SUMMARY
Aims: Several studies have documented an involvement of Neuropeptide Y (NPY) in stress‐related disorders. Stress‐related disorders are also characterized by changes in brain‐derived neurotrophic factor (BDNF) and nerve growth factor (NGF), neurotrophins implicated in the survival and function of neurons. Thus the aim of this study was to investigate whether an NPY intraperitoneal treatment has antidepressant‐like effects in rats subjected to a classical stress paradigm, the Forced Swim Test (FST), in association with changes in local brain neurotrophin production. Methods: Rats were intraperitoneally injected with either NPY (60 μg/kg) or a vehicle for three consecutive days between two FST sessions and then tested for time spent (or delay onset) in immobile posture. Moreover, we measured by enzyme‐linked immunosorbent assay (ELISA) neurotrophin levels in the hypothalamus and corticosterone levels in plasma. Results: The data showed that NPY induced a significant delay in the onset and a significant reduction in the duration of the immobility posture in FST. We also found that NPY decreased BDNF levels in the hypothalamus and corticosterone levels in plasma. Discussion: Immobility posture in FST can be reduced by antidepressant drugs. Thus, our data show an antidepressant‐like effect of NPY associated with changes in BDNF levels in the hypothalamus and reduced activity of hypothalamic–pituitary–adrenal (HPA) axis. Conclusion: These findings, while confirming the involvement of the NPY system in stress‐related disorders, suggest that a less invasive route of administration, such as an intraperitoneal injection, may be instrumental in coping with stressful events in animal models and perhaps in humans.
Keywords: BDNF, Forced Swim Test, NPY, Neurotrophins, Rat Brain
Introduction
Neuropeptide Y (NPY) is a 36‐amino acid molecule belonging to the pancreatic polypeptide family that is widely distributed in the central (CNS) and peripheral nervous system (PNS) [1]. It is associated with numerous biological functions including food intake, cardiovascular regulation, cognition, seizure activity, circadian rhythms, memory and learning [2].
Several studies have documented an NPY involvement in stress‐related disorders, such as depression and anxiety [3, 4, 5, 6, 7, 8, 9]. According to these studies, NPY may exert its anxiolytic and antidepressant action by antagonizing the hypothalamic–pituitary–adrenal (HPA) axis [10, 11, 12, 13] which is chronically hyperactivated in stress‐related disorders [2, 14]. Stress‐related disorders are also characterized by changes in brain‐derived neurotrophic factor (BDNF) and nerve growth factor (NGF), neurotrophins implicated in the growth, survival, and function of CNS and PNS neurons [15]. In the hippocampus of animal models of depression increased BDNF [16] or decreased [17] BDNF and NGF [18] levels have been reported, though the effects of antidepressant agents may not always be associated with changes in hippocampal BDNF expression [19]. Decreased BDNF levels in depressed patients [20] and increased NGF levels have been also reported in the serum of anxious patients [21, 22]. Interestingly, these neurotrophins also exert a regulatory action on the HPA axis. It has been shown that either intracerebroventricular administration of BDNF [23, 24] or intravenous administration of NGF [25] in rats produce an increase in HPA axis activity.
In accordance with all these evidences, a recent study from our group [26] showed that NPY intraperitoneal administration was able to induce changes in hypothalamic neurotrophin levels. In fact, injection of NPY in rats for three consecutive days decreased BDNF and increased NGF production in the hypothalamus. In that study, we hypothesized that the BDNF decrease was involved with NPY antidepressant action possibly through the inhibition of the HPA axis.
In the present study, this hypothesis has been further investigated in rats subjected to a classical stress paradigm, the Forced Swim Test (FST) [27, 28], one of the most widely used models to assess the effects of antidepressant drugs [29, 30]. In the FST, we tested the rats for time spent (or delay onset) in immobile posture that is defined as “behavioral despair” and interpreted as a depressive‐like behavior [31, 32, 33]. Our goal was to evaluate whether NPY given intraperitoneally induces an antidepressant‐like response in rats subjected to FST and whether this effect is associated with neurotrophin and/or HPA axis activity changes. Therefore, we measured, by enzyme‐linked immunosorbent assay (ELISA), BDNF and NGF levels in the hypothalamus and other brain regions to investigate whether NPY antidepressant action is associated with neurotrophin changes in the rat brain. To determine HPA axis activity we measured corticosterone plasma levels in NPY and vehicle‐treated rats and compared them with corticosterone plasma levels of untreated rats.
Materials and Methods
Animals
Adult male 60‐day‐old Wistar rats (250–300 g; Harlan, Italy) were used in the study. Rats were kept under standard conditions on a 12/12h dark/light cycle and were allowed access to water and food during acclimation ad libitum. Animals were maintained according to the guidelines for ethical conduct developed by the European Communities Council Directive of November 24, 1986 (86/609/EEC). All efforts were made to minimize animal pain and discomfort.
Forced Swim Test and Behavioral Analysis
In this procedure, rats are exposed to an acute and short‐duration stress by forcing them to swim in a narrow cylinder of water, from which they cannot escape. After an initial period of active escape behaviors, the animals develop the characteristic immobile posture which can be used to test the effects of an antidepressant treatment.
The procedure used was similar to that described by Porsolt et al. [28] and consisted in two swim sessions. Swim sessions were conducted by placing each rat in a glass cylinder (height: 60 cm; diameter: 22.5 cm) containing water at 25 ± 2°C, with a depth of 30 cm, for which the hind limbs could not reach the floor.
The first session (pretest) was performed two days before the test session, to acclimatize the animals to the test situation, thereby providing a stable level of immobile behavior during the test session, as reported by others [33, 34]. Rats were forced to swim for 15 min and then removed from the cylinder and dried before they return to their home cage. During the test session rats were placed again in the cylinder for 5 min.
Test sessions were recorded with a video‐camera (mod.: SMX‐C10RP; Samsung Electronics Co., Ltd.) placed at 1 m from the cylinder. An operator blind to treatment groups subsequently acquired behavioral scores from tape recording. Latency (defined as time elapsed between placing the animal in the cylinder and the first analyzed behavior) and immobility (defined as the lack of movements except those necessary to keep the head above the water) were evaluated. NPY was administered between the two session according to the protocol described below.
NPY Treatment
Neuropeptide Y (NPY; human, rat–cat. n. H‐6375) was purchased from Bachem AG, Switzerland. It was reconstituted in sterile distilled H2O at a concentration of 0.1 mM, filtered/sterilized and stored at −20°C.
Rats were intraperitoneally injected with either vehicle (saline) or NPY (60 μg/kg) for three consecutive days (n = 5 animals/group) between the two test sessions. The NPY dose was selected on the basis of a previous study where NPY was administered for three days to naive rats and found to affect hypothalamic BDNF and NGF levels [26].
The first injection was made on the pretest day, immediately after the swim session. The last injection was made 1 h before the test session.
Because NPY may also affect food intake, during the time of treatment we measured body weight and food intake in NPY‐ and saline‐treated rats.
Tissue Dissection
Twenty‐four hours after the last injection, rats of saline‐ and NPY‐treated groups were decapitated and the brains were quickly removed and dissected on ice using a binocular dissection microscope. Hypothalamus and the other brain regions (frontal cortex, striatum, parietal cortex, occipital cortex, and cerebellum) were collected according to Glowinski and Iversen's method [35]. Brain regions were extracted in 1 mL extraction buffer /100 mg tissue and homogenized in an ice‐cold lysis buffer containing 137mM NaCl, 20mM Tris–HCl (pH 8.0), 1% NP40, 10% glycerol, 1mM PMSF 10 μg/mL aprotinin, 1 μg/mL leupetin and 0.5mM sodium vanadate. The tissue homogenate solutions were centrifuged with 14,000 × g for 25 min at 4°C. The supernatants were collected and used for neurotrophin quantification.
BDNF and NGF Determination by Enzyme‐Linked Immunosorbent Assay (ELISA)
Concentrations of BDNF and NGF proteins were assessed using a two‐site enzyme immunoassay kit (Promega, Madison, WI, USA). In brief, 96‐well immunoplates (NUNC) were coated with 50 μL/well with the corresponding capture antibody which binds the neurotrophin of interest, and stored overnight at 4°C. The next day serial dilutions of known amounts of BDNF and NGF ranging from 0 to 500 pg/mL were performed in duplicate to generate a standard curve. Then the plates were washed three times with wash buffer and the standard curves and supernatants of brain tissue homogenates were incubated in the coated wells (100 μL each) for 2 h at room temperature (RT) with shaking. After additional washes, the antigen was incubated with second specific antibody for 2 h at RT (BDNF) or overnight at 4°C (NGF), as specified in the protocol. The plates were washed again with wash buffer and then incubated with an anti‐IgY HRP for 1 h at RT. After another wash, the plates were incubated with a TMB/Peroxidase substrate solution for 15 min and phosphoric acid 1M (100 μL/well) was added to the wells. The colorimetric reaction product was measured at 450 nm using a microplate reader (Dynatech MR 5000, Germany). Neurotrophin concentrations were determined from the regression line for the neurotrophin standard (ranging from 7.8 to 500 pg/mL‐purified mouse BDNF or NGF) incubated under similar conditions in each assay. Cross‐reactivity with other related neurotrophic factors, for example, NT‐3 and NT‐4 was less than 3%. Neurotrophin concentration was expressed as pg/g wet weight and all assays were performed in triplicate.
Corticosterone Determination in Plasma
For corticosterone determination, additional rats were exposed to the same experimental procedure (NPY and saline treatments) or left untreated (n = 7/group). Trunk blood was collected after decapitation in vacutainers containing sodium heparine. Samples were then spined at 2000 g for 20 min at 4°C to obtain plasma. The samples were stored at −70°C until dosage of the hormone with an ELISA kit (Cayman Chemical Co., Ann Arbor, MI, USA) following manufacturer's instructions. All the samples were processed in duplicate at a dilution of 1:5.
Statistical Analysis
Data on behavioral scoring, neurotrophin, and corticosterone levels were analyzed by one‐way analysis of variance (ANOVA) considering NPY and saline treatments as variables. Differences in body weight and food intake were evaluated with ANOVA for repeated measures. Post hoc comparisons were performed using Tukey's HSD test. P‐values ≤0.05 were considered statistically significant.
Results
Effects of NPY Treatment on Body Weight and Food Intake
Data on body weights and food intake are shown in Table 1.
Table 1.
Body weight and food intake of saline‐ and NPY‐treated rats at each time point of treatment and behavioral testing. n = 5 animals/group. Values are expressed in grams
| Time | Body weight | Food intake | ||
|---|---|---|---|---|
| Saline | NPY | Saline | NPY | |
| 1st day (Forced swim pretest + 1st injection) | 272 ± 9.87 | 271.54 ± 11.2 | – | – |
| 2nd day (2nd injection) | 271 ± 10.2 | 273.44 ± 11.7 | 25 ± 0.34 | 27 ± 1.76 |
| 3rd day (3rd injection + Forced swim test) | 276 ± 10.6 | 277.48 ± 12.9 | 24 ± 0.31 | 24 ± 1.56 |
| 4th day (sacrifice) | 277 ± 10.1 | 279.02 ± 12.9 | 22. ± 0.75 | 24 ± 1.92 |
Data are shown as the mean ± standard error.
NPY treatment did not affect body weight (F‐value = 0.01; P‐value = 0.93), and no interaction between treatment and time was found (F‐value = 0.38; P‐value = 0.77). An effect of time was present (F‐value = 15.9; P‐value < 0.00001). Post hoc analysis showed that rats of both groups gained weight during the experiment. In fact, the rat weight was higher on the third and the fourth days of treatment as compared to the first (1st day vs. 3rd day: P‐value < 0.001; 1st day vs. 4th day: P‐value < 0.0005) and the second day (2nd day vs. 3rd day: P‐value < 0.005; 2nd day vs. 4th day: P‐value < 0.0005).
NPY treatment also did not affect food intake (F‐value = 0.39; P‐value = 0.55), and no interaction between treatment and time was found (F‐value = 0.60; P‐value = 0.56). An effect of time was present (F‐value = 8.26; P‐value < 0.005). According to body weight data, post hoc tests showed that rats of both groups ate more food on the fourth day of the experiment than on the second one (P‐value < 0.005).
Effects of NPY Treatment on Forced Swim Test
Data on Forced Swim Test are shown in Figure 1. ANOVA showed that NPY‐treated rats had a significantly higher latency time than saline‐treated rats (F‐value = 7.05; P‐value < 0.05). ANOVA also showed that NPY treatment significantly affected immobility as NPY‐treated rats showed decreased time spent in immobile posture as compared to saline‐treated rats (F‐value = 6.68; P‐value < 0.05; Figure 1).
Figure 1.
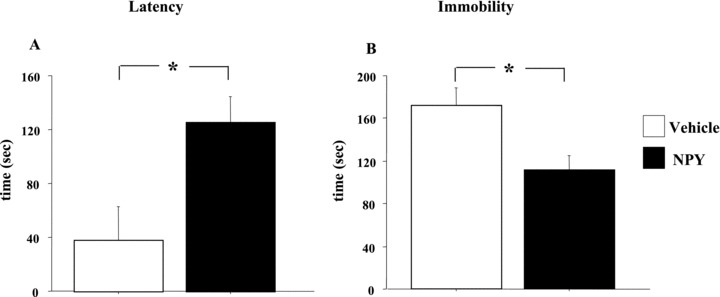
Effect of NPY peripheral administration on rat performances in the Forced Swim Test. Rats were intraperitoneally injected with either vehicle (saline) or NPY (60 μg /kg) for three consecutive days (n = 5 animals/group) between pretest and test sessions. Figure shows latency (A) and duration of immobility (B) (in seconds). Data represent means ± SEM. Asterisks indicate significant differences between groups (*P < 0.05).
Effects of NPY Treatment on Neurotrophin Synthesis
Neurotrophin levels in the hypothalamus are shown in Figure 2. ANOVA showed that NPY‐treated rats had significantly lower BNDF levels than saline‐treated rats (F‐value = 6.44; P < 0.05). No significant effects of NPY were found on hypothalamic NGF levels (F‐value = 0.29; P= 0.61).
Figure 2.
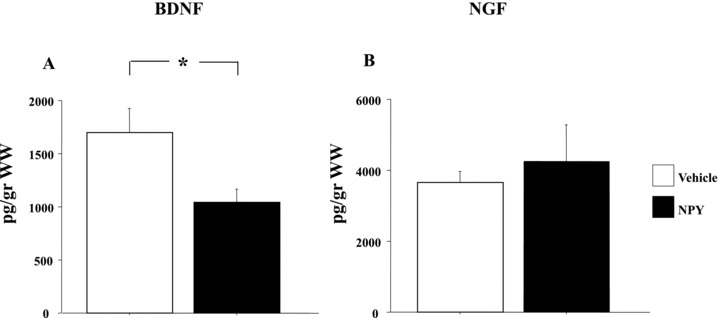
Effect of NPY peripheral administration on neurotrophin production in the rat hypothalamus. Rats were intraperitoneally injected with either vehicle (saline) or NPY (60 μg /kg) for three consecutive days (n = 5 animals/group). Figure shows BDNF (A) and NGF (B) levels. Data represent means ± SEM. WW: wet weight. Asterisks indicate significant differences between groups (*P < 0.05).
BDNF and NGF levels in the other brain regions examined are shown in Table 2. We found that NPY treatment did not affect BDNF and NGF levels in frontal, parietal and occipital cortices, hippocampus, striatum, and cerebellum (see Table 2).
Table 2.
Levels of BDNF and NGF in frontal, parietal and occipital cortices, hippocampus, striatum, and cerebellum of saline‐ and NPY‐treated rats. n = 5 animals/group. Values are expressed in pg/g of wet weight
| Brain regions | BDNF | NGF | ||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Statistic | Treatment | Statistic | |||||
| Saline | NPY | F‐value | P‐value | Saline | NPY | F‐value | p‐value | |
| Frontal cortex | 286 ± 28.4 | 257 ± 14.4 | 0.82 | 0.39 | 1690 ± 330.84 | 1853 ± 442 | 0.09 | 0.77 |
| Parietal cortex | 162 ± 14.1 | 200 ± 12.1 | 4.11 | 0.07 | 860 ± 103.79 | 1194 ± 220 | 1.89 | 0.21 |
| Occipital cortex | 263 ± 44.0 | 201 ± 17.5 | 1.67 | 0.23 | 1643 ± 327.08 | 1745 ± 341 | 0.05 | 0.83 |
| Hippocampus | 714 ± 38.7 | 680 ± 28.6 | 0.51 | 0.49 | 2923 ± 301.37 | 3215 ± 494 | 0.25 | 0.63 |
| Striatum | 329 ± 45.4 | 289 ± 39.8 | 0.44 | 0.53 | 1503 ± 201.56 | 1745 ± 417 | 0.27 | 0.61 |
| Cerebellum | 132 ± 12.3 | 144 ± 12.9 | 0.47 | 0.51 | 924 ± 271.07 | 3177 ± 1339 | 2.71 | 0.14 |
Data are shown as the mean ± standard error.
Corticosterone Plasma Levels
Corticosterone plasma levels in saline‐ and NPY‐treated rats and untreated controls are shown in Table 3. There was a significant effect of the treatment (F‐value = 0.59; P < 0.05). Post hoc analysis showed that rats of the saline group had significantly higher plasma levels of corticosterone as compared to NPY‐treated rats (P < 0.05) and untreated rats (P < 0.01). No significant differences were noted between NPY‐treated and untreated rats (Table 3).
Table 3.
Corticosterone plasma levels in saline‐ and NPY‐treated rats and untreated controls (n = 7 animals/group).
| Treatment | Corticosterone plasma levels | P‐value |
|---|---|---|
| Saline | 7417 ± 1583 | – |
| NPY | 3004 ± 946 | 0.01* |
| untreated controls | 1336 ± 338 | 0.001** |
Data are shown as the mean ± standard error. Values are expressed in pg/mL. Asterisks indicate significant differences versus Saline treated rats.
*P < 0.05; **P < 0.01.
Discussion
The aim of this study was to investigate whether NPY intraperitoneal treatment in rats has an antidepressant‐like effect in the classical stress paradigm of FST, in association with changes in local brain neurotrophin production and/or hypothalamic—pituitary–adrenal (HPA) axis activity.
The data showed that NPY (60 μg/kg for three consecutive days) was able to induce a significant delay in the onset and a significant reduction in the duration of the immobility posture in FST. We also found that NPY selectively decreased BDNF levels in the hypothalamus, without affecting other brain regions. In addition, the levels of corticosterone in NPY‐treated rats were reduced to levels comparable to those of untreated rats suggesting that these changes (antidepressant effects and decreased BDNF in hypothalamus) are due to a direct effect of NPY on the HPA axis. As for the NGF levels, no changes were found in all analyzed brain areas. We also found that during the time of treatment body weight and food intake were not affected by NPY administration.
Our data show an antidepressant‐like effect of NPY. These findings are in line with previous studies showing that an intracerebroventricular NPY injection in rodents reduces immobility time in FST [8, 31, 36, 37]. Therefore, our results, although confirming the involvement of the NPY system in stress‐related disorders, suggest that a less invasive route of administration, such as an intraperitoneal injection, may be instrumental to deal with stressful events. This finding may be of relevance for the development of a therapeutic use of NPY in humans, without the need of a direct local brain administration. Although at a level of preclinical observations, it is encouraging the fact that no relevant side effects such as changes in food intake between NPY‐ and saline‐ treated rats were detected.
The other finding of this study was the decrease of BDNF in the hypothalamus. In a previous study [26] performed in rats not exposed to FST, we found the same alteration in the hypothalamus and hypothesized that it could be part of the NPY antidepressant action. The present data support this hypothesis, in view of the fact that the decrease in BDNF is present in rats showing an antidepressant‐like response in the FST. A consequence of decreased hypothalamic BDNF levels may be the inhibition of the HPA axis activity [16] and possibly one of the mechanisms by which NPY exerts its antidepressant action. Further support of this hypothesis comes from our data on corticosterone plasma levels which can be used to determine the activity of HPA.
In a previous study [26], we also observed in naive rats that the three‐day NPY treatment at the same dose used in this experiment induced a significant increase in hypothalamic NGF levels. This effect was not present in rats exposed to FST. Human studies documented increased NGF serum concentrations in subjects exposed to acute stressful situations [38] but also in association with a positive response to generalized anxiety disorder therapy [22]. These findings suggest that levels of NGF may be different in rats exposed to stressful conditions from those of naive rats.
In conclusion, the present study demonstrated that NPY, given intraperitoneally, has antidepressant‐like effects associated with changes in BDNF levels in the hypothalamus and reduced corticosterone plasma levels. These data provide further evidence for the role of NPY in depression and, given the absence of relevant side effects on food intake, for its use in animal models of stress‐related disorders.
Author Contributions
Authors Francesco Angelucci and Francesca Gelfo designed the study and wrote the protocols. Authors Sergio Bernardini, Laura Petrosini, and Carlo Caltagirone managed the literature searches and analyses. Authors Paola De Bartolo, Paola Tirassa, Nicoletta Croce, and Francesca Gelfo managed the experimental procedures and undertook the statistical analysis. Authors Francesco Angelucci and Francesca Gelfo wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Disclosure
Supported by nEUROsyn Italian Ministry of Health (RC2008) in the frame of ERA‐Net Neuron.
Acknowledgment
We thank Dr. Amanda Formosa for English editing.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1. Beck B. Neuropeptide Y in normal eating and in genetic and dietary‐induced obesity. Philos Trans R Soc Lond B Biol Sci 2006;361:1159–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paschos KA, Veletza S, Chatzaki E. Neuropeptide and sigma receptors as novel therapeutic targets for the pharmacotherapy of depression. CNS Drugs 2009;23:755–772. [DOI] [PubMed] [Google Scholar]
- 3. Redrobe JP, Dumont Y, Quirion R. Neuropeptide Y (NPY) and depression: From animal studies to the human condition. Life Sci 2002;71:2921–2937. [DOI] [PubMed] [Google Scholar]
- 4. Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides 2004;38:213–224. [DOI] [PubMed] [Google Scholar]
- 5. Jiménez‐Vasquez PA, Diaz‐Cabiale Z, Caberlotto L, Bellido I, Overstreet D, Fuxe K, Mathé AA. Electroconvulsive stimuli selectively affect behavior and neuropeptide Y (NPY) and NPY Y(1) receptor gene expressions in hippocampus and hypothalamus of Flinders Sensitive Line rat model of depression. Eur Neuropsychopharmacol 2007;17:298–308. [DOI] [PubMed] [Google Scholar]
- 6. Nikisch G, Mathé AA. CSF monoamine metabolites and neuropeptides in depressed patients before and after electroconvulsive therapy. Eur Psychiatry 2008;23:356–359. [DOI] [PubMed] [Google Scholar]
- 7. Bjørnebekk A, Mathé AA, Brené S. The antidepressant effects of running and escitalopram are associated with levels of hippocampal NPY and Y1 receptor but not cell proliferation in a rat model of depression. Hippocampus 2010;20:820–828. [DOI] [PubMed] [Google Scholar]
- 8. Redrobe JP, Dumont Y, Fournier A, Quirion R. The neuropeptide Y (NPY) Y1 receptor subtype mediates NPY‐induced antidepressant‐like activity in the mouse forced swimming test. Neuropsychopharmacology 2002;26:615–624. [DOI] [PubMed] [Google Scholar]
- 9. Morales‐Medina JC, Dumont Y, Quirion R. A possible role of neuropeptide Y in depression and stress. Brain Res 2010;1314:194–205. [DOI] [PubMed] [Google Scholar]
- 10. Britton KT, Southerland S, Van Uden E, Kirby D, Rivier J, Koob G. Anxiolytic activity of NPY receptor agonists in the conflict test. Psychopharmacology (Berl) 1997;132:6–13. [DOI] [PubMed] [Google Scholar]
- 11. Ehlers CL, Somes C, Seifritz E, Rivier JE. CRF/NPY interactions: A potential role in sleep dysregulation in depression and anxiety. Depress Anxiety 1997;6:1–9. [PubMed] [Google Scholar]
- 12. Kask A, Rägo L, Harro J. Alpha‐helical CRF(9–41) prevents anxiogenic‐like effect of NPY Y1 receptor antagonist BIBP3226 in rats. Neuroreport 1997;8:3645–3647. [DOI] [PubMed] [Google Scholar]
- 13. Thorsell A, Slawecki CJ, Ehlers CL. Effects of neuropeptide Y and corticotropin‐releasing factor on ethanol intake in Wistar rats: Interaction with chronic ethanol exposure. Behav Brain Res 2005;161:133–140. [DOI] [PubMed] [Google Scholar]
- 14. Tsigos C, Chrousos GP. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J Psychosom Res 2002;53:865–871. [DOI] [PubMed] [Google Scholar]
- 15. Pardon MC. Role of neurotrophic factors in behavioral processes: Implications for the treatment of psychiatric and neurodegenerative disorders. Vitam Horm 2010;82:185–200. [DOI] [PubMed] [Google Scholar]
- 16. Naert G, Ixart G, Maurice T, Tapia‐Arancibia L, Givalois L. Brain‐derived neurotrophic factor and hypothalamic‐pituitary‐adrenal axis adaptation processes in a depressive‐like state induced by chronic restraint stress. Mol Cell Neurosci 2011;46:55–66. [DOI] [PubMed] [Google Scholar]
- 17. Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci 2007;10:1089–1093. [DOI] [PubMed] [Google Scholar]
- 18. Marais L, van Rensburg SJ, van Zyl JM, Stein DJ, Daniels WM. Maternal separation of rat pups increases the risk of developing depressive‐like behavior after subsequent chronic stress by altering corticosterone and neurotrophin levels in the hippocampus. Neurosci Res 2008;61:106–112. [DOI] [PubMed] [Google Scholar]
- 19. Hansson AC, Rimondini R, Heilig M, Mathé AA, Sommer WH. Dissociation of antidepressant‐like activity of escitalopram and nortriptyline on behaviour and hippocampal BDNF expression in female rats. J Psychopharmacol; in press. doi: 10.1177/0269881110393049. [DOI] [PubMed] [Google Scholar]
- 20. Hashimoto K. Brain‐derived neurotrophic factor as a biomarker for mood disorders: an historical overview and future directions. Psychiatry Clin Neurosci 2010;64:341–357. [DOI] [PubMed] [Google Scholar]
- 21. Lang UE, Jockers‐Scherübl MC, Hellweg R. State of the art of the neurotrophin hypothesis in psychiatric disorders: implications and limitations. J Neural Transm 2004;111:387–411. [DOI] [PubMed] [Google Scholar]
- 22. Jockers‐Scherübl MC, Zubraegel D, Baer T, Linden M, et al Nerve growth factor serum concentrations rise after successful cognitive‐behavioural therapy of generalized anxiety disorder. Prog Neuropsychopharmacol Biol Psychiatry 2007;31:200–204. [DOI] [PubMed] [Google Scholar]
- 23. Givalois L, Naert G, Rage F, Ixart G, Arancibia S, Tapia‐Arancibia L. A single brain‐derived neurotrophic factor injection modifies hypothalamo–pituitary–adrenocortical axis activity in adult male rats. Mol Cell Neurosci 2004;27:280–295. [DOI] [PubMed] [Google Scholar]
- 24. Naert G, Ixart G, Tapia‐Arancibia L, Givalois L. Continuous i.c.v. infusion of brain‐derived neurotrophic factor modifies hypothalamic–pituitary–adrenal axis activity, locomotor activity and body temperature rhythms in adult male rats. Neuroscience 2006;139:779–789. [DOI] [PubMed] [Google Scholar]
- 25. Scaccianoce S, Lombardo K, Nicolai R, Affricano D, Angelucci L. Studies on the involvement of histamine in the hypothalamic–pituitary–adrenal axis activation induced by nerve growth factor. Life Sci 2000;67:3143–3152. [DOI] [PubMed] [Google Scholar]
- 26. Gelfo F, De Bartolo P, Tirassa P, Croce N, Caltagirone C, Petrosini L, Angelucci F. Intraperitoneal injection of neuropeptide Y (NPY) alters neurotrophin rat hypothalamic levels: Implications for NPY potential role in stress‐related disorders. Peptides 2011;32:1320–1323. [DOI] [PubMed] [Google Scholar]
- 27. Porsolt RD, Le Pichon M, Jalfre M. Depression: A new model sensitive to antidepressant treatments. Nature 1977;266:730–732. [DOI] [PubMed] [Google Scholar]
- 28. Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioral despair in rats: A new model sensitive to antidepressant treatments. Eur J Pharmacol 1978;47:379–391. [DOI] [PubMed] [Google Scholar]
- 29. Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev 2005;29:547–569. [DOI] [PubMed] [Google Scholar]
- 30. Rotzinger S, Lovejoy DA, Tan LA. Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides 2010;31:736–756. [DOI] [PubMed] [Google Scholar]
- 31. Goyal SN, Kokare DM, Chopde CT, Subhedar NK. Alpha‐melanocyte stimulating hormone antagonizes antidepressant‐like effect of neuropeptide Y in Porsolt's test in rats. Pharmacol Biochem Behav 2006;85:369–377. [DOI] [PubMed] [Google Scholar]
- 32. Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci 2010;13:1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Castagné V, Moser P, Roux S, Porsolt RD. Rodent models of depression: Forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci 2011;55:8.10A.1–8.10A.14. [DOI] [PubMed] [Google Scholar]
- 34. Detke MJ, Johnson J, Lucki I. Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp Clin Psychopharmacol 1997;5:107–112. [DOI] [PubMed] [Google Scholar]
- 35. Glowinski J, Iversen LL. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem 1966;13:655–669. [DOI] [PubMed] [Google Scholar]
- 36. Husum H, Mikkelsen JD, Hogg S, Mathé AA, Mørk A. Involvement of hippocampal neuropeptide Y in mediating the chronic actions of lithium, electroconvulsive stimulation and citalopram. Neuropharmacology 2000;39:1463–1473. [DOI] [PubMed] [Google Scholar]
- 37. Stogner KA, Holmes PV. Neuropeptide‐Y exerts antidepressant‐like effects in the forced swim test in rats. Eur J Pharmacol 2000;387:R9–R10. [DOI] [PubMed] [Google Scholar]
- 38. Aloe L, Bracci‐Laudiero L, Alleva E, Lambiase A, Micera A, Tirassa P. Emotional stress induced by parachute jumping enhances blood nerve growth factor levels and the distribution of nerve growth factor receptors in lymphocytes. Proc Natl Acad Sci U S A 1994;91:10440–10444. [DOI] [PMC free article] [PubMed] [Google Scholar]


