SUMMARY
Aim: Human cytomegalovirus (HCMV) is implicated in several cardiovascular disorders, including atherosclerosis, coronary heart disease, and cardiac transplant arteriopathy. We aimed to evaluate the relationship between HCMV and stroke. Methods: Real‐time polymerase chain reaction (PCR) and ELISA were performed on plasma samples isolated from 200 patients diagnosed with stroke and 200 controls. All participants belonged to the Stroke Hypertension Investigation in Genetics (SHINING) study. Results: HCMV seropositivity was higher in the stroke group than in controls (55.0% vs. 23.5%; P < 0.0001). The presence of HCMV DNA increased the risk of stroke (unadjusted odds ratio [OR], 3.98; 95% confidence interval [CI], 2.59 to 6.11; P < 0.0001). Risks were also increased for the subtypes ischemic stroke (unadjusted OR, 4.01; 95% CI, 2.57–6.24; P < 0.0001) and hemorrhagic stroke (unadjusted OR, 3.80; 95% CI, 1.64–8.78; P= 0.0018). Increased risk with HCMV remained significant after adjustment for age, sex, body mass index, hypertension, and smoking (ischemic stroke: adjusted OR, 4.07; 95% CI, 2.52–6.32; P < 0.0001; hemorrhagic stroke: adjusted OR, 3.88; 95% CI, 1.61–9.36; P= 0.0026). Conclusions: We demonstrate a novel link between HCMV infection and stroke. These findings may provide important insights into the pathogenesis of stroke.
Keywords: Human cytomegalovirus, Infection, Seropositivity, Stroke
Aims
Stroke is one of the leading causes of death worldwide [1]. In 2005, stroke caused an estimated 5.7 million deaths; 87% of these were in low‐ and middle‐income countries [2]. Stroke is generally classified as ischemic or hemorrhagic, and is a complex disease influenced by genetic and environmental factors and their interactions [3]. In recent years, accumulating evidence indicated that inflammation and atherosclerosis play important roles in stroke development [4, 5].
Human cytomegalovirus (HCMV), a ubiquitous DNA virus of the Herpesviridae family that only replicates in human, has been implicated in several cardiovascular disorders, including atherosclerosis, coronary heart disease, hypertension, and cardiac transplant arteriopathy [6, 7, 8, 9]. Previous studies suggested an association of HCMV with cardiovascular disorders through impaired endothelial nitric oxide synthase function and subsequent endothelial dysfunction [10, 11]. However, direct links between HCMV infection and stroke remain undefined and are only documented by evaluation of anti‐HCMV antibodies (IgG and IgA) [12, 13]. The purpose of this study was to address the association between HCMV infection and ischemic and hemorrhagic stroke by means of highly sensitive quantitative real‐time polymerase chain reaction (PCR).
Material and Methods
Plasma samples of 200 patients diagnosed with stroke and 200 controls were randomly chosen from 3,119 participants (1,559 patients with stroke and 1,293 controls) in the Stroke Hypertension Investigation in Genetics (SHINING) study. The Beijing Hypertension League Institute conducted the SHINING study [14]. From 1997 to 2000, patients and control subjects from six geographical regions in China were recruited for the case‐control study. The SHINING study included patients of Chinese Han ethnicity only. Patients with ischemic stroke and hemorrhagic stroke diagnosed by computed tomography or magnetic resonance imaging were included. Control subjects were selected according to the case‐control study criteria: control subjects were matched to cases by sex, age within 3 years, geographic location, and blood pressure category (<140/90, ≥140/90 and ≤180/105, >180/105 mmHg). Data collected included age, sex, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), and hypertension. All study participants provided written informed consent. The ethics committees of the Beijing Hypertension League Institute approved the study.
DNA was purified from 400‐μl plasma using the QIAamp DNA Mini kit (Qiagen, Valencia, CA, USA; Cat. No.51306) according to the manufacturer's protocol. HCMV DNA was amplified by TaqMan real‐time PCR with HCMV‐specific primers as follows: HCMV‐F:5'‐CACGGTCCCGGTTTAGCA‐3'; HCMV‐R:5'‐CGTAACGTGGACCTGACGTTT‐3'; HCMV Probe:5'‐FAM‐TATCTGCCCGAGGATCGCGGTTACA‐TAMRA‐3'. The two‐step thermocycling procedure consisted of 45 cycles of denaturation at 95°C for 15 second and annealing and extension at 60°C for 60 second. The PCR product was hybridized to a HCMV‐specific, FAM‐labeled probe. Serving as a positive control, plasmid DNA containing the HCMV target sequence was used in separate reactions on each TaqMan assay plate. Results were expressed as copies/mL plasma. A negative results means no DNA was detected. Any positive PCR result was regarded as positive irrespective of viral load.
Tests for anti‐HCMV IgG and IgM antibodies were performed with an enzyme‐linked immunosorbent assay (ELISA) kit (HCMV Diagnostic Kit, Haitai Biotech Inc., Zhuhai, China) according to the manufacturer's instructions. An ELISA value of less than 0.9 units was considered negative, and a value of 1.1 unit or higher was considered positive, indicating prior exposure to HCMV (anti‐HCMV IgG) or acute infection with HCMV (anti‐HCMV IgM); values between 0.9 and 1.1 were considered equivocal.
Continuous variables are expressed as mean ± standard deviation (SD). Univariate associations were explored with frequency tables and Pearson's χ2 ‐tests for independent proportions. Continuous variables were compared with t‐tests or appropriate non‐parametric tests, depending on their distribution. Initial analyses were performed on the entire study group; patients were then stratified into the two major stroke subtypes: ischemic and hemorrhagic. Odds ratios (ORs) with corresponding 95% confidence intervals (CI) and adjusted OR for age, sex, BMI, hypertension, and smoking were performed by a logistic regressions model with age, sex, BMI, hypertension, and smoking as independent variables. Data were analyzed using SAS statistical software (version 9.2, SAS Institute Inc, Drive Cany, NC, USA). P < 0.05 was considered statistically significant.
Results
Table 1 reports patients’ clinical characteristics. Mean age, BMI, and frequency of smoking among patients were not significantly different from controls. SBP and DBP in stroke patients were significantly higher than in controls.
Table 1.
Clinical characteristics of study participants
| Patients with stroke (n = 200) | Controls (n = 200) | P value | |
|---|---|---|---|
| Age (years)a | 63.7 ± 8.3 | 63.4 ± 10.9 | 0.718 |
| Sex (% male) | 46.5 | 67.5 | <0.0001 |
| BMI (kg/m2)a | 25.3 ± 3.5 | 24.7 ±3.3 | 0.115 |
| SBP(mmHg)a | 148 ± 22.4 | 139 ± 21.3 | <0.0001 |
| DBP (mmHg)a | 88.1 ± 13.6 | 84.1 ± 11.9 | 0.002 |
| Hypertension (% yes) | 85.0 | 65.5 | 0.0001 |
| Smokers (%) | 30.8 | 32.4 | 0.754 |
aContinuous variables are expressed as mean ± standard deviation.
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Using quantitative PCR assays, we detected HCMV DNA in 110 of 200 plasma samples from patients with stroke and in 47 of 200 control samples (55.0% vs. 23.5%; P < 0.0001; Figure 1A). HCMV seropositivity increased the risk of stroke (unadjusted OR, 3.98; 95% CI, 2.59–6.11; P < 0.0001; Table 2). Risk was also increased for the individual stroke subtypes (ischemic stroke: unadjusted OR, 4.01; 95% CI, 2.57–6.24; P < 0.0001; hemorrhagic stroke: unadjusted OR, 3.80; 95% CI, 1.64–8.78; P= 0.0018). Positivity for HCMV DNA still increased the risk of ischemic and hemorrhagic stroke after adjustment for age, sex, BMI, hypertension, and smoking (ischemic stroke: adjusted OR, 4.07; 95% CI, 2.52–6.32; P < 0.0001; hemorrhagic stroke: adjusted OR, 3.88; 95% CI, 1.61–9.36; P= 0.0026). HCMV seropositivity was significantly higher in patients compared to controls, but the geometric mean of HCMV titers in stroke patients and controls did not show significant differences (224 copies/mL vs. 254 copies/mL; P= 0.496). The rate of anti‐HCMV lgG and anti‐HCMV lgM positivity were not significantly different between stroke patients and controls (98.50% vs. 97.50%, P= 0.48; 1.00% vs. 2.00%, P= 0.41; respectively; Figure 1B).
Figure 1.
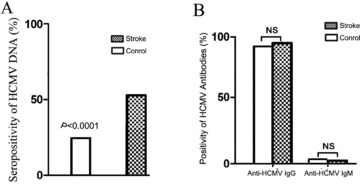
HCMV status in patients with stroke and controls. (A) Comparison of HCMV seropositivity in the stroke group versus the control group; P values calculated by chi‐square test. (B) Rate of anti‐HCMV IgG and anti‐HCMV IgM seropositivity in stroke patients and controls; P values were calculated by Fisher's exact test.
Table 2.
Case‐control study showing association between HCMV and stroke
| Unadjusted OR (95% CI) | P value | OR (95% CI) | Adjusted P value | |
|---|---|---|---|---|
| All stroke | 3.98 (2.59–6.11) | <0.0001 | 3.98 (2.50–6.32) | <0.0001 |
| Ischemic | 4.01 (2.57–6.24) | <0.0001 | 4.07 (2.52–6.58) | <0.0001 |
| Hemorrhagic | 3.80 (1.64–8.78) | 0.0018 | 3.88 (1.61–9.36) | 0.0026 |
Odds ratio adjusted for age, sex, body mass index (BMI), hypertension, and smoking.
Discussion
This is the first report demonstrating a direct link between HCMV infection and stroke, including both ischemic and hemorrhagic types. In the present work, we concentrated on DNA detection because it can reveal both productive and latent infections. After logistic regression adjusting for confounding risk factors, HCMV DNA seropositivity remained the strongest predictor of stroke. Moreover, among the various factors potentially involved in the pathogenesis of stroke, a latent HCMV infection has been recently emphasized by Yi et al. [15]. These authors reported that the presence of HCMV DNA and antigens in the internal carotid arteries collected from 35 patients with ischemic stroke. Levels of HCMV IE gene/protein were significantly higher in the stroke group than in control group detected by the three methods (immunohistochemistry 34.3% vs. 10.0%; hybridization in situ 40.0% vs. 10.0; PCR 60.0% vs. 30.0%) [15].
The underlying pathophysiological mechanism linking HCMV with stroke is yet to be determined. HCMV infection exerts an inhibitory effect on eNOS activation and reduces nitric oxide (NO) production via activation of the stress‐response signal P38‐MAPK pathway and upregulation of PTEN, leading to endothelial dysfunction [11, 16]. HCMV infection stimulates renin and angiotensin II expression in human vascular endothelial cells [17], thus enhancing NAD(P)H oxidase activity, and elicits the production of reactive oxygen species (ROS). HCMV infection of smooth muscle cells also generates ROS through a COX‐2‐dependent pathway, thereby activating NF‐κB [18]. NF‐κB is associated with endothelial dysfunction and vascular inflammation, as well as vascular smooth muscle cell proliferation [19]. HCMV‐infected cells also secrete a number of cytokines (interleukin‐6, tumor necrosis factor‐α), chemokines (monocyte chemoattractant protein‐1, macrophage inflammatory protein‐1α and macrophage inflammatory protein‐1β), growth factors (fibroblast growth factor, platelet‐derived growth factor, vascular endothelial growth factor, transforming growth factor‐β), and adhesion molecules (intercellular adhesion molecule, E‐adherin), some of which play important roles in the migration and proliferation of smooth muscle and endothelial cells [20]. These findings may provide a mechanistic basis for the association between HCMV infection and vascular diseases, including stroke.
There are some limitations of the present study. Our findings from Chinese stroke patients may not be generalizable, as HCMV infection rates may vary across populations. Furthermore, whether ongoing HCMV infection is related to plaque instability, and subsequent acute cerebral ischemia, is relatively unknown. Because the present study was an observational cross‐sectional study, further large‐scale studies with longer observation periods are needed to determine a definite cause‐and‐effect relationship between HCMV and stroke.
In conclusion, HCMV seropositivity is positively associated with stroke and is independent of other stroke risk factors. Our study provides important insights into the mechanisms of stroke and may offer potential therapeutic targets.
Disclosures
None.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
The authors thank the staff and participants of the SHINING Study for their important contributions. We also thank Dr. Hongxi Zhao (Shanghai ZJ Bio‐Tech Co., Ltd.) for technical assistance. This work is supported by grants from the National Natural Science Foundation of China (No.81170244 and 81170090), Beijing Nova Program (No.2009B39), Beijing Natural Science Foundation (No.7102057), Science Foundation for Distinguished Young Scholars of Fujian Province (No.2009D015), Science Foundation for Distinguished Young Scholars of Xiamen (No.3502Z20116009).
The first two authors contributed equally to this study.
References
- 1. Lloyd‐Jones D, Adams R, Carnethon M, et al Heart disease and stroke statistics—2009 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009;119:480–486. [DOI] [PubMed] [Google Scholar]
- 2. Strong K, Mathers C, Bonita R. Preventing stroke: Saving lives around the world. Lancet Neurol 2007;6:182–187. [DOI] [PubMed] [Google Scholar]
- 3. Jiang B, Wang WZ, Chen H, et al Incidence and trends of stroke and its subtypes in China: Results from three large cities. Stroke 2006;37:63–68. [DOI] [PubMed] [Google Scholar]
- 4. Stoll G, Bendszus M. Inflammation and atherosclerosis: Novel insights into plaque formation and destabilization. Stroke 2006;37:1923–1932. [DOI] [PubMed] [Google Scholar]
- 5. Zhang XH, Lei H, Liu AJ, Zou YX, Shen FM, Su DF. Increased oxidative stress is responsible for severer cerebral infarction in stroke‐prone spontaneously hypertensive rats. CNS Neurosci Ther 2011;17:590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Speir E, Modali R, Huang ES, et al Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science 1994;265:391–394. [DOI] [PubMed] [Google Scholar]
- 7. Zhou YF, Leon MB, Waclawiw MA, et al Association between prior cytomegalovirus infection and the risk of restenosis after coronary atherectomy. N Engl J Med 1996;335:624–630. [DOI] [PubMed] [Google Scholar]
- 8. Li S, Zhu J, Zhang W, et al Signature microRNA expression profile of essential hypertension and its novel link to human cytomegalovirus infection. Circulation 2011;124:175–184. [DOI] [PubMed] [Google Scholar]
- 9. Haarala A, Kähönen M, Lehtimäki T, et al Relation of high cytomegalovirus antibody titers to blood pressure and brachial artery flow‐mediated dilation in young men:the cardiovascular risk in young finns study. Clin Exp Immunol 2012;167:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weis M, Kledal TN, Lin KY, et al Cytomegalovirus infection impairs the nitric oxide synthase pathway: Role of asymmetric dimethylarginine in transplant arteriosclerosis. Circulation 2004;109:500–505. [DOI] [PubMed] [Google Scholar]
- 11. Grahame‐Clarke C. Human cytomegalovirus, endothelial function and atherosclerosis. Herpes 2005;12:42–45. [PubMed] [Google Scholar]
- 12. Ridker PM, Hennekens CH, Stampfer MJ, Wang F. Prospective study of herpes simplex virus, cytomegalovirus, and the risk of future myocardial infarction and stroke. Circulation 1998;98:2796–2799. [DOI] [PubMed] [Google Scholar]
- 13. Haider AW, Wilson PW, Larson MG, et al The association of seropositivity to Helicobacter pylori, Chlamydia pneumoniae, and cytomegalovirus with risk of cardiovascular disease: A prospective study. J Am Coll Cardiol 2002;40:1408–1413. [DOI] [PubMed] [Google Scholar]
- 14. Wu L, Shen Y, Liu X, et al The 1425G/A SNP in PRKCH is associated with ischemic stroke and cerebral hemorrhage in a Chinese population. Stroke 2009;40:2973–2976. [DOI] [PubMed] [Google Scholar]
- 15. Yi L, Lin JY, Gao Y, Feng ZJ, Wang DX. Detection of human cytomegalovirus in the atherosclerotic cerebral arteries in Han population in China. Acta Virol 2008;52:99–106. [PubMed] [Google Scholar]
- 16. Shen YH, Zhang L, Utama B, et al Human cytomegalovirus inhibits Akt‐mediated eNOS activation through upregulating PTEN (phosphatase and tensin homolog deleted on chromosome 10). Cardiovasc Res 2006;69:502–511. [DOI] [PubMed] [Google Scholar]
- 17. Cheng J, Ke Q, Jin Z, et al Cytomegalovirus infection causes an increase of arterial blood pressure. PLoS Pathog 2009;5:e1000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Speir E, Yu ZX, Ferrans VJ, Huang ES, Epstein SE. Aspirin attenuates cytomegalovirus infectivity and gene expression mediated by cyclooxygenase‐2 in coronary artery smooth muscle cells. Circ Res 1998;83:210–216. [DOI] [PubMed] [Google Scholar]
- 19. Kowalik TF, Wing B, Haskill JS, Azizkhan JC, Baldwin AS, Jr ., Huang ES. Multiple mechanisms are implicated in the regulation of NF‐kappa B activity during human cytomegalovirus infection. Proc Natl Acad Sci U S A 1993;90:1107–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dumortier J, Streblow DN, Moses AV, et al Human cytomegalovirus secretome contains factors that induce angiogenesis and wound healing. J Virol 2008;82:6524–6535. [DOI] [PMC free article] [PubMed] [Google Scholar]


