decreased significantly compared with the non‐irradiated ischemic animals, whether they were treated with CIMT or not. Moreover, in the present study, the results of detectable beneficial effects on behavioral recovery were no longer seen after a combination of irradiation before stroke and CIMT following stroke. All the results indicate that neurogenesis might contribute to the functional outcome induced by CIMT after experimental stroke in adult rats. Nevertheless, enhanced functional recovery might be promoted partly by processes of other plasticity, such as dendritic arborization, synaptogenesis and axonogenesis, which are all known to be highly correlated with neurogenesis and stroke recovery.
In summary, the gain‐ and loss‐of‐function experiments by CIMT and focal brain irradiation provide new evidence for the functional contribution of neurogenesis to stroke recovery induced by CIMT. Elucidation of the mechanisms behind CIMT efficacy will help further and provide a new theoretical basis for clinical rehabilitation.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 30500611, No. 30872736).
References
- 1. Cramer SC, Riley JD. Neuroplasticity and brain repair after stroke. Curr Opin Neurol 2008;21:76–82. [DOI] [PubMed] [Google Scholar]
- 2. Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003;301:805–809. [DOI] [PubMed] [Google Scholar]
- 3. Lazarini F, Mouthon MA, Gheusi G, et al. Cellular and behavioral effects of cranial irradiation of the subventricular zone in adult mice. PLoS ONE 2009;4:e7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Windle V, Szymanska A, Granter‐Button S, et al. An analysis of four different methods of producing focal cerebral ischemia with endothelin‐1 in the rat. Exp Neurol 2006;201:324–334. [DOI] [PubMed] [Google Scholar]
- 5. Muller HD, Hanumanthiah KM, Diederich K, Schwab S, Schabitz W‐R, Sommer C. Brain‐derived neurotrophic factor but not forced arm use improves long‐term outcome after photothrombotic stroke and transiently upregulates binding densities of excitatory glutamate receptors in the rat brain. Stroke 2008;39:1012–1021. [DOI] [PubMed] [Google Scholar]
- 6. Lee SR, Kim HY, Rogowska J, et al. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci 2006;26:3491–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao C, Wang J, Zhao S, Nie Y. Constraint‐induced movement therapy enhanced neurogenesis and behavioral recovery after stroke in adult rats. Tohoku J Exp Med 2009;218:301–308. [DOI] [PubMed] [Google Scholar]
- 8. Raber J, Fan Y, Matsumori Y, et al. Irradiation attenuates neurogenesis and exacerbates ischemia‐induced deficits. Ann Neurol 2004;55:381–389. [DOI] [PubMed] [Google Scholar]
- 9. Wang X, Mao X, Xie L, Sun F, Greenberg DA, Jin K. Conditional depletion of neurogenesis inhibits long‐term recovery after experimental stroke in mice. PLoS ONE 2012;7:e38932. [DOI] [PMC free article] [PubMed] [Google Scholar]


