Summary
Cerebral ischemia is a severe outcome that could cause cognitive and motor dysfunction, neurodegenerative diseases and even acute death. Although the existence of autophagy in cerebral ischemia is undisputable, the consensus has not yet been reached regarding the exact functions and influence of autophagy in cerebral ischemia. Whether the activation of autophagy is beneficial or harmful in cerebral ischemia injury largely depends on the balance between the burden of intracellular substrate targeted for autophagy and the capacity of the cellular autophagic machinery. Furthermore, the mechanisms underlying the autophagy in cerebral ischemia are far from clear yet. This brief review focuses on not only the current understanding of biological effects of autophagy, but also the therapeutic potentials of autophagy in ischemic stroke. There are disputes over the exact role of autophagy in cerebral ischemia. Application of chemical autophagy inhibitor (e.g., 3‐methyladenine) or inducer (e.g., rapamycin) in vitro and in vivo was reported to protect or harm neuronal cell. Knockdown of autophagic protein, such as Beclin 1, was also reported to modulate the cerebral ischemia‐induced injury. Moreover, autophagy inhibitor abolished the neuroprotection of ischemic preconditioning, implying a neuroprotective effect of autophagy. To clarify these issues on autophagy in cerebral ischemia, future investigations are warranted.
Keywords: Autophagy, Cell death, Cerebral ischemia
Introduction
Ischemic stroke, often resulted from hypoxic ischemic encephalopathy and acute cerebrovascular accident, is a leading cause of mortality and morbidity worldwide 1. It could induce severe cognitive and motor dysfunction, neurodegenerative diseases and even acute death 1. The tissue plasminogen activator (tPA) is the only therapy for acute cerebral ischemia approved by the Food and Drug Administration of United States at present 2, 3. However, the strict 3‐h time window for the tPA treatment is the main barrier to acute intravenous thrombolysis 2, 3. Thus, identification or exploration of novel therapeutic targets becomes a major challenge and task in the field.
Many molecular mechanisms, including excitotoxicity, periinfarct depolarization, inflammation, oxidative stress, calcium overload and programmed cell death, contribute to ischemic cerebral damage 4. The apoptosis and necrosis attributing to the neuronal cell death caused by ischemia have been intensively studied 5. In recent years, autophagy has been discovered to be an important mechanism adopted by many different types of cells for determining their fate. In cells, autophagy is responsible for degradation of most superfluous proteins and organelles. The cytoplasmic constituents, including organelles, are sequestered into double‐membrane autophagosomes, which subsequently fuse with lysosomes where their contents are degraded. Although it is still a topic of debate whether autophagy is a mechanism of cell survival or cell death, the importance of autophagy in various biological and pathological processes is widely accepted 6.
As an intracellular bulk degradation system that is found ubiquitously in eukaryotes, autophagy plays important roles in many physiological processes, including development/differentiation 7, 8, 9, 10, immunity 11, 12, 13, 14, metabolism 15, 16, 17, 18, 19 and aging 20, 21, 22, 23, as well as pathophysiological processes, including neurodegeneration 24, 25, 26, 27, 28, diabetes 16, 29, 30, 31, obesity 32, 33, cancer 34, 35, 36, 37, 38, and inflammation 11, 39, 40, 41. Also, autophagic flux has been found in multiple ischemic diseases, such as myocardial infarction 42, 43, 44, kidney ischemia 22, liver ischemia 45, 46, and cerebral ischemia 47, 48. Generally, in the neuronal system, moderate autophagy is thought to be neuroprotective because autophagy helps to clear aggregated‐protein associated with neurodegeneration 49, 50, 51, 52, 53, 54. Inadequate or defective autophagy may lead to neuronal cell death, while excess autophagy, often triggered by intensive stress, can also promote neuronal cell death 47, 55. The existence of autophagy in ischemic stroke has been found for many years; however, it is not sure whether autophagy plays a protective role in ischemic cerebral injury or not yet 56, 57. More and more reports regarding the involvement of autophagy in cerebral ischemic stroke have been published in recent years. These reports have brought attention to the novel recruitment and elaborate regulatory mechanisms of autophagy in cerebral ischemia. Thus, this review paper focuses on the updated role of autophagy in cerebral ischemia and neuronal damage.
Autophagy in Central Nervous System
Autophagy, [from the Greek roots “auto” (self) and “phagy” (eating)] which was first described in 1963, mainly refers to the cellular catabolic processes in which cytoplasmic target material is transported to lysosomes for degradation as an evolutionarily conserved mechanism from yeast to mammals 58. At least three forms, which include macroautophagy, chaperone‐mediated autophagy and microautophagy, have been identified in mammals 58. The macroautophagy is the most well‐studied form of autophagy. In macroautophagy, double‐membraned vacuoles are generated, called autophagosomes, which sequester cytoplasmic material before delivering it to the lysosome for degradation 59. Macroautophagy is regulated by nutrition status and AMP‐activated protein kinase (AMPK) 60, 61, 62, 63, a sensor of cellular energy. In the process of macroautophagy, several autophagic factors, such as Beclin 1 64, 65, LC3 66, p62 67, 68, 69, 70, and ULK1 71, 72, 73, 74, regulate the intensity and duration of macroautophagy. Chaperone‐mediated autophagy 25, 75 involves selective translocation of the cytosolic proteins that are marked by a pentapeptide motif with a consensus sequence similar to KFERQ across the lysosomal membrane, while cytosolic chaperones aid in the target recognition and unfolding. In this process, the lysosomal‐associated membrane protein‐2α (LAMP‐2α) is thought to be rate‐limiting for target translocation into lysosomes. Microautophagy, a poorly understood phenomenon in mammalian cells, refers to a process where the lysosome itself takes up small portions of cytoplasm by pinching off a vesicle 76, 77. Because macroautophagy is the major autophagy–lysosomal proteolytic pathway identified in central nervous system 78, the term “autophagy” refers to the macroautophagy in this review. All the above‐mentioned observation of autophagy was from animal model and there is no direct evidence of autophagy in ischemic stroke in human yet. However, the autophagy in other neurological diseases, such as Alzheimer's disease, has been showed in human brain tissue 26, 79.
Evidence for the Autophagy in Cerebral Ischemic Injury
Accumulating evidence has shown that autophagy is activated in brain tissues or neuronal cells after ischemic stimulation. Individual or combined evidence is provided mainly by morphological observation from electronic microscope, immunohistochemistry, immunofluorescence, and immunoblotting assays.
Evidence from Electronic Microscope
Electronic microscope is widely used in autophagy research and discovered the first autophagy more than 50 years ago 80. The use of electron microscopy is a valid and important method for observation of changes in various autophagic structures that sequentially form the phagophore, autophagosome, and autolysosome 81. Electronic microscope validated the first autophagy in the hippocampus CA1 pyramidal neurons after transient global cerebral ischemia in 1995 82. It was found that the volume density of cathepsin B‐positive lysosomes markedly increased 3 days after ischemic insult, while the autophagic vacuole‐like structures also increased at this stage. Adhami et al. 83 reported vacuole associated cortical neurons damage in an adult mouse ischemia‐hypoxia model, which ranges from cells harboring multiple cytoplasmic vacuoles to cells completely lacking cytoplasmic contents, suggesting the existence of autophagosomal–lysosomal in neurons in ischemic stroke. Many later documents also reported similar results 84, 85, 86, 87, 88, 89. Electronic microscopy demonstrated that autophagy was not only induced in neurons, but also in glial cells during ischemic stroke 90.
Evidence from Assays of Autophagosomal Marker Proteins
LC3 is a mammalian homolog of the yeast Atg8, which is a specific constituent of the autophagosomal membrane 91. Therefore, the immunohistochemistry or immunofluorescence of LC3 was used to observe the “punctate LC3” after autophagy, while the immunoblotting is applied to detect the ratio of two forms of LC3 (LC3‐II to LC3‐I) that could be used to estimate the abundance of autophagosomes. Moreover, Beclin 1, a mammalian ortholog of the yeast Atg6 that is required for autophagosome formation, is also used as a marker of autophagy activation.
Using transgenic GFP‐LC3 mice, Adhami et al. 83 found that cerebral ischemia–hypoxia causes redistribution of LC3 proteins. In addition, the difference of LC3 fluorescence intensity between the ischemic and contralateral brain tissue on neonatal or adult rodents has been observed at different time points after hypoxia or ischemia 86, 92, 93, 94, 95. Upregulation of Beclin 1 induced by cerebral ischemia was also showed by many reports 48, 96, 97. Recently, using in vivo imaging technology, Tian et al. 98 showed that autophagic GFP‐LC3‐positive cells were primarily neurons, not astroglial or microglial cells, and the number of autophagic GFP‐LC3 cells was greater in the peri‐ischemic area than in the core.
A Double‐Edged Sword: The Role of Autophagy in Ischemic Cerebral Injury
Numerous data have demonstrated that autophagy is activated by ischemic insult in various models, and the elevated autophagic activity could be regulated by a wide range of interventions, mainly including pharmacological and genetic methods (summarized in Table 1). There is no question that disrupting the autophagic process in brain is deleterious, particularly for the lifespan of the animal, resulting in the accumulation of dysfunctional or aging macromolecules and organelles 99, 100. However, upon the acute cerebral ischemia stress, whether autophagy plays a beneficial or harmful role in the survival of neuronal cells is not an easy question. Adhami et al. 83 showed for the first time that many damaged neurons displayed features of autophagic/lysosomal cell death, and very few cells completed the apoptosis process in cerebral ischemic stress. This result suggested that the damaged neuronal cells can exhibit multiple forms of cell death morphological features, and autophagy is only one kind of cell death during ischemic injury. Alternatively, autophagy may protect neurons by degrading damaged organelles to abrogate apoptosis or generating energy to delay the onset of ionic imbalance and necrosis after cerebral ischemia–hypoxia. However, these early reports did not determine the exact role of autophagy. Dozens of later investigations pointed out the complex effects of autophagy in cerebral ischemia. The autophagy and the controversial impacts of autophagy on cerebral ischemic injury as a double‐edged sword have been uncovered.
Table 1.
In vivo role of autophagy in cerebral ischemic injury
| Animals | Model | Phenotypes | Effect of autophagy in cerebral ischemia injury | References |
|---|---|---|---|---|
| CD‐1 mice | tMCAO | NAD+ inhibited autophagy | Harmful | 105 |
| ICR mice | tMCAO | Edaravone inhibited autophagy | Harmful | 113 |
| CBS+/− mice | tMCAO | Tetrahydrocurcumin inhibited autophagy | Harmful | 112 |
| SOD2−/+ mice | tMCAO | SOD2 knockdown inhibited autophagy | Protective | 118 |
| STZ‐induced diabetic mice | tCCAO | Autophagy is associated with amyloid‐beta generation in diabetic mellitus | Harmful | 115 |
| tCCAO | 3‐n‐Butylphthalide inhibited autophagy | Harmful | 116 | |
| Adult SD rats | pMCAO | GSK‐3 inhibitor enhanced autophagy | Protective | 119 |
| pMCAO | Nampt overexpression enhanced autophagy in early stage | Protective | 95 | |
| tMCAO | Hyperbaric oxygen preconditioning enhanced autophagy | Protective | 96 | |
| pMCAO | Focal cerebral ischemic preconditioning enhanced autophagy | Protective | 127 | |
| 4VO | 2‐methoxyestradiol inhibited autophagy | Harmful | 111 | |
| pMCAO | Autophagy inhibitors is neuroprotective | Harmful | 102 | |
| tMCAO | Beclin 1 knockdown is neuroprotective | Harmful | 89 | |
| tMCAO | β‐asarone inhibited autophagy | Harmful | 107 | |
| tMCAO | TMEM166 induced autophagy | Harmful | 109 | |
| pMCAO | Autophagy inhibitors are neuroprotective | Harmful | 103 | |
| Adult Wistar rats | tMCAO | GNDF and HGF inhibited autophagy | Harmful | 104 |
| Adult Zucker fatty rats | tMCAO | Amlodipine and atorvastatin inhibited autophagy | Harmful | 111 |
| Neonatal SD rats | ischemia‐hypoxia | Rapamycin enhanced autophagy | Protective | 86, 93 |
| dMCAO | Autophagy inhibitor is neuroprotective | Harmful | 88 | |
| Neonatal Wistar rats | ischemia‐hypoxia | Lithium inhibited autophagy | Harmful | 108 |
SD, Sprague Dawley; 2VO, occlusion of bilateral common carotid arteries; 4VO, 4 vessels occlusion; tMCAO/pMCAO/dMCAO, transient/permanent/distal middle cerebral artery occlusion; tCCAO, transient common carotid artery occlusion; IPC, ischemic preconditioning; SOD2, manganese superoxide dismutase; GNDF, glial gell line‐derived neurotrophic factor; HGF, hepatocyte growth factor; Nampt, nicotinamide phosphoribosyltransferase; NAD+, nicotinamide adenine dinucleotide; TMEM166, transmembrane protein 166.
Detrimental role of Autophagy in Ischemic Cerebral Injury
In 2001, Uchiyama 101 showed that autophagy was induced from the early stage of the glucose–oxygen deprivation in PC12 neuron cells. Administration of autophagy inhibitor 3‐methyladenine (3‐MA) protected the PC12 cells from apoptosis, indicating that autophagy may lead to neuronal cell death. Mice deficient in Atg7, a necessary catalyst in both conjugation systems for autophagy, showed nearly complete protection from cerebral ischemia‐induced caspase‐3 activation and neuron death, supporting that autophagy plays an essential role in triggering neuronal death execution after ischemic injury in brain 102. In addition, focal cerebral ischemia induced by permanent middle cerebral artery occlusion increased the formation of autophagosomes and autolysosomes and expression of LC3‐II and cathepsin B 102. Autophagy inhibitor 3‐MA reduced infarct volume, brain edema and motor deficits via inhibiting the ischemia‐induced upregulation of LC3‐II and cathepsin B 102, 103. Also, in a study in neonatal cerebral ischemia, postischemic intracerebroventricular injections of 3‐MA reduced the lesion volume even when given > 4 h after the beginning of the ischemia 88. RNA interference‐mediated downregulation of Beclin 1 inhibited autophagy and attenuated cerebral ischemic injury in rats 89. Two neurotrophic factors, glial cell line‐derived neurotrophic factor (GDNF) and hepatocyte growth factor (HGF), decreased the numbers of LC3‐positive cells, suggesting that the protective effects of GDNF and HGF were closely associated with their antiautophagic effects 104. Moreover, various agents, including NAD+ 105, propofol 106, β‐asarone 107, lithium 108, transmembrane protein 166 109, ginsenoside Rb1 110, 2‐methoxyestradiol 111, tetrahydrocurcumin 112, edaravone 113, and selenite 114, were reported to decrease ischemic brain damage by blocking autophagy process. In these reports, autophagy caused energy depletion, DNA fragmentation, apoptotic signaling pathways activation and severe damage in intracellular components.
Autophagy is also found to be involved in diseases associated with cerebral ischemia. Zhang et al. 115 showed that exacerbation of ischemia‐induced amyloid‐beta generation by diabetes might be associated with autophagy activation in mouse brain. Also, 3‐n‐butylphthalide attenuated amyloid‐beta protein generation promoted by diabetes in ischemia through inhibiting abnormally activated neuronal autophagy 116. Similarly, Zhang et al. 117 demonstrated that neuroprotective effects of amlodipine and atorvastatin in metabolic syndrome model of Zucker fatty rats involved their antiautophagic effect.
Beneficial Role of Autophagy in Cerebral Ischemic Injury
A lot of direct evidence has also been demonstrated on the beneficial role of autophagy in ischemic injury. In focal cerebral ischemia, the brain neuronal cells over‐expressing Beclin 1 were found to exhibit damaged DNA but without changes in nuclear morphology, indicating that not all the autophagic cells are predestined to die 48. 3‐MA and wortmannin, two autophagy inhibitors, significantly reduced Beclin 1 expression and switched the mechanism of the cell death mode from apoptosis to necrosis 85, 93. Conversely, rapamycin, which increases autophagy, augmented Beclin 1 expression, reduced necrotic cell death, and decreased brain injury 93, suggesting that inhibition of autophagy might help to switch the mechanism of cell death from apoptotic to necrotic 93. The same research group provided further evidence showing that the inhibition of Akt/CREB signaling pathway by wortmannin could influence autophagy, and autophagy can be part of an integrated prosurvival signaling, which includes the PI3K‐Akt‐mammalian target of rapamycin (mTOR) axis 86. Many other mechanisms were also discovered. SOD2 knockdown exacerbated ischemic brain damage under hyperglycemic conditions via increased oxidative stress and DNA oxidation, which was associated with suppression of autophagy regulators 118. GSK‐3β inhibitor suppressed neuroinflammation by activating autophagy after ischemic brain injury, thus suggesting that GSK‐3β is a new target for prevention of ischemic brain injury 119. Accumulation of p62 under hypoxic stress promotes neuronal cell death, which was partly blocked by autophagy inducer lithium chloride 120, supporting that autophagy promotes neuronal cell survival under hypoxic stress. Melatonin, an antioxidant product, promoted neuron cell survival in glucose–oxygen deprivation, while autophagy inhibitor 3‐MA totally blocked the neuroprotection of melatonin 121, suggesting that autophagy is possibly one of the mechanisms underlying neuroprotection of melatonin. Our group demonstrated that induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase (Nampt) in the early stage of cerebral ischemia 95. Overexpression of Nampt increased LC3 puncta immunochemistry staining, LC3‐II/Beclin 1 expression and autophagosomes number both in vivo and in vitro at 2 h after cerebral ischemia. At the early stage of OGD, autophagy inducer rapamycin protected against neuronal injury induced by Nampt knockdown, whereas autophagy inhibitor 3‐MA abolished the neuroprotective effect of Nampt partly. Overexpression or knockdown of Nampt regulated the phosphorylation of mTOR and S6K1 signaling pathway upon OGD stress through enhancing phosphorylation of TSC2 at Ser1387 but not Thr1462 site. All these phenotypes are SIRT1 dependent. Of note, the beneficial effect of autophagy in glial cells, such as astocytes following glucose and oxygen deprivation and focal cerebral ischemia, was also observed 90.
Essential Role of Autophagy in Ischemic Preconditioning and Hyperbaric Oxygen Preconditioning
Ischemic preconditioning is a short period of ischemia followed by a brief period of reperfusion before a sustained ischemic insult, which was found to be a powerful method for limiting cerebral ischemia‐induced tissue damage 122. According to several recent studies, autophagy was believed to play an essential role in ischemic preconditioning‐induced protection in many organs 22, 45, 123, 124. Atg3, an autophagic gene, was found to be upregulated by ischemic preconditioning but downregulated by prolonged ischemia 125, suggesting that the activation of autophagy is a specific response to ischemic preconditioning. In cultured PC12 cells, Park et al. 126 showed that ischemic preconditioning markedly increased LC3‐II bands, cathepsin D positive cells, lysosomal activity and autophagic vacuoles, and inhibition of autophagy by 3‐MA ameliorated the neuroprotective effects of ischemic preconditioning. This phenotype was confirmed in an in vivo rat model of focal cerebral ischemia 127. Moreover, endoplasmic reticulum (ER) stress inhibitor recovered ischemic preconditioning‐induced neuroprotection in the presence of 3‐MA 128, suggesting that preactivation of autophagy by ischemic preconditioning can boost endogenous defense mechanisms to upregulate molecular chaperones, and hence reduce excessive ER stress during fatal ischemia. Activation of PI3K‐Akt‐mTOR axis by autophagy might also be crucial for ischemic preconditioning 129.
Hyperbaric oxygen preconditioning has been used for multiple neurological diseases including ischemic stroke and proved to be a safe treatment in all age and gender groups 130. The protein expression of LC3‐II and Beclin 1 and the formation of autophagosomes were increased by hyperbaric oxygen preconditioning, even higher than those in ischemia. Blockade of autophagy by 3‐MA attenuated the neuroprotection of hyperbaric oxygen preconditioning against cerebral ischemia 96.
Issues in the Autophagy‐Related Studies on Cerebral Ischemia
In this field, two things are irrefutable. First, autophagy is a fundamental intracellular process and disruption of autophagy in brain for long durations such as knockout autophagy‐related genes is detrimental. Second, activation of autophagy in various types of neuronal cells is observed in experimental models of brain injury. Nevertheless, it seems that it is very hard to conduct an unambiguous conclusion on the role of autophagy in cerebral ischemic injury. In our opinion, there may be several issues in the current autophagy‐related studies on cerebral ischemia.
One of the key issues is that the used chemical agents are nonspecific to autophagy. For example, it seemed that 3‐MA was used widely as an autophagy inhibitor in both in vitro and in vivo studies. In fact, 3‐MA inhibits autophagy via inhibiting PI3K activation 131. In another word, the observed effects of 3‐MA in many studies might not truly reflect the inhibition of autophagy but the inhibition of PI3K‐class III. We know that Akt‐PI3K signaling indeed critically contributes to autophagy 132, 133, 134, 135, 136, 137, 138, 139; however, Akt‐PI3K signaling also has impacts on other biological functions, such as apoptosis and necrosis 140, 141. Thus, the nonspecific effects of 3‐MA may not be excluded in autophagy‐related researches. In contrast to 3‐MA, rapamycin is an autophagy inducer. It has been used to augment autophagy after cerebral ischemia insult to examine the effect of enhanced autophagy on neuronal injury. The proautophagic activity of rapamycin is due to its inhibitory effect on mTOR, which also results in immunosuppressive and antiproliferative properties 142, 143, 144. Besides the regulatory effect on autophagy, the mTOR plays important roles in growth and metabolism 142, 143. Therefore, it may be hard to separate out these multiple influences in some studies. Therefore, the experimental conditions of inhibitor application and their side effects must be carefully considered.
Another important issue is the reliability of the assays for monitoring autophagy in mouse or rat cerebral ischemia models. Importantly, there are no absolute criteria for evaluating the autophagy activation that apply to every situation. This is because some assays are inappropriate, problematic or may not work at all in particular cells, tissues, or organisms 81, 145, 146. There are many acceptable methods to measure macroautophagy in higher eukaryotes summarized in three recent reviews 81, 145, 146. Here, we emphasize that no individual assay is guaranteed to be the most appropriate one in every situation, and we strongly recommend the use of multiple assays to monitor autophagy.
Conclusions and Future Perspectives
Although there are disputes over the exact role of autophagy in cerebral ischemia, there is no doubt that autophagy critically contributes to the neuronal fate upon cerebral ischemic stress. How do we reconcile the divergent experimental data on autophagy in ischemic stroke? Cerebral ischemia results in damages to proteins, lipids, and all intracellular components. As a repair mechanism, autophagy is activated to eliminate damaged proteins that accumulate within the neuronal cells. At this stage, autophagy is prosurvival (Figure 1). If the ischemic stress persists for a long time, the autophagy intensity is strengthened consistently. In this case, not only the autophagy is further increased (“supply”), but also the cellular burden of damaged and/or dysfunctional macromolecules and organelles (“demand”) is increased (Figure 1). The neuronal cells can not remove all the autophagosomes to return to its basal state, which at last induces neuronal cell death.
Figure 1.
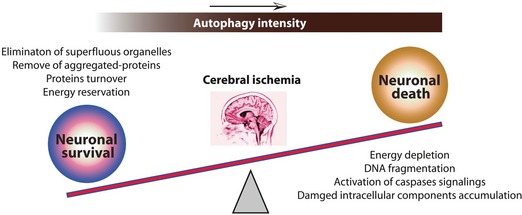
A balance between autophagy‐regulated neuronal death and survival in cerebral ischemic stress. Upon cerebral ischemic stress, basal autophagy in neuronal cells acts as a cytoprotective mechanism and serves homeostatic functions such as superfluous organelles removal, aggregated‐proteins turnover/elimination and energy reservation to provide metabolic substrates for survival. However, the long‐term, uncontrolled and strong, autophagy can digest vital amounts of cell components and survival factors, thus leading to the energy depletion, DNA fragmentation and activation of apoptotic/necrotic signaling pathways, and thereby cell death.
However, there are several questions that need to be clarified in the future. Some questions left unanswered are (1) What is the association between autophagy and apoptosis/necrosis during cerebral ischemia? (2) Is there any special autophagic mechanism in neuronal cells triggered by ischemia? (3) Does the autophagy in ischemic/hyperbaric oxygen preconditioning really contribute to the neuroprotection? (4) Can autophagy inducer mimic the ischemic/hyperbaric oxygen preconditioning? To answer these questions is a challenging task!
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by grants from the National Basic Research Program of China (2009CB521902 to C.‐Y.M.), the National Natural Science Foundation of China (81100866 to P.W., and 81130061 to C.‐Y.M.), the Program of Shanghai Subject Chief Scientist (10XD1405300 to C.‐Y.M.), the Shanghai “Shu Guang” Project (10GG19 to C.‐Y.M.) and the SMMU Young Investigator Foundation (to P.W.).
References
- 1. Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet 2008;371:1612–1623. [DOI] [PubMed] [Google Scholar]
- 2. Yepes M, Roussel BD, Ali C, Vivien D. Tissue‐type plasminogen activator in the ischemic brain: More than a thrombolytic. Trends Neurosci 2009;32:48–55. [DOI] [PubMed] [Google Scholar]
- 3. Liang BA, Lew R, Zivin JA. Review of tissue plasminogen activator, ischemic stroke, and potential legal issues. Arch Neurol 2008;65:1429–1433. [DOI] [PubMed] [Google Scholar]
- 4. Moskowitz MA, Lo EH, Iadecola C. The science of stroke: Mechanisms in search of treatments. Neuron 2010;67:181–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke 2009;40:e331–e339. [DOI] [PubMed] [Google Scholar]
- 6. Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell 2008;132:27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Le Bot N. Autophagy: A new regulator of development. Nat Cell Biol 2007;9:741. [DOI] [PubMed] [Google Scholar]
- 8. Mortensen M, Simon AK. Nonredundant role of Atg7 in mitochondrial clearance during erythroid development. Autophagy 2011;6:423–425. [DOI] [PubMed] [Google Scholar]
- 9. Mizushima N, Levine B. Autophagy in mammalian development and differentiation. Nat Cell Biol 2010;12:823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qu X, Zou Z, Sun Q, et al. Autophagy gene‐dependent clearance of apoptotic cells during embryonic development. Cell 2007;128:931–946. [DOI] [PubMed] [Google Scholar]
- 11. Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature 2011;469:323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lenz HD, Haller E, Melzer E, Gust AA, Nurnberger T. Autophagy controls plant basal immunity in a pathogenic lifestyle‐dependent manner. Autophagy 2011;7:773–774. [DOI] [PubMed] [Google Scholar]
- 13. Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe 2009;5:527–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Virgin HW, Levine B. Autophagy genes in immunity. Nat Immunol 2009;10:461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rabinowitz JD, White E. Autophagy and metabolism. Science 2010;330:1344–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gonzalez CD, Lee MS, Marchetti P, et al. The emerging role of autophagy in the pathophysiology of diabetes mellitus. Autophagy 2011;7:2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xiao S, Chye ML. The Arabidopsis thaliana ACBP3 regulates leaf senescence by modulating phospholipid metabolism and ATG8 stability. Autophagy 2010;6:802–804. [DOI] [PubMed] [Google Scholar]
- 18. Kovsan J, Bashan N, Greenberg AS, Rudich A. Potential role of autophagy in modulation of lipid metabolism. Am J Physiol Endocrinol Metab 2010;298:E1–E7. [DOI] [PubMed] [Google Scholar]
- 19. Kang R, Livesey KM, Zeh HJ 3rd, Lotze MT, Tang D. Metabolic regulation by HMGB1‐mediated autophagy and mitophagy. Autophagy 2011;7:1256–1258. [DOI] [PubMed] [Google Scholar]
- 20. Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell 2011;146:682–695. [DOI] [PubMed] [Google Scholar]
- 21. Lipinski MM, Zheng B, Lu T, et al. Genome‐wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer's disease. Proc Natl Acad Sci U S A 2010;107:14164–14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Isaka Y, Kimura T, Takabatake Y. The protective role of autophagy against aging and acute ischemic injury in kidney proximal tubular cells. Autophagy 2011;7:1085–1087. [DOI] [PubMed] [Google Scholar]
- 23. Bartlett BJ, Isakson P, Lewerenz J, et al. p62, Ref(2)P and ubiquitinated proteins are conserved markers of neuronal aging, aggregate formation and progressive autophagic defects. Autophagy 2011;7:572–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harris H, Rubinsztein DC. Control of autophagy as a therapy for neurodegenerative disease. Nat Rev Neurol 2012;8:108–117. [DOI] [PubMed] [Google Scholar]
- 25. Wang Y, Martinez‐Vicente M, Kruger U, et al. Synergy and antagonism of macroautophagy and chaperone‐mediated autophagy in a cell model of pathological tau aggregation. Autophagy 2010;6:182–183. [DOI] [PubMed] [Google Scholar]
- 26. Scheper W, Nijholt DA, Hoozemans JJ. The unfolded protein response and proteostasis in Alzheimer disease: Preferential activation of autophagy by endoplasmic reticulum stress. Autophagy 2011;7:910–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vila M, Bove J, Dehay B, Rodriguez‐Muela N, Boya P. Lysosomal membrane permeabilization in Parkinson disease. Autophagy 2011;7:98–100. [DOI] [PubMed] [Google Scholar]
- 28. Gray SG. Targeting histone deacetylases for the treatment of Huntington's disease. CNS Neurosci Ther 2010;16:348–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen ZF, Li YB, Han JY, et al. The double‐edged effect of autophagy in pancreatic beta cells and diabetes. Autophagy 2011;7:12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ost A, Svensson K, Ruishalme I, et al. Attenuated mTOR signaling and enhanced autophagy in adipocytes from obese patients with type 2 diabetes. Mol Med 2010;16:235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xie Z, He C, Zou MH. AMP‐activated protein kinase modulates cardiac autophagy in diabetic cardiomyopathy. Autophagy 2011;7:1254–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goldman S, Zhang Y, Jin S. Autophagy and adipogenesis: Implications in obesity and type II diabetes. Autophagy 2010;6:179–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou L, Liu F. Autophagy: Roles in obesity‐induced ER stress and adiponectin downregulation in adipocytes. Autophagy 2010;6:1196–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer 2005;5:726–734. [DOI] [PubMed] [Google Scholar]
- 35. Dewaele M, Maes H, Agostinis P. ROS‐mediated mechanisms of autophagy stimulation and their relevance in cancer therapy. Autophagy 2010;6:838–854. [DOI] [PubMed] [Google Scholar]
- 36. Dalby KN, Tekedereli I, Lopez‐Berestein G, Ozpolat B. Targeting the prodeath and prosurvival functions of autophagy as novel therapeutic strategies in cancer. Autophagy 2010;6:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gargini R, Garcia‐Escudero V, Izquierdo M. Therapy mediated by mitophagy abrogates tumor progression. Autophagy 2011;7:466–476. [DOI] [PubMed] [Google Scholar]
- 38. Guo JY, Chen HY, Mathew R, et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev 2011;25:460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abdulrahman BA, Khweek AA, Akhter A, et al. Autophagy stimulation by rapamycin suppresses lung inflammation and infection by Burkholderia cenocepacia in a model of cystic fibrosis. Autophagy 2011;7:1359–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy‐inflammation‐cell death axis in organismal aging. Science 2011;333:1109–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Luciani A, Villella VR, Esposito S, et al. Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS‐mediated autophagy inhibition. Nat Cell Biol 2010;12:863–875. [DOI] [PubMed] [Google Scholar]
- 42. Matsui Y, Takagi H, Qu X, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of AMP‐activated protein kinase and Beclin 1 in mediating autophagy. Circ Res 2007;100:914–922. [DOI] [PubMed] [Google Scholar]
- 43. Przyklenk K, Undyala VV, Wider J, Sala‐Mercado JA, Gottlieb RA, Mentzer RM Jr. Acute induction of autophagy as a novel strategy for cardioprotection: Getting to the heart of the matter. Autophagy 2011;7:432–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang Y, Ren J. Autophagy in ALDH2‐elicited cardioprotection against ischemic heart disease: Slayer or savior? Autophagy 2010;6:1212–1213. [DOI] [PubMed] [Google Scholar]
- 45. Esposti DD, Domart MC, Sebagh M, et al. Autophagy is induced by ischemic preconditioning in human livers formerly treated by chemotherapy to limit necrosis. Autophagy 2010;6:172–174. [DOI] [PubMed] [Google Scholar]
- 46. Wang JH, Ahn IS, Fischer TD, et al. Autophagy suppresses age‐dependent ischemia and reperfusion injury in livers of mice. Gastroenterology 2011;141(2188–2199):e2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Uchiyama Y, Koike M, Shibata M. Autophagic neuron death in neonatal brain ischemia/hypoxia. Autophagy 2008;4:404–408. [DOI] [PubMed] [Google Scholar]
- 48. Rami A, Langhagen A, Steiger S. Focal cerebral ischemia induces upregulation of Beclin 1 and autophagy‐like cell death. Neurobiol Dis 2008;29:132–141. [DOI] [PubMed] [Google Scholar]
- 49. Zhang X, Li L, Chen S, et al. Rapamycin treatment augments motor neuron degeneration in SOD1 (G93A) mouse model of amyotrophic lateral sclerosis. Autophagy 2011;7:412–425. [DOI] [PubMed] [Google Scholar]
- 50. Jaeger PA, Wyss‐Coray T. All‐you‐can‐eat: Autophagy in neurodegeneration and neuroprotection. Mol Neurodegener 2009;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Madeo F, Eisenberg T, Kroemer G. Autophagy for the avoidance of neurodegeneration. Genes Dev 2009;23:2253–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Medeiros R, Baglietto‐Vargas D, LaFerla FM. The role of tau in Alzheimer's disease and related disorders. CNS Neurosci Ther 2011;17:514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Balin B, Abrams JT, Schrogie J. Toward a unifying hypothesis in the development of Alzheimer's disease. CNS Neurosci Ther 2011;17:587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Berezniuk I, Fricker LD. A defect in cytosolic carboxypeptidase 1 (Nna1) causes autophagy in Purkinje cell degeneration mouse brain. Autophagy 2010;6:558–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chu CT. Eaten alive: Autophagy and neuronal cell death after hypoxia‐ischemia. Am J Pathol 2008;172:284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Smith CM, Chen Y, Sullivan ML, Kochanek PM, Clark RS. Autophagy in acute brain injury: Feast, famine, or folly? Neurobiol Dis 2011;43:52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xu M, Zhang HL. Death and survival of neuronal and astrocytic cells in ischemic brain injury: A role of autophagy. Acta Pharmacol Sin 2011;32:1089–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 2009;43:67–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yang Z, Klionsky DJ. Eaten alive: A history of macroautophagy. Nat Cell Biol 2010;12:814–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 2011;13:1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Loffler AS, Alers S, Dieterle AM, et al. Ulk1‐mediated phosphorylation of AMPK constitutes a negative regulatory feedback loop. Autophagy 2011;7:696–706. [DOI] [PubMed] [Google Scholar]
- 62. Hardie DG. AMPK and autophagy get connected. EMBO J 2011;30:634–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shang L, Wang X. AMPK and mTOR coordinate the regulation of Ulk1 and mammalian autophagy initiation. Autophagy 2011;7:924–926. [DOI] [PubMed] [Google Scholar]
- 64. Djavaheri‐Mergny M, Maiuri MC, Kroemer G. Cross talk between apoptosis and autophagy by caspase‐mediated cleavage of Beclin 1. Oncogene 2010;29:1717–1719. [DOI] [PubMed] [Google Scholar]
- 65. Proikas‐Cezanne T, Codogno P. Beclin 1 or not Beclin 1. Autophagy 2011;7:671–672. [DOI] [PubMed] [Google Scholar]
- 66. Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol 2004;36:2503–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pankiv S, Clausen TH, Lamark T, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 2007;282:24131–24145. [DOI] [PubMed] [Google Scholar]
- 68. Mathew R, Karp CM, Beaudoin B, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell 2009;137:1062–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Komatsu M, Ichimura Y. Physiological significance of selective degradation of p62 by autophagy. FEBS Lett 2010;584:1374–1378. [DOI] [PubMed] [Google Scholar]
- 70. Larsen KB, Lamark T, Øvervatn A, Harneshaug I, Johansen T, Bjørkøy G. A reporter cell system to monitor autophagy based on p62/SQSTM1. Autophagy 2010;6:784–793. [DOI] [PubMed] [Google Scholar]
- 71. Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 2011;13:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jung CH, Seo M, Otto NM, Kim DH. ULK1 inhibits the kinase activity of mTORC1 and cell proliferation. Autophagy 2011;7:1212–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dunlop EA, Hunt DK, Acosta‐Jaquez HA, Fingar DC, Tee AR. ULK1 inhibits mTORC1 signaling, promotes multisite Raptor phosphorylation and hinders substrate binding. Autophagy 2011;7:737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lee JW, Park S, Takahashi Y, Wang HG. The association of AMPK with ULK1 regulates autophagy. PLoS One 2010;5:e15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dice JF. Chaperone‐mediated autophagy. Autophagy 2007;3:295–299. [DOI] [PubMed] [Google Scholar]
- 76. Mijaljica D, Prescott M, Devenish RJ. Microautophagy in mammalian cells: Revisiting a 40‐year‐old conundrum. Autophagy 2011;7:673–682. [DOI] [PubMed] [Google Scholar]
- 77. Santambrogio L, Cuervo AM. Chasing the elusive mammalian microautophagy. Autophagy 2011;7:652–654. [DOI] [PubMed] [Google Scholar]
- 78. Rosello A, Warnes G, Meier UC. Cell death pathways and autophagy in the central nervous system and its involvement in neurodegeneration, immunity and central nervous system infection: To die or not to die–that is the question. Clin Exp Immunol 2012;168:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Haapasalo A, Viswanathan J, Bertram L, Soininen H, Tanzi RE, Hiltunen M. Emerging role of Alzheimer's disease‐associated ubiquilin‐1 in protein aggregation. Biochem Soc Trans 2010;38:150–155. [DOI] [PubMed] [Google Scholar]
- 80. Eskelinen EL, Reggiori F, Baba M, Kovacs AL, Seglen PO. Seeing is believing: The impact of electron microscopy on autophagy research. Autophagy 2011;7:935–956. [DOI] [PubMed] [Google Scholar]
- 81. Klionsky DJ, Abeliovich H, Agostinis P, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 2008;4:151–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nitatori T, Sato N, Waguri S, et al. Delayed neuronal death in the CA1 pyramidal cell layer of the gerbil hippocampus following transient ischemia is apoptosis. J Neurosci 1995;15:1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Adhami F, Liao G, Morozov YM, et al. Cerebral ischemia‐hypoxia induces intravascular coagulation and autophagy. Am J Pathol 2006;169:566–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Liu C, Gao Y, Barrett J, Hu B. Autophagy and protein aggregation after brain ischemia. J Neurochem 2010;115:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang JY, Xia Q, Chu KT, et al. Severe global cerebral ischemia‐induced programmed necrosis of hippocampal CA1 neurons in rat is prevented by 3‐methyladenine: A widely used inhibitor of autophagy. J Neuropathol Exp Neurol 2011;70:314–322. [DOI] [PubMed] [Google Scholar]
- 86. Carloni S, Girelli S, Scopa C, Buonocore G, Longini M, Balduini W. Activation of autophagy and Akt/CREB signaling play an equivalent role in the neuroprotective effect of rapamycin in neonatal hypoxia‐ischemia. Autophagy 2010;6:366–377. [DOI] [PubMed] [Google Scholar]
- 87. Grishchuk Y, Ginet V, Truttmann AC, Clarke PG, Puyal J. Beclin 1‐independent autophagy contributes to apoptosis in cortical neurons. Autophagy 2010;7:1115–1131. [DOI] [PubMed] [Google Scholar]
- 88. Puyal J, Vaslin A, Mottier V, Clarke PG. Postischemic treatment of neonatal cerebral ischemia should target autophagy. Ann Neurol 2009;66:378–389. [DOI] [PubMed] [Google Scholar]
- 89. Zheng YQ, Liu JX, Li XZ, Xu L, Xu YG. RNA interference‐mediated downregulation of Beclin1 attenuates cerebral ischemic injury in rats. Acta Pharmacol Sin 2009;30:919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Qin AP, Liu CF, Qin YY, et al. Autophagy was activated in injured astrocytes and mildly decreased cell survival following glucose and oxygen deprivation and focal cerebral ischemia. Autophagy 2010;6:738–753. [DOI] [PubMed] [Google Scholar]
- 91. Kabeya Y, Mizushima N, Ueno T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 2000;19:5720–5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ginet V, Puyal J, Clarke PG, Truttmann AC. Enhancement of autophagic flux after neonatal cerebral hypoxia‐ischemia and its region‐specific relationship to apoptotic mechanisms. Am J Pathol 2009;175:1962–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Carloni S, Buonocore G, Balduini W. Protective role of autophagy in neonatal hypoxia‐ischemia induced brain injury. Neurobiol Dis 2008;32:329–339. [DOI] [PubMed] [Google Scholar]
- 94. Koike M, Shibata M, Tadakoshi M, et al. Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic‐ischemic injury. Am J Pathol 2008;172:454–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wang P, Guan YF, Du H, Zhai QW, Su DF, Miao CY. Induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemia. Autophagy 2012;8:77–87. [DOI] [PubMed] [Google Scholar]
- 96. Yan W, Zhang H, Bai X, Lu Y, Dong H, Xiong L. Autophagy activation is involved in neuroprotection induced by hyperbaric oxygen preconditioning against focal cerebral ischemia in rats. Brain Res 2011;1402:109–121. [DOI] [PubMed] [Google Scholar]
- 97. Wang YC, Zhang S, Du TY, Wang B, Sun XQ. Hyperbaric oxygen preconditioning reduces ischemia‐reperfusion injury by stimulating autophagy in neurocyte. Brain Res 2010;1323:149–151. [DOI] [PubMed] [Google Scholar]
- 98. Tian F, Deguchi K, Yamashita T, et al. In vivo imaging of autophagy in a mouse stroke model. Autophagy 2010;6:1107–1114. [DOI] [PubMed] [Google Scholar]
- 99. Komatsu M, Waguri S, Ueno T, et al. Impairment of starvation‐induced and constitutive autophagy in Atg7‐deficient mice. J Cell Biol 2005;169:425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Komatsu M, Waguri S, Chiba T, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 2006;441:880–884. [DOI] [PubMed] [Google Scholar]
- 101. Uchiyama Y. Autophagic cell death and its execution by lysosomal cathepsins. Arch Histol Cytol 2001;64:233–246. [DOI] [PubMed] [Google Scholar]
- 102. Wen YD, Sheng R, Zhang LS, et al. Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy 2008;4:762–769. [DOI] [PubMed] [Google Scholar]
- 103. Kubota C, Torii S, Hou N, et al. Constitutive reactive oxygen species generation from autophagosome/lysosome in neuronal oxidative toxicity. J Biol Chem 2010;285:667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Shang J, Deguchi K, Yamashita T, et al. Antiapoptotic and antiautophagic effects of glial cell line‐derived neurotrophic factor and hepatocyte growth factor after transient middle cerebral artery occlusion in rats. J Neurosci Res 2010;88:2197–2206. [DOI] [PubMed] [Google Scholar]
- 105. Zheng C, Han J, Xia W, Shi S, Liu J, Ying W. NAD(+) administration decreases ischemic brain damage partially by blocking autophagy in a mouse model of brain ischemia. Neurosci Lett 2012;512:67–71. [DOI] [PubMed] [Google Scholar]
- 106. Cui D, Wang L, Qi A, Zhou Q, Zhang X, Jiang W. Propofol prevents autophagic cell death following oxygen and glucose deprivation in PC12 cells and cerebral ischemia‐reperfusion injury in rats. PLoS One 2012;7:e35324. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 107. Liu L, Fang YQ, Xue ZF, He YP, Fang RM, Li L. Beta‐asarone attenuates ischemia‐reperfusion‐induced autophagy in rat brains via modulating JNK, p‐JNK, Bcl‐2 and Beclin 1. Eur J Pharmacol 2012;680:34–40. [DOI] [PubMed] [Google Scholar]
- 108. Li Q, Li H, Roughton K, et al. Lithium reduces apoptosis and autophagy after neonatal hypoxia‐ischemia. Cell Death Dis 2010;1:e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Li L, Khatibi NH, Hu Q, et al. Transmembrane protein 166 regulates autophagic and apoptotic activities following focal cerebral ischemic injury in rats. Exp Neurol 2012;234:181–190. [DOI] [PubMed] [Google Scholar]
- 110. Lu T, Jiang Y, Zhou Z, et al. Intranasal ginsenoside Rb1 targets the brain and ameliorates cerebral ischemia/reperfusion injury in rats. Biol Pharm Bull 2011;34:1319–1324. [DOI] [PubMed] [Google Scholar]
- 111. Xin XY, Pan J, Wang XQ, et al. 2‐methoxyestradiol attenuates autophagy activation after global ischemia. Can J Neurol Sci 2011;38:631–638. [DOI] [PubMed] [Google Scholar]
- 112. Tyagi N, Qipshidze N, Munjal C, et al. Tetrahydrocurcumin ameliorates homocysteinylated cytochrome‐c mediated autophagy in hyperhomocysteinemia mice after cerebral ischemia. J Mol Neurosci 2012;47:128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Liu N, Shang J, Tian F, Nishi H, Abe K. In vivo optical imaging for evaluating the efficacy of edaravone after transient cerebral ischemia in mice. Brain Res 2011;1397:66–75. [DOI] [PubMed] [Google Scholar]
- 114. Mehta SL, Kumari S, Mendelev N, Li PA. Selenium preserves mitochondrial function, stimulates mitochondrial biogenesis, and reduces infarct volume after focal cerebral ischemia. BMC Neurosci 2012;13:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhang T, Liu X, Li Q, Wang J, Jia W, Sun X. Exacerbation of ischemia‐induced amyloid‐beta generation by diabetes is associated with autophagy activation in mice brain. Neurosci Lett 2010;479:215–220. [DOI] [PubMed] [Google Scholar]
- 116. Zhang T, Yan W, Li Q, et al. 3‐n‐Butylphthalide (NBP) attenuated neuronal autophagy and amyloid‐beta expression in diabetic mice subjected to brain ischemia. Neurol Res 2011;33:396–404. [DOI] [PubMed] [Google Scholar]
- 117. Zhang X, Deguchi S, Deguchi K, et al. Amlodipine and atorvastatin exert protective and additive effects via antiapoptotic and antiautophagic mechanisms after transient middle cerebral artery occlusion in Zucker metabolic syndrome rats. J Neurosci Res 2011;89:1228–1234. [DOI] [PubMed] [Google Scholar]
- 118. Mehta SL, Lin Y, Chen W, et al. Superoxide dismutase deficiency exacerbates ischemic brain damage under hyperglycemic conditions by altering autophagy. Transl Stroke Res 2011;2:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Zhou X, Zhou J, Li X, Guo C, Fang T, Chen Z. GSK‐3beta inhibitors suppressed neuroinflammation in rat cortex by activating autophagy in ischemic brain injury. Biochem Biophys Res Commun 2011;411:271–275. [DOI] [PubMed] [Google Scholar]
- 120. Tanabe F, Yone K, Kawabata N, et al. Accumulation of p62 in degenerated spinal cord under chronic mechanical compression: Functional analysis of p62 and autophagy in hypoxic neuronal cells. Autophagy 2011;7:1462–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Guo Y, Wang J, Wang Z, Yang Y, Wang X, Duan Q. Melatonin protects N2a against ischemia/reperfusion injury through autophagy enhancement. J Huazhong Univ Sci Technolog Med Sci 2010;30:1–7. [DOI] [PubMed] [Google Scholar]
- 122. Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci 2006;7:437–448. [DOI] [PubMed] [Google Scholar]
- 123. Huang C, Yitzhaki S, Perry CN, et al. Autophagy induced by ischemic preconditioning is essential for cardioprotection. J Cardiovasc Transl Res 2010;3:365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Yeh CH, Hsu SP, Yang CC, Chien CT, Wang NP. Hypoxic preconditioning reinforces HIF‐alpha‐dependent HSP70 signaling to reduce ischemic renal failure‐induced renal tubular apoptosis and autophagy. Life Sci 2010;86:115–123. [DOI] [PubMed] [Google Scholar]
- 125. Wu BX, Darden AG, Laser M, et al. The rat Apg3p/Aut1p homolog is upregulated by ischemic preconditioning in the retina. Mol Vis 2006;12:1292–1302. [PubMed] [Google Scholar]
- 126. Park HK, Chu K, Jung KH, et al. Autophagy is involved in the ischemic preconditioning. Neurosci Lett 2009;451:16–19. [DOI] [PubMed] [Google Scholar]
- 127. Sheng R, Zhang LS, Han R, Liu XQ, Gao B, Qin ZH. Autophagy activation is associated with neuroprotection in a rat model of focal cerebral ischemic preconditioning. Autophagy 2010;6:482–494. [DOI] [PubMed] [Google Scholar]
- 128. Sheng R, Liu XQ, Zhang LS, et al. Autophagy regulates endoplasmic reticulum stress in ischemic preconditioning. Autophagy 2012;8:310–325. [DOI] [PubMed] [Google Scholar]
- 129. Balduini W, Carloni S, Buonocore G. Autophagy in hypoxia‐ischemia induced brain injury. J Matern Fetal Neonatal Med 2012;25(Suppl 1):30–34. [DOI] [PubMed] [Google Scholar]
- 130. Matchett GA, Martin RD, Zhang JH. Hyperbaric oxygen therapy and cerebral ischemia: Neuroprotective mechanisms. Neurol Res 2009;31:114–121. [DOI] [PubMed] [Google Scholar]
- 131. Klionsky DJ. Autophagy: From phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol 2007;8:931–937. [DOI] [PubMed] [Google Scholar]
- 132. Hu G, Hacham M, Waterman SR, et al. PI3K signaling of autophagy is required for starvation tolerance and virulenceof Cryptococcus neoformans. J Clin Invest 2008;118:1186–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wu YT, Tan HL, Huang Q, Ong CN, Shen HM. Activation of the PI3K‐Akt‐mTOR signaling pathway promotes necrotic cell death via suppression of autophagy. Autophagy 2009;5:824–834. [DOI] [PubMed] [Google Scholar]
- 134. Cheng Y, Yan L, Ren X, Yang JM. eEF‐2 kinase, another meddler in the “yin and yang” of Akt‐mediated cell fate? Autophagy 2011;7:660–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Qin L, Wang Z, Tao L, Wang Y. ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy 2010;6:239–247. [DOI] [PubMed] [Google Scholar]
- 136. Fan QW, Weiss WA. Autophagy and Akt promote survival in glioma. Autophagy 2011;7:536–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Barre B, Perkins ND. The Skp2 promoter integrates signaling through the NF‐kappaB, p53, and Akt/GSK3beta pathways to regulate autophagy and apoptosis. Mol Cell 2010;38:524–538. [DOI] [PubMed] [Google Scholar]
- 138. Fan QW, Cheng C, Hackett C, et al. Akt and autophagy cooperate to promote survival of drug‐resistant glioma. Sci Signal 2010;3:ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Dutta S, Baehrecke EH. Warts is required for PI3K‐regulated growth arrest, autophagy, and autophagic cell death in Drosophila. Curr Biol 2008;18:1466–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Duronio V. The life of a cell: Apoptosis regulation by the PI3K/PKB pathway. Biochem J 2008;415:333–344. [DOI] [PubMed] [Google Scholar]
- 141. Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta 2011;1813:1978–1986. [DOI] [PubMed] [Google Scholar]
- 142. Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 2006;124:471–484. [DOI] [PubMed] [Google Scholar]
- 143. Ma XM, Blenis J. Molecular mechanisms of mTOR‐mediated translational control. Nat Rev Mol Cell Biol 2009;10:307–318. [DOI] [PubMed] [Google Scholar]
- 144. Menzies FM, Rubinsztein DC. Broadening the therapeutic scope for rapamycin treatment. Autophagy 2010;6:286–287. [DOI] [PubMed] [Google Scholar]
- 145. Galluzzi L, Aaronson SA, Abrams J, et al. Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ 2009;16:1093–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell 2010;140:313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]


