Summary
Aims
We investigated whether CHADS2 or CHA2DS2‐VASc scores could be used to predict 1‐year prognosis in stroke recurrence, mortality, and mortality of ischemic stroke or transient ischemic attack (TIA) patients with nonvalvular atrial fibrillation (NVAF).
Methods
Patients were selected from a national prospective registry in China. The clinical prediction of the scores was examined using the C statistic. Univariate and multivariate logistic regressions were performed to analyze the relevant risk factors.
Results
Thousand two hundred and ninety‐seven of 22,216 patients were enrolled in the study. For stroke recurrence rate, the C statistic value was 0.53 (odds ratio [OR] 1.15, 95% confidence interval [CI]: 1.01 to 1.32) for CHADS2 and 0.55 (OR 1.14, 95% CI: 1.05 to 1.24) for CHA2DS2‐VASc; adding baseline National Institutes of Health Stroke Scale (NIHSS) score to these two scores, the value of C statistic was 0.58 (OR 1.25 95% CI: 1.14 to 1.37) and 0.58 (OR 1.19, 95% CI: 1.11 to 1.27), respectively.
Conclusions
Both CHADS2 and CHA2DS2‐VASc scores have limitations in predicting the 1‐year prognosis of stroke/TIA patients with NVAF in China. The predictive value of these two scores improved by adding the baseline NIHSS score.
Keywords: Atrial fibrillation, Clinical prediction, Risk stratification, Stroke
Introduction
Cardioembolic stroke accounts for 20–30% of all ischemic strokes worldwide and 8–13% nationwide in China, with atrial fibrillation (AF) being the leading risk factor 1, 2, 3. CHADS2 and CHA2DS2‐VASc are scores commonly used for stroke risk stratification in AF patients. The acronym for CHADS2 is congestive heart failure, hypertension, age > 75 years, diabetes mellitus, and prior stroke or transient ischemic attack (TIA). CHA2DS2‐VASc score adds two additional risk factors, vascular disease and gender, to CHADS2 score and is separated into two subgroups according to age (≥75 years and 65–74 years). Recent treatment guidelines divided subjects into low‐, moderate‐, and high‐risk strata based on the CHADS2 or CHA2DS2‐VASc score 4, 5. In patients with a high risk of cardioembolic stroke, the new oral anticoagulants (NOACs, Dabigatran and Rivaroxaban) were recommended unless NOACs was contraindicated 6. Anticoagulant therapy was not recommended in patients with CHA2DS2‐VASc score of 0 7. CHADS2 and CHA2DS2‐VASc scores have been recognized worldwide, and the moderate C statistic values were 0.60–0.63 in predicting the risk of ischemic stroke in NVAF patients 8, 9, 10. These two scores were suggested to be directly related to stroke severity and early outcome in patients with NVAF 11, 12, 13. However, the treatment guidelines for the secondary prevention of ischemic stroke and TIA in patients with NVAF did not thoroughly study the predictive values of these two scores 14.
The aim of this study was to investigate whether the CHADS2 or CHA2DS2‐VASc scores were suitable for predicting the 1‐year outcome in stroke recurrence, death, and disability in ischemic stroke/TIA patients with NVAF in China.
Patients and Methods
The current cohort was a subset study from the China National Stroke Registry (CNSR) 15. The CNSR was a well‐designed, nationwide, prospective registry. Patients hospitalized for cerebrovascular events from 2007 to 2008 were enrolled in the study and followed for 1 year. The ethics committees at all participating hospitals approved the study, and all patients or their designated relatives signed the informed consent forms.
The eligible NVAF patients were defined as patients with atrial fibrillation or atrial flutter by self‐report or by electrocardiography (ECG) on admission, without mitral or aortic valve disease. Acute ischemic stroke or TIA was diagnosed according to World Health Organization criteria 16 combined with brain computed tomography or magnetic resonance confirmation. Patients were grouped according to the presence of hypertension (HTN), diabetes mellitus (DM), or dyslipidemia (HLD) if there was a self‐reported history or the use of antihypertensive, antihyperglycemic, or lipid‐lowering agents during hospitalization or at discharge. The definition of baseline vascular disease was the presence of coronary heart diseases or peripheral artery vascular disease by self‐report or diagnosis during hospitalization. The diagnostic criteria were consistent across all participating hospitals.
The primary endpoint was 1‐year self‐reported stroke recurrence rate, defined as new or worsened primary neurologic deficit, or readmission due to ischemic stroke, intracerebral hemorrhage, or subarachnoid hemorrhage. The secondary endpoints were 1‐year disability rate (disability was defined as modified Rankin Scale (mRS) score from 2 to 5) and all‐cause mortality. The outcome data were obtained through follow‐up phone calls conducted by well‐trained interviewers using standardized questionnaires. The interviewers were blinded to the baseline characteristics for all patients.
Statistical Analysis
The data collected from CNSR were retrospectively assessed. The demographic and clinical characteristics of patients on vitamin K antagonists (VKA) were compared with those who were not on VKA using chi‐squared tests. The area under the curve (AUC) by C statistic was used to validate the discrimination of the CHADS2 and CHA2DS2‐VASc scores for predicting 1‐year outcomes. The associations between the risk factors and the outcomes were analyzed in univariate and multivariate logistic regression models. The data were analyzed with statistical software SAS version 9.1.3 (SAS Institute Inc., Cary, NC, USA), and a two‐sided significance level of α = 0.05 was assumed. The statistical scientist was blinded to the patients' information.
Results
Of the 22,216 hospitalized patients with acute stroke from 132 participating hospitals in the CNSR, 1297 patients with NVAF completed the follow‐up were enrolled in our study (Figure 1). Table 1 showed the baseline characteristics for the analyzed patients. The clinical characteristics were compared between the patients who were on VKA to those who were not. The demographic characteristics, risk factors, and drug interventions were similar in the two groups. However, older patients were more likely to be on VKA therapy (P < 0.0010). The lowest CHADS2 and CHA2DS2‐VASc scores were 2 because all the patients had history of ischemic stroke or TIA. The cumulative 1‐year stroke recurrence rate was 32.35% (95% confidence interval [CI]: 29.73 to 35.06%), all‐cause mortality from any cause was 34.23% (95 CI: 31.65 to 36.89%), and disability rate was 51.58% (95% CI: 48.17 to 54.99%). The rate of recurrent stroke and poor outcome increased with rising CHADS2 and CHA2DS2‐VASc scores. For recurrent stroke rate, the C statistic value at 1‐year follow‐up was 0.532 (odds ratio [OR] 1.150, 95% CI: 1.005 to 1.315) for CHADS2 and 0.551 (OR 1.138, 95% CI: 1.045 to 1.240) for CHA2DS2‐VASc. For all‐cause mortality, the C statistic value was 0.525 (OR 1.122, 95% CI: 0.987 to 1.276) for CHADS2, and 0.574 (OR 1.201, 95% CI: 1.105 to 1.305) for CHA2DS2‐VASc. For disability rate, the C statistic value was 0.542 (OR 1.195, 95% CI: 1.023 to 1.397) and 0.593 (OR 1.276, 95% CI: 1.156 to 1.409) for these two scores, respectively (Table 2).
Figure 1.
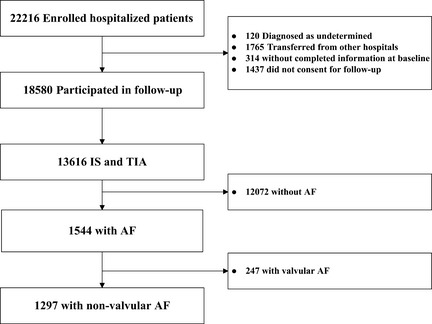
Flow chart of study population. Abbreviation: IS, ischemic stroke, TIA, transient ischemic attack, AF, atrial fibrillation.
Table 1.
Baseline characteristics of patients with and without anticoagulants
| Characteristics | Overall | With anticoagulants | Without anticoagulants | P value |
|---|---|---|---|---|
| Sample size | 1297 | 185 | 1112 | |
| Heart failure | 120 (9.3%) | 28 (15.1%) | 92 (8.3%) | 0.0029 |
| Hypertension | 947 (73.0%) | 129 (69.7%) | 818 (73.6%) | 0.2770 |
| Age (years) | <0.0010 | |||
| ≥75 | 243 (18.7%) | 54 (29.2%) | 189 (17.0%) | |
| 65–74 | 408 (31.5%) | 65 (35.1%) | 343 (30.8%) | |
| ≤64 | 646 (49.8%) | 66 (35.7%) | 580 (52.2%) | |
| Diabetes mellitus | 532 (41%) | 82 (44.3%) | 450 (40.5%) | 0.3234 |
| Vascular diseasea | 479 (36.9%) | 65 (35.1%) | 414 (37.2%) | 0.5846 |
| Female sex | 679 (52.4%) | 91 (49.2%) | 588 (52.9%) | 0.3523 |
| Hyperlipidemia | 1279 (98.6%) | 179 (96.8%) | 1100 (98.9%) | 0.0198 |
| BMI (kg/m2) | 24.02 ± 4.33 | 23.9 ± 4.02 | 24.04 ± 4.38 | 0.6973 |
| NIHSS | 13.02 ± 23.56 | 10.29 ± 8.51 | 13.43 ± 25.04 | 0.1335 |
| Smoking | 185 (14.3%) | 28 (15.1%) | 157 (14.1%) | 0.7143 |
| Heavy drinkingb | 79 (6.1%) | 13 (7.0%) | 66 (5.9%) | 0.5653 |
| CHADS2 | ||||
| 2 | 107 (8.2%) | 23 (12.4%) | 84 (7.6%) | |
| 3 | 436 (33.6%) | 59 (31.9%) | 377 (33.9%) | |
| 4 | 517 (39.9%) | 66 (35.7%) | 451 (40.6%) | |
| 5 | 218 (16.8%) | 35 (18.9%) | 183 (16.5%) | |
| 6 | 19 (1.5%) | 2 (1.1%) | 17 (1.5%) | |
| CHA2DS2‐VASc | ||||
| 2 | 27 (2.1%) | 12 (6.5%) | 15 (1.3%) | |
| 3 | 88 (6.8%) | 15 (8.1%) | 73 (6.6%) | |
| 4 | 210 (16.2%) | 34 (18.4%) | 176 (15.8%) | |
| 5 | 336 (25.9%) | 40 (21.6%) | 296 (26.6%) | |
| 6 | 337 (26.0%) | 42 (22.7%) | 295 (26.5%) | |
| 7 | 211 (16.3%) | 31 (16.8%) | 180 (16.2%) | |
| 8 | 78 (6.0%) | 11 (5.9%) | 67 (6.0%) | |
| 9 | 10 (0.8%) | 0 (0.0%) | 10 (0.9%) | |
| Drugs | ||||
| Antiplatelet agents | 916 (70.6%) | 125 (67.6%) | 791 (71.1%) | 0.3242 |
| Antihypertensive agents | 864 (66.6%) | 118 (63.8%) | 746 (67.1%) | 0.3778 |
| Hypoglycemic agents | 512 (39.5%) | 80 (43.2%) | 432 (38.8%) | 0.2575 |
| Lipid‐lowering agents | 1269 (97.8%) | 176 (95.1%) | 1093 (98.3%) | 0.0062 |
BMI, body mass index; NIHSS, the National Institutes of Health Stroke Scale.
The vascular diseases excluded cerebrovascular disease.
Heavy drinking indicates ≥2 standard alcohol intake per day.
Table 2.
Predictive value of the CHADS2 and CHA2DS2‐VASc scores compared with the modified scores taking the NIHSS score at baseline into account
| Stroke recurrence | Death | Disability | ||||
|---|---|---|---|---|---|---|
| C statistic | OR (95% CI)* | C statistic | OR (95% CI)* | C statistic | OR (95% CI)a | |
| Original scores | ||||||
| CHADS2 | 0.53 | 1.15 (1.01–1.32) | 0.53 | 1.12 (0.99–1.28) | 0.54 | 1.20 (1.02–1.40) |
| CHA2DS2‐VASc | 0.55 | 1.14 (1.05–1.24) | 0.57 | 1.20 (1.11–1.31) | 0.59 | 1.28 (1.16–1.41) |
| Modified scores | ||||||
| CHADS2N | 0.58 | 1.25 (1.14–1.37) | 0.69 | 1.81 (1.64–2.01) | 0.67 | 1.84 (1.60–2.12) |
| CHA2DS2‐VAScN | 0.58 | 1.19 (1.11–1.27) | 0.69 | 1.53 (1.42–1.65) | 0.68 | 1.58 (1.43–1.74) |
NIHSS, the National Institutes of Health Stroke Scale, OR, odds ratio, CI, confidence interval, CHADS2N, adding NIHSS into CHADS2, CHA2DS2‐VAScN, adding NIHSS into CHA2DS2‐VASc.
OR means odds of getting the event increased with each extra point of the score.
Table 3 showed the univariate logistic regression analysis results of the risk factors. Older patients (age ≥ 75 years, OR 1.713, 95% CI: 1.217 to 2.411), strokes of higher severity (NIHSS scores ≥ 15, OR 1.912, 95% CI: 1.408 to 2.596), and the presence of DM (OR 1.336, 95% CI: 1.049 to 1.702) were associated with a higher risk for stroke recurrence. Interestingly, patient's level of education was inversely related to the stroke recurrence rate (OR 0.726, 95% CI: 0.532 to 0.991). The multivariate logistic regression analysis (Table 4) demonstrated that older age (≥75 years) and a higher NIHSS score (≥15) were associated with a higher risk for 1‐year stroke recurrence with the adjusted odds ratio of 1.659 (95% CI: 1.144 to 2.405) and 1.886 (95% CI: 1.341 to 2.653) after controlling other confounders. These two risk factors were also associated with the all‐cause death (OR 2.167, 95% CI: 1.417 to 3.317 for age ≥ 75 years; OR 7.916, 95% CI: 5.435 to 11.529 for the NIHSS score ≥ 15) and disability (OR 2.322, 95% CI: 1.500 to 3.594 for age ≥ 75 years; OR 14.829, 95% CI: 7.762 to 28.330 for the NIHSS score ≥ 15). After adding NIHSS into the risk stratification scores (Table 5), the C statistic values of CHADS2N and CHA2DS2‐VAScN were 0.578 (OR 1.250, 95% CI: 1.137 to 1.374) and 0.580 (OR 1.185, 95% CI: 1.105 to 1.272) for stroke recurrence rate, 0.691 (OR 1.183, 95% CI: 1.635 to 2.010) and 0.689 (OR 1.528, 95% CI: 1.023 to 1.397) for all‐cause mortality, and 0.668 (OR 1.1842 95% CI: 1.603 to 2.116) and 0.681 (OR 1.579, 95% CI: 1.430 to 1.743) for disability rate, respectively (Table 2).
Table 3.
Risk factors for stroke recurrence by univariate logistic regression
| Risk factor | OR (95% CI) |
|---|---|
| Heart failure | 0.866 (0.569–1.318) |
| Hypertension | 0.825 (0.631–1.078) |
| Age ≥ 75 versus <65 (years) | 1.713 (1.217–2.411) |
| Age 65–74 versus <65 (years) | 1.332 (0.920–1.929) |
| Diabetes mellitus | 1.336 (1.049–1.702) |
| Vascular disease | 1.144 (0.894–1.464) |
| Female sex versus male sex | 1.239 (0.974–1.577) |
| Hyperlipidemia | 0.533 (0.204–1.392) |
| BMI 25–29 versus <25(kg/m2) | 0.926 (0.696–1.231) |
| BMI ≥ 30 versus <25(kg/m2) | 0.695 (0.372–1.299) |
| Smoking | 0.919 (0.647–1.305) |
| Heavy drinkinga | 0.960 (0.577–1.597) |
| NIHSS 25–42 versus ≤3 | 2.121(1.398–3.219) |
| NIHSS 15–24 versus ≤3 | 1.811(1.288–2.546) |
| NIHSS 4–14 versus ≤3 | 1.097(0.811–1.484) |
| NIHSS | 1.032(1.019–1.045) |
| Ethnicb | 0.950 (0.445–2.025) |
| Secondary educationc versus elementary educationd | 0.842 (0.619–1.147) |
| Higher educatione versus elementary education | 0.726 (0.532–0.991) |
| Antihypertensive agents | 0.846 (0.657–1.090 |
| Hypoglycemic agents | 1.386 (1.087–1.767) |
| Lipid‐lowering agents | 0.809 (0.367–1.784) |
| Antiplatelet agents | 0.652 (0.505–0.843) |
| Anticoagulants | 0.809 (0.567–1.155) |
OR, odds ratio; CI, confidence interval; BMI, body mass index; NIHSS, the National Institutes of Health Stroke Scale.
Heavy drinking indicates standard alcohol intake per day ≥ 2, OR represented standard alcohol intake per day ≥2 versus <2.
Ethnic indicates other ethnic groups outside the Han in China, OR represented other groups versus Han.
Secondary education indicates middle school.
Elementary education indicates elementary school or below.
Higher education indicates high school or above.
Table 4.
Risk factors for stroke recurrence, death and disability by multivariate logistic regressiona
| Risk factor | OR (95% CI) | ||
|---|---|---|---|
| Stroke recurrence | Death | Disability | |
| Age ≥ 75 (year) | 1.659 (1.144–2.405) | 2.167 (1.417–3.317) | 2.322 (1.500–3.594) |
| Age 65–74 (year) | 1.272 (0.852–1.899) | 1.260 (0.800–1.983) | 1.356 (0.865–2.125) |
| Female sex | 1.972 (1.414–2.750) | ||
| NIHSS 25–42 | 1.833 (1.131–2.971) | 14.036 (8.020–24.564) | |
| NIHSS 15–24 | 1.886 (1.341–2.653) | 7.916 (5.435–11.529) | 14.829 (7.762–28.330) |
| NIHSS 4–14 | 1.247 (0.899–1.728) | 1.629 (1.127–2.354) | 1.918 (1.358–2.710) |
OR, odds ratio; CI, confidence interval; NIHSS, the National Institutes of Health Stroke Scale.
Reference for age was <65 years old. Reference for NIHSS score was ≤3. Reference for female sex was male sex. Adjusted for gender, ethnicity, educational background, smoking and heavy drinking, adiposity, history of disease including heart failure, hypertension, diabetes mellitus, hyperlipidemia, vascular disease, drug intervention such as antihypertensive agents use, hypoglycemic agents use, lipid‐lowering agents use, antiplatelet agents use, and anticoagulants use.
Table 5.
The component of the modified scores
| Risk Factor | Score |
|---|---|
| CHADS2N | |
| Congestive heart failure | 1 |
| Hypertension | 1 |
| Age > 75 years | 1 |
| Diabetes | 1 |
| History of stroke/TIA | 2 |
| NIHSS scores 4–14 | 1 |
| NIHSS scores 15–24 | 2 |
| NIHSS scores 25–42 | 3 |
| Total | 9 |
| CHA2DS2‐VAScN | |
| Congestive heart failure | 1 |
| Hypertension | 1 |
| Age ≥ 75 years | 2 |
| Diabetes | 1 |
| History of stroke/TIA | 2 |
| Vascular disease | 1 |
| Age 65–74 years | 1 |
| Female gender | 1 |
| NIHSS scores 4–14 | 1 |
| NIHSS scores 15–24 | 2 |
| NIHSS scores 25–42 | 3 |
| Total | 12 |
NIHSS, the National Institutes of Health Stroke Scale.
Discussion
This study was conducted primarily to determine whether the CHADS2 or CHA2DS2‐VASc score could sufficiently predict the 1‐year outcome in ischemic stroke/TIA patients with NVAF in China. To date, there were limited data available for this purpose 17, 18. The predictive values of the CHADS2 and CHA2DS2‐VASc scores in our study were lower than those from previous systematic review and meta‐analysis from USA, Japan, Spain, and China 8. CHA2DS2‐VASc score was more accurate than CHADS2 score to identify low to intermediate risk patients with NVAF after stroke and consequently guide clinicians in decision making of using VKA 5, 19, 20. In our study, <14.3% of the patients received VKA treatment, while 25% patients received neither VKA nor antiplatelet agents because of contraindication such as hemorrhage risk. The relatively low use of VKA and antiplatelet agents may skew the 1‐year outcome of ischemic stroke/TIA patients with NVAF and influence predictive value of the CHADS2 and CHA2DS2‐VASc scores. We found that CHA2DS2‐VASc score performed better than CHADS2 in predicting prognoses, especially in predicting disability. The highest C statistic value among the outcome was approximately 0.6, indicating the limited predicative use of these two scores in stroke/TIA patients with NVAF in China. The CHADS2 and CHA2DS2‐VASc scores were initially used to evaluate the risk of cardioembolic stroke. The risk factors included in these two scores (such as hypertension, older age, and DM) were also atherosclerosis risk factors. The two risk stratification systems may also predict the risk of atherosclerosis stroke 21. Most of our study population suffered atherosclerosis stroke. It suggested that CHADS2 and CHA2DS2‐VASc scores might be more applicable to risk prediction in patients with cardioembolic stroke.
The overall 1‐year event rate in this study was significantly higher than that reported in a previous article 2. The low use of anticoagulant therapy may have played a role. In addition, all patients in our study had history of ischemic stroke/TIA, which is the most important risk factor of stroke recurrence and poor outcome 22. Several studies have reported four consistent risk factors for predicting stroke in nonvalvular atrial fibrillation patients: prior stroke/TIA, older age, hypertension, and DM 22, 23. Our study population had a mean age of 73 years, and 18.7% of patients were older than 75 years of age. The NVAF prevalence is age dependent, which rises by 1% per year in patients under 60 and 15% per year in those over 85. The prevalence of other cerebrovascular risk factors also increases with age 24, 25. In the NVAF patients without other risk factors and without the use of OAC, the stroke event rate in patients older than 75 years of age is twice that of the patients ages 65–74 26. The Birmingham Atrial Fibrillation Treatment of the Aged Study (BAFTA) supported VKA therapy for NVAF patients aged over 75, unless there were contraindications or patient refusal 27. Our study confirmed that age of 75 or greater was an independent risk factor for stroke recurrence, death, and disability. In our study, age is the only significant different baseline characteristic in the patients with and without the use of VKA, suggesting that age was one of the most influential factors on the use of anticoagulant in clinical practice. Only 22.22% of the patients 75 years or older were treated with VKA in our study, which may have resulted in a high stroke recurrence rate and poor outcomes.
The NIHSS score is the most commonly used standardized stroke scale 28, 29, which is associated with mortality 30. Previous studies have reported that stroke patients with AF may have higher NIHSS scores than those without AF 31, 32. In addition, stroke patients with AF with higher CHADS2 or CHA2DS2‐VASc scores had worse outcome (worse neurologic deficit or death) 11, 13. Our study found that the NIHSS score was an independent risk factor for stroke recurrence, death, and disability in ischemic stroke/TIA patients with NVAF. The NIHSS score had the greatest impacted on disability, followed by death, and then stroke recurrence. The NIHSS score was included in many deaths predictive models before 33, 34. After adding the NIHSS score into the CHADS2 and CHA2DS2‐VASc scores, the predictive ability for death and disability improved and the C statistic value reached near 0.7, while the predictive ability for stroke recurrence remained <0.6.
There are several limitations in this study. First, all the hospitals involved in the registry are from urban areas of China. They have better medical resources than the ones in rural areas. There is no data available on the hospitals in the vast rural areas of China. Second, the diagnosis of NVAF in the CNSR may have been suboptimal, as paroxysmal AF is more difficult to detect and the diagnosis can be easily missed relying only on self‐reporting and a single ECG. Third, the subtype of AF was not distinguished in our study. Previous research suggested that patients with paroxysmal AF had a similar risk for thromboembolic events when compared to the patients with persistent AF 35. Other studies found that paroxysmal AF occurred more often than persistent AF in stroke/TIA patients 36. However, our study did not classify the subtype of AF.
In conclusion, our study suggests that, in China, the clinical utilization of the CHADS2 and CHA2DS2‐VASc scores in predicting the long‐term outcome of the ischemic stroke or TIA patients with NVAF is limited. A one‐point increase in the CHADS2 or CHADS2‐VASc score was associated with an approximate 1.1 to 1.3‐fold increase of the risk for poor outcomes. Both age and stroke severity were independent risk factors of 1‐year stroke recurrence, death, and disability. Adding the baseline NIHSS score to the CHADS2 and CHA2DS2‐VASc scores improved the predictive value, especially for mortality and disability.
Conflict of Interest
The authors have no conflict of interest.
Acknowledgments
This study was funded by the Ministry of Science and Technology and the Ministry of Health of the People's Republic of China: National Science and Technology Major Project of China (2008ZX09312–008) and State Key Development Program of (for) Basic Research of China (2009CB521905).
References
- 1. Ihle‐Hansen H, Thommessen B, Wyller TB, Engedal K, Fure B. Risk factors for and incidence of subtypes of ischemic stroke. Funct Neurol 2012;27:35–40. [PMC free article] [PubMed] [Google Scholar]
- 2. Lin S, Wu B, Hao ZL, et al. Characteristics, treatment and outcome of ischemic stroke with atrial fibrillation in a chinese hospital‐based stroke study. Cerebrovasc Dis 2011;31:419–426. [DOI] [PubMed] [Google Scholar]
- 3. Wen‐Hang QI. Retrospective investigation of hospitalised patients with atrial fibrillation in mainland china. Int J Cardiol 2005;105:283–287. [DOI] [PubMed] [Google Scholar]
- 4. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: Results from the national registry of atrial fibrillation. JAMA 2001;285:2864–2870. [DOI] [PubMed] [Google Scholar]
- 5. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: The euro heart survey on atrial fibrillation. Chest 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 6. Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: The task force for the management of atrial fibrillation of the european society of cardiology (esc). Eur Heart J 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 7. Lip GY, Halperin JL. Improving stroke risk stratification in atrial fibrillation. Am J Med 2010;123:484–488. [DOI] [PubMed] [Google Scholar]
- 8. Keogh C, Wallace E, Dillon C, Dimitrov BD, Fahey T. Validation of the chads2 clinical prediction rule to predict ischaemic stroke. A systematic review and meta‐analysis. Thromb Haemost 2011;106:528–538. [DOI] [PubMed] [Google Scholar]
- 9. Olesen JB, Lip GY, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: Nationwide cohort study. BMJ 2011;342:d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lip GY, Frison L, Halperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation: A comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke 2010;41:2731–2738. [DOI] [PubMed] [Google Scholar]
- 11. Hong HJ, Kim YD, Cha MJ, et al. Early neurological outcomes according to CHADS(2) score in stroke patients with non‐valvular atrial fibrillation. Eur J Neurol 2012;19:284–290. [DOI] [PubMed] [Google Scholar]
- 12. Sato S, Yazawa Y, Itabashi R, Tsukita K, Fujiwara S, Furui E. Pre‐admission CHADS2 score is related to severity and outcome of stroke. J Neurol Sci 2011;307:149–152. [DOI] [PubMed] [Google Scholar]
- 13. Giralt‐Steinhauer E, Cuadrado‐Godia E, Ois A, et al. CHA(2)DS (2)‐VASc score and prognosis in ischemic strokes with atrial fibrillation. J Neurol 2012;259:745–751. [DOI] [PubMed] [Google Scholar]
- 14. Wang YJ, Zhang SM, Zhang L, et al. Chinese guidelines for the secondary prevention of ischemic stroke and transient ischemic attack 2010. CNS Neurosci Ther 2012;18:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Y, Cui L, Ji X, et al. The china national stroke registry for patients with acute cerebrovascular events: Design, rationale, and baseline patient characteristics. Int J Stroke 2011;6:355–361. [DOI] [PubMed] [Google Scholar]
- 16. WHO Task Force on stroke and other cerebrovascular disorders . Stroke‐1989: Recommendations on stroke prevention, diagnosis and therapy. Stroke 1989;20:1407–1421. [DOI] [PubMed] [Google Scholar]
- 17. Guo Y, Wu Q, Zhang L, et al. Antithrombotic therapy in very elderly patients with atrial fibrillation: Is it enough to assess thromboembolic risk? Clin Interv Aging 2010;5:157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ho LY, Siu CW, Yue WS, Lau CP, Lip GY, Tse HF. Safety and efficacy of oral anticoagulation therapy in chinese patients with concomitant atrial fibrillation and hypertension. J Hum Hypertens 2011;25:304–310. [DOI] [PubMed] [Google Scholar]
- 19. Olesen JB, Torp‐Pedersen C, Hansen ML, Lip GY. The value of the cha2ds2‐vasc score for refining stroke risk stratification in patients with atrial fibrillation with a chads2 score 0–1: A nationwide cohort study. Thromb Haemost 2012;107:1172–1179. [DOI] [PubMed] [Google Scholar]
- 20. Pieri A, Lopes TO, Gabbai AA. Stratification with cha2ds2‐vasc score is better than chads2 score in reducing ischemic stroke risk in patients with atrial fibrillation. Int J Stroke 2011;6:466. [DOI] [PubMed] [Google Scholar]
- 21. Kim YD, Cha MJ, Kim J, et al. Increases in Cerebral Atherosclerosis According to CHADS2 Scores in Patients With Stroke With Nonvalvular Atrial Fibrillation. Stroke 2011;42:930–934. [DOI] [PubMed] [Google Scholar]
- 22. Hart RG, Pearce LA. Current status of stroke risk stratification in patients with atrial fibrillation. Stroke 2009;40:2607–2610. [DOI] [PubMed] [Google Scholar]
- 23. Independent predictors of stroke in patients with atrial fibrillation . A systematic review. Neurology 2007;69:546–554.17679673 [Google Scholar]
- 24. Heeringa J, van der Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: The rotterdam study. Eur Heart J 2006;27:949–953. [DOI] [PubMed] [Google Scholar]
- 25. Deng YX, Wang YL, Gao BQ, et al. Age Differences in Clinical Characteristics, Health care, and Outcomes after Ischemic Stroke in China. CNS Neurosci Ther 2012;18:819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Olesen JB, Fauchier L, Lane DA, Taillandier S, Lip GY. Risk factors for stroke and thromboembolism in relation to age among patients with atrial fibrillation: The loire valley atrial fibrillation project. Chest 2012;141:147–153. [DOI] [PubMed] [Google Scholar]
- 27. Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the birmingham atrial fibrillation treatment of the aged study, bafta): A randomised controlled trial. Lancet 2007;370:493–503. [DOI] [PubMed] [Google Scholar]
- 28. Muir KW, Weir CJ, Murray GD, Povey C, Lees KR. Comparison of neurological scales and scoring systems for acute stroke prognosis. Stroke 1996;27:1817–1820. [DOI] [PubMed] [Google Scholar]
- 29. Goldstein LB, Samsa GP. Reliability of the national institutes of health stroke scale. Extension to non‐neurologists in the context of a clinical trial. Stroke 1997;28:307–310. [DOI] [PubMed] [Google Scholar]
- 30. Smith EE, Shobha N, Dai D, et al. Risk score for in‐hospital ischemic stroke mortality derived and validated within the get with the guidelines‐stroke program. Circulation 2010;122:1496–1504. [DOI] [PubMed] [Google Scholar]
- 31. Lamassa M, Di Carlo A, Pracucci G, et al. Characteristics, outcome, and care of stroke associated with atrial fibrillation in europe: Data from a multicenter multinational hospital‐based registry (the european community stroke project). Stroke 2001;32:392–398. [DOI] [PubMed] [Google Scholar]
- 32. Hannon N, Sheehan O, Kelly L, et al. Stroke associated with atrial fibrillation–incidence and early outcomes in the north dublin population stroke study. Cerebrovasc Dis 2010;29:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johnston KC, Wagner DP, Wang XQ, et al. Validation of an acute ischemic stroke model: Does diffusion‐weighted imaging lesion volume offer a clinically significant improvement in prediction of outcome? Stroke 2007;38:1820–1825. [DOI] [PubMed] [Google Scholar]
- 34. Weimar C, Konig IR, Kraywinkel K, Ziegler A, Diener HC. Age and national institutes of health stroke scale score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: Development and external validation of prognostic models. Stroke 2004;35:158–162. [DOI] [PubMed] [Google Scholar]
- 35. Hohnloser SH, Pajitnev D, Pogue J, et al. Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: An ACTIVE W substudy. J Am Coll Cardiol 2007;50:2156–2161. [DOI] [PubMed] [Google Scholar]
- 36. Rizos T, Wagner A, Jenetzky E, et al. Paroxysmal atrial fibrillation is more prevalent than persistent atrial fibrillation in acute stroke and transient ischemic attack patients. Cerebrovasc Dis 2011;32:276–282. [DOI] [PubMed] [Google Scholar]


