Summary
Introduction
The delta opioid peptide [D‐Ala2, D‐Leu5]enkephalin (DADLE) plays a key role in neuronal protection against both hypoxic and ischemic conditions. However, the cellular mechanisms of action of DADLE under these conditions remain unclear.
Methods
Ischemia was simulated with perfusing the brain slices with glucose‐free artificial cerebrospinal fluid. Apoptosis was examined using an in situ cell death detection kit and expressed as the percentage of positively labeled neurons relative to total number of neurons. PCR was performed by adding cDNA, 5 pm dNTP, 1 μL Taqase, and primers. PCR products were separated with electrophoresis, stained with ethidium bromide, and visualized under ultraviolet light.
Aims
To investigate the potential effects of DADLE in an ex vivo model of cerebral ischemia/reperfusion.
Results
DADLE attenuated lactic dehydrogenase release and neuronal apoptosis in a concentration‐dependent manner. The protective effects of DADLE were attenuated by representative selective delta2, but not delta1 opioid antagonists. Treatment with PD98059, a selective inhibitor of ERK kinase (MEK), also blocked the protective effect of DADLE as well as ERK phosphorylation induced by DADLE.
Conclusions
Endogenous opioid peptides could promote cell survival via delta2 opioid receptors, possibly through the downstream MEK‐ERK pathway.
Keywords: Apoptosis; Brain slices; D‐Ala2, D‐Leu5; ERK1/2; Ischemia/reperfusion
Introduction
[D‐Ala2,D‐Leu5]enkephalin (DADLE), a delta opioid peptide, protects the brain against ischemia‐induced insults 1, 2. Putative mechanisms underlying the protective properties of DADLE include antioxidation 3. Other studies have indicated that the neuroprotective effects of DADLE against methamphetamine‐induced dopaminergic terminal damage are mediated by opioid receptors 4, 5. Antiapoptotic effects apparently also contribute to the protective action of delta opioids both in vitro and in vivo 2, 5. Delta opioid receptors could stimulate extracellular signal‐regulated protein kinases (ERK), which are members of the mitogen‐activated protein kinases (MAPK) 6. Because ERKs are implicated in cell survival 7, 8, it is possible that DADLE might induce an antiapoptotic property by activating the MAPK pathway via opioid receptors 8. Indeed, it is suggested that ERK activation may promote cell survival via the MEK‐ERK pathway perhaps through delta2 opioid receptors 9.
In the current study, we examined the protective effects of DADLE against ischemia/reperfusion injury in a model system using rat brain slices. Ischemia/reperfusion was simulated by removing oxygen/glucose from the perfusate, followed by re‐oxygenation 10, 11. Potential role of delta receptor subtypes and the MAPK pathway were also investigated.
Methods
Animals
Male Sprague–Dawley rats (Grade II, Certificate No. 152) weighing 90–120 g were purchased from Shanghai Experimental Animal Center, Chinese Academy of Sciences. All animal experiments were approved by the Institutional Animal Care and Use Committee.
Reagents and Drugs
Lactic dehydrogenase (LDH) detection kit was purchased from Jiancheng Bioengineering Institution (Jiancheng, Nanjing, China). [D‐Ala, D‐Leu]enkephalinamide (DADLE, a selective delta opioid receptor agonist), naltrindole (a selective delta opioid receptor antagonist), 7‐benzylidene‐naltrexone (BNTX; a selective delta1 antagonist), naltriben (a selective delta2 opioid antagonist), and naloxone (a nonselective opioid antagonist) were all purchased from Sigma‐Aldrich (St. Louis, MO, USA).
Cerebral Cortical Slice Preparation
Brain slices (400 μm thick) were prepared as previously described 11 using a vibrating tissue slicer (ZQP‐86; Zhixin Co., Ltd., Shanghai, China), with minor modifications. Briefly, rats were decapitated, and the brain was quickly removed and immersed in ice‐cold oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid (ACSF) with the following composition (in mM): NaCl 119, KC1 2.5, CaCl2 2, MgSO4 1, NaH2PO4 1.25, NaHCO3 26.2, glucose 10, with pH 7.4. Slices were equilibrated at room temperature (24°C) for 90 min and then at 37°C for 30 min prior to experiments.
Ischemia/Reperfusion Experiments
Ischemia was simulated with perfusing the brain slices with glucose‐free ACSF (glucose was substituted by sucrose of equimolar concentration) and saturated with 95% N2 + 5% CO2 for 10 min. Reperfusion was simulated with perfusion in oxygenated normal ACSF (containing glucose) and saturated with 95% O2/5% CO2 for 2 h.
DADLE (10−9–10−5 M), in the presence or absence of one of the following compounds, was included in the ACSF in some experiments: naltrindole (10 μM), BNTX (10 μM), NTB (10 μM), and naloxone (10 μM).
Lactate Dehydrogenase Assay
Lactic dehydrogenase in the perfusate was examined as previously described 12 using a commercial kit from Jiancheng Bioengineering Institution and used to reflect neuronal damage. LDH concentration in brain slices was subjected to ischemia/reperfusion, but not any other drug treatment was set at 100%.
In Situ Labeling of DNA Fragmentation
At the end of the experiments, brain slices were fixed at 4°C in 4% paraformaldehyde for 2 h. Sections (6‐μm thick) were prepared with routine procedures. Apoptosis was examined using an in situ cell death detection kit from Roche Applied Science (Mannheim, Germany). Briefly, tissue sections were rehydrated by heating at 60°C, followed by washing in xylene and rehydration through a graded series of ethanol. Sections were then incubated with protease for 15 min at 37°C, followed by 1% Triton X‐100 for 8 min. Slides where then rinsed twice with PBS. Fifty microliter of TUNEL reaction mixture was added to the tissue, and incubation lasted for 60 min at 37°C in a humidified atmosphere in the absence of light. The slides were rinsed three times in PBS prior to addition of 50 μL converter‐POD. Tissue sections were incubated in a humidified chamber for 30 additional min at 37°C and rinsed three times with PBS prior to DAB color reaction and microscopic examination using an Axioplan 2 imaging system (ZEISS, Jena, Germany). Apoptosis is expressed as the percentage of positively labeled neurons relative to total number of neurons.
RT‐PCR Procedures
Total RNA was extracted from brain slices using a Trizol reagent (Invitrogen, CA, USA). cDNA was synthesized from 1 μg of pooled mRNA using Oligo dT Primer (50 pmol), 20 pmol dNTPs, 20 U RNase Inhibitor (Promega, San Luis Obispo, CA, USA), and 200 μM‐MLV Rtase (Promega). PCR was performed by adding cDNA, 5 pm dNTP, 1 μL Taqase, and one of the following primers:
Bcl‐2 sense, 5′‐CTGGTGGACAACATCGCTCTG‐3′ and antisense, 5′‐GGTCTGCTGACCTCACTTGTG‐3′; Bax sense, 5′‐TCCAGGATCGAGCAGA‐3′ and antisense, 5′‐AAGTAGAAGAGGGCAACC‐3′; β‐actin sense, 5′‐ATTGTAACCAACTGGGACG‐3′ and antisense, 5′‐TTGCCGATAGTGATGACCT‐3′. All primers were designed using the DNAstar PrimerSelect program (Lasergene, Madison, WI, USA) and synthesized by CASarray (Shanghai, China). PCR products were separated with electrophoresis, stained with ethidium bromide, and visualized under ultraviolet light. Optical density was semiquantitatively analyzed with a Tanon GIS gel imaging system (Bio‐Tanon, Shanghai, China). mRNA expression was normalized using β‐actin as an internal control.
Western Blot Analysis
Brain slices were homogenized in 20 mM HEPES (pH 7.5) buffer containing: 250 mM sucrose, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride, 2 μg/mL aprotinin, 10 μg/mL leupeptin, 5 μg/mL pepstatin, and 12.5 μg/mL Nacetyl‐leu‐leu‐norleucinal, and 1:100 dilution phosphatase inhibitor cocktail I and II (Sigma‐Aldrich, St. Louis, MO, USA). The homogenate was centrifuged at 8000 × g for 20 min at 4°C. Protein concentration of the supernatant was determined using a Bradford method (Bio‐Rad, Hercules, CA, USA). Twenty micrograms of protein from each sample was separated by 12% SDS‐PAGE and transferred to a polyvinylidene difluoride membrane, prior to incubation with a primary antibody against one of the following proteins: phospho‐ versus total ERK1, phospho‐ versus total ERK2 (all at 1:1000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After incubation with an appropriate secondary antibody and color reaction, the bands of interest were quantified using an image analysis system (Image; NIH, Bethesda, MD, USA).
Statistical Analysis
Morphologic assessments and biochemical assays were performed in a minimum of eight slices for each treatment condition. All data are presented as mean ± SEM and analyzed with one‐way ANOVA followed by Fisher's PLSD post hoc test. Statistical significance was set at P < 0.05.
Results
DADLE attenuated LDH release from the brain slices in a concentration‐dependent manner (Figure 1A). The inhibitory effects of DADLE at a representative concentration (10 mM) were attenuated by nonselective opioid antagonists naltrindole and naloxone, the selective delta2 opioid antagonist naltriben, but not the selective delta1 antagonist BNTX (Figure 1B). Consistent with the LDH results, DADLE (10−6 and 10−5 M) significantly attenuated neuronal apoptosis (Figure 2).
Figure 1.
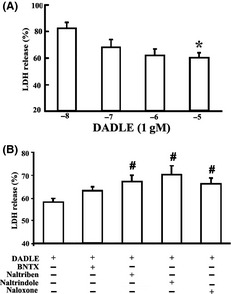
Effects of DADLE on I/R injury in brain slices. (A) Effects of DADLE alone. *P < 0.05 versus blank control. (B) Interaction of DADLE (10−5 M) with representative opioid antagonists (all at 10 μM). #P < 0.05 versus I/R plus DADLE. The values represent mean ± SD from five to six independent experiments.
Figure 2.
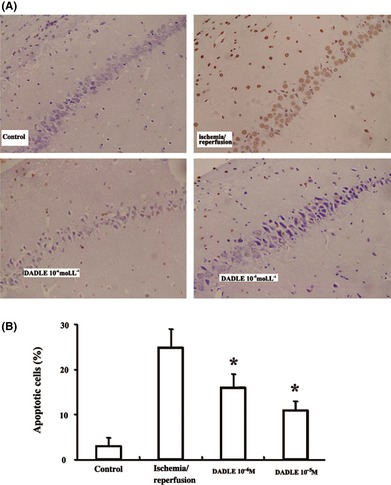
Effects of DADLE on neuronal apoptosis. (A) Representative slides of TUNEL staining; ×400. (B) Summary of the results. The values represent mean ± SD of four independent experiments. *P < 0.05 versus I/R injury alone.
Ischemia/reperfusion increased the mRNAs (Figure 3A) and protein (Figure 4A) level for both Bcl‐2 and Bax. Treatment with DADLE (10−6 and 10−5 M) significantly enhanced Bcl‐2 mRNA expression but suppressed Bax mRNA expression (Figure 3B). As a result, the Bax/Bcl‐2 ratio was reduced with DADLE (Figure 4B).
Figure 3.
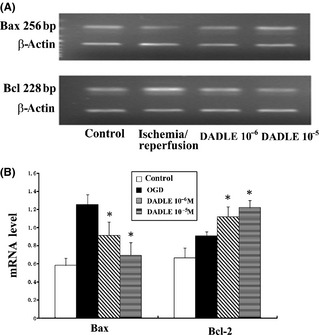
Effects of DADLE on Bax and Bcl‐2 mRNA expression. (A) Representative mRNA blots. (B) Summary of results. All values represent mean ± SD of three independent experiments. *P < 0.05 versus I/R injury alone.
Figure 4.
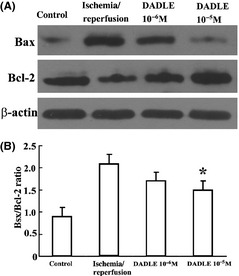
Effects of DADLE on Bax and Bcl‐2 protein expression. (A) Representative immunoblots. (B) Effects on Bax/Bcl‐2 ration; a summary of results. *P < 0.05 versus I/R injury alone. All values represent mean ± SD of three independent experiments.
DADLE increased ERK phosphorylation (Figure 5). Increased ERK phosphorylation by DADLE was abolished by naltrindole (Figure 6A,B). Treatment of the brain slices with PD98059, a selective MEK inhibitor upstream of ERK, also abolished the DADLE‐induced phosphorylation of ERK (Figure 6C,D).
Figure 5.
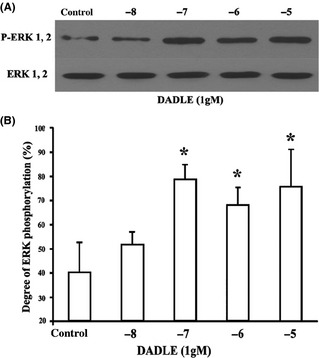
Effects of DADLE on ERK phosphorylation. (A) Representative immunoblots. (B) Summary of results. All values represent mean ± SD of four independent experiments. *P < 0.05 versus I/R injury alone.
Figure 6.
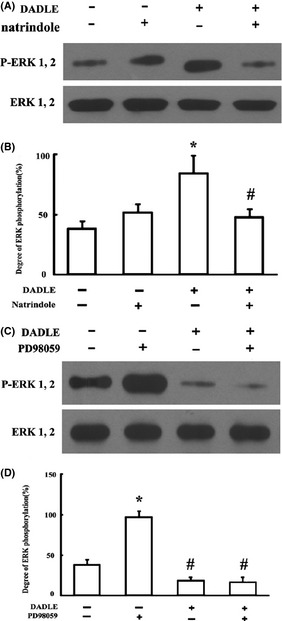
Interaction of DADLE with natrindole and PD98059 on ERK phosphorylation. (A) Effects of DADLE in the absence versus presence of naltrindole (10 μM), representative immunoblots. (B) Summary of results. *P < 0.05 versus I/R injury alone (DADLE −; natrindole −); #P < 0.05 versus I/R plus DADLE. (C) Effects of DADLE in the absence versus presence of PD98059 (100 mM), representative immunoblots. (D) Summary of results. *P < 0.05 versus I/R injury alone (DADLE −; natrindole −); #P < 0.05 versus I/R plus DADLE. All values represent mean ± SD of four independent experiments.
Conclusion
Results from this study suggested that the delta opioid peptide DADLE could protect the brain from ischemia/reperfusion injury in a concentration‐dependent manner, most likely by attenuating apoptosis via the MEK/ERK antiapoptotic pathway. Consistent with a previous study 13, the protective effects of DADLE seemed to be mediated by delta2 opioid receptor. It is noteworthy that DADLE has been reported to exhibit dual pharmacological actions in neocortical neurons 14, 15. It was recently reported that the delta opioid peptide 4‐((2,5‐dimethyl‐4‐(2‐propenyl)‐1‐piperazinyl)(3‐methoxyphenyl)methyl)‐N, N‐diethylbenzamide (2S‐(1(S*),2alpha,5beta))‐(9Cl) (SNC‐80) could inhibit HIV‐1 expression in DOR‐transfected lymphocytes, whereas the delta2 antagonist naltrindole could block this effect 16.
The fact that the effects of DADLE could be attenuated by naltrindole supports the notion that the antiapoptotic action of DADLE is mediated by the activation of delta2 opioid receptors 17, 18. Although two delta opioid receptor subtypes have been defined pharmacologically 12, only one gene (DOR‐1) was cloned 19. A recent study on DOR‐1 knockout mice demonstrated that both delta1 and delta2 subtypes of opioid receptors were abolished in these mice, suggesting that the pharmacologically distinct delta1 and delta2 subtypes of opioid receptors are encoded by the DOR‐1 gene 20. The different pharmacological profiles observed for the two delta subtypes might arise from posttranslational protein modification and/or alternative splicing 21.
In our study, the protective effects of DADLE were observed at the micromolar range; such results were consistent with the previous findings in primary culture of neocortical neurons 22 but not in PC12 cells 13, suggesting that the results from immortalized cell line should be extrapolated with caution.
The results from this study demonstrated a role of MEK/ERK in the antiapoptotic action of DADLE. Potential role of Akt, also known as protein kinase B, a serine/threonine protein kinase that plays an important role in multiple cellular processes 23, in the action of DADLE cannot be ruled out 23. Activation of the phosphoinositide 3‐kinase (PI3K), and its downstream signaling molecules, for example, the MEK/ERK and the Akt/p70S6 pathways, produces potent antiapoptotic action 24. It will be important that further studies clarify the potential involvement of Akt with DADLE action.
One recent study suggested that the cardioprotective effect of ÿ‐opioid receptor agonists is mediated by Gi/Go proteins and mitochondrial K channels 25, 26. More specifically, functional inhibition or blocking of Gi/Go proteins and K channels could attenuate ÿ‐opioid receptor‐induced cardioprotection 25, 26. Opening of plasma membrane K channels has been shown to be protective in neuronal adaptation to hypoxic stress 26, but whether the G protein–K channel pathway is involved in the protective effects of ÿ‐opioid receptors remains unclear. It is also possible that delta opioid receptor could produce neuroprotection by modulating protein kinase C (PKC) activity 25. In fact, there is evidence showing that the cardioprotective effect of opioid receptors is mediated through a PKC pathway 27. In addition, modulation of voltage‐dependent Ca2+ channels may be involved in delta opioid receptor–mediated neuronal protection 28.
In summary, results from the current study indicated that endogenous opioid peptides may play important roles in regulating both cell survival and cell death through distinct subtypes of opioid receptors. Our findings also encourage development of delta opioids in the treatment of ischemic brain diseases.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1. Iwata M, Inoue S, Kawaguchi M, Nakamura M, Konishi N, Furuya H. Effects of delta‐opioid receptor stimulation and inhibition on hippocampal survival in a rat model of forebrain ischaemia. Br J Anaesth 2007;99: 538–546. [DOI] [PubMed] [Google Scholar]
- 2. Duan YL, Wang SY, Zeng QW, et al. Astroglial reaction to delta opioid peptide [d‐Ala2, d‐Leu5] enkephalin confers neuroprotection against global ischemia in the adult rat hippocampus. Neuroscience 2011;192: 81–90. [DOI] [PubMed] [Google Scholar]
- 3. Yang Y, Xia X, Zhang Y, et al. Delta‐opioid receptor activation attenuates oxidative injury in the ischemic rat brain. BMC Biol 2009;26: 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borlongan CV, Wang Y, Su TP. Delta opioid peptide (D‐Ala 2, D‐Leu 5) enkephalin: Linking hibernation and neuroprotection. Front Biosci 2004;9: 3392–3398. [DOI] [PubMed] [Google Scholar]
- 5. Ke S, Su DS, Wang XR. Delta opioid agonist [D‐Ala2, D‐Leu5] enkephalin (DADLE) reduced oxygen‐glucose deprivation caused neuronal injury through the MAPK pathway. Brain Res 2009;1292: 100–106. [DOI] [PubMed] [Google Scholar]
- 6. Xie N, Li H, Wei D, et al. Glycogen synthase kinase‐3 and p38 MAPK are required for opioid‐induced microglia apoptosis. Neuropharmacology 2010;59: 444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kang KA, Wang ZH, Zhang R, et al. Myricetin protects cells against oxidative stress‐induced apoptosis via regulation of PI3K/Akt and MAPK signaling pathways. Int J Mol Sci 2010;11: 4348–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu XS, Jiang J. Induction of apoptosis and regulation of the MAPK pathway by ursolic acid in human leukemia K562 cells. Planta Med 2007;73: 1192–1194. [DOI] [PubMed] [Google Scholar]
- 9. Eisinger DA, Ammer H. Delta‐opioid receptors activate ERK/MAP kinase via integrin‐stimulated receptor tyrosine kinases. Cell Signal 2008;20: 2324–2331. [DOI] [PubMed] [Google Scholar]
- 10. Serbinek D, Ullrich C, Pirchl M, Hochstrasser T, Schmidt‐Kastner R, Humpel C. S100b counteracts neurodegeneration of rat cholinergic neurons in brain slices after oxygen‐glucose deprivation. Cardiovasc Psychiatry Neurol. DOI: 10.1155/2010/106123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ricci L, Valoti M, Sgaragli G, Frosini M. Protection by taurine of rat brain cortical slices against oxygen glucose deprivation‐and reoxygenation‐induced damage. Eur J Pharmacol 2009;621: 26–32. [DOI] [PubMed] [Google Scholar]
- 12. Wild KD, Porreca F, Yamamura HI, Raffa RB. Differentiation of receptor subtypes by thermodynamic analysis: Application to opioid delta receptors. Proc Natl Acad Sci USA 1994;91: 12018–12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayashi T, Tsao LI, Su TP. Antiapoptotic and cytotoxic properties of delta opioid peptide [D‐Ala(2),D‐Leu(5)]enkephalin in PC12 cells. Synapse 2002;43: 86–94. [DOI] [PubMed] [Google Scholar]
- 14. Tao R, Auerbach SB. Opioid receptor subtypes differentially modulate serotonin efflux in the rat central nervous system. J Pharmacol Exp Ther 2002;303: 549–556. [DOI] [PubMed] [Google Scholar]
- 15. Zhang J, Haddad GG, Xia Y. Delta‐, but not mu‐ and kappa‐, opioid receptor activation protects neocortical neurons from glutamate‐induced excitotoxic injury. Brain Res 2000;885: 143–153. [DOI] [PubMed] [Google Scholar]
- 16. Sharp BM, Gekker G, Li MD, Chao CC, Peterson PK. Delta‐opioid suppression of human immunodeficiency virus‐1 expression in T cells (Jurkat). Biochem Pharmacol 1998;56: 289–292. [DOI] [PubMed] [Google Scholar]
- 17. Borlongan CV, Hayashi T, Oeltgen PR, Su TP, Wang Y. Hibernation‐like state induced by an opioid peptide protects against experimental stroke. BMC Biol 2009;7: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yao LL, Wang YG, Cai WJ, Yao T, Zhu YC. Survivin mediates the anti‐apoptotic effect of delta‐opioid receptor stimulation in cardiomyocytes. J Cell Sci 2007;120: 895–907. [DOI] [PubMed] [Google Scholar]
- 19. Kieffer BL, Befort K, Gaveriaux‐Ruff C, Hirth CG. The delta‐opioid receptor: Isolation of a cDNA by expression cloning and pharmacological characterization. Proc Natl Acad Sci U S A 1992;89: 12048–12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhu Y, King MA, Schuller AG, et al. Retention of supraspinal delta‐like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron 1999;24: 243–252. [DOI] [PubMed] [Google Scholar]
- 21. Allouche S, Hasbi A, Ferey V, Sola B, Jauzac P, Polastron J. Pharmacological delta1‐and delta2‐opioid receptor subtypes in the human neuroblastoma cell line SK‐N‐BE: No evidence for distinct molecular entities. Biochem Pharmacol 2000;59: 915–925. [DOI] [PubMed] [Google Scholar]
- 22. Zhang J, Qian H, Zhao P, Hong SS, Xia Y. Rapid hypoxia preconditioning protects cortical neurons from glutamate toxicity through delta‐opioid receptor. Stroke 2006;37: 1094–1099. [DOI] [PubMed] [Google Scholar]
- 23. Wang S, Duan Y, Su D, et al. Delta opioid peptide [D‐Ala2, D‐Leu5] enkephalin (DADLE) triggers postconditioning against transient forebrain ischemia. Eur J Pharmacol 2011;658: 140–144. [DOI] [PubMed] [Google Scholar]
- 24. Shao Y, Aplin AE. Akt3‐mediated resistance to apoptosis in B‐RAF‐targeted melanoma cells. Cancer Res 2010;70: 6670–6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fryer RM, Hsu AK, Eells JT, Nagase H, Gross GJ. Opioid‐induced second window of cardioprotection: Potential role of mitochondrial KATP channels. Circ Res 1999;84: 846–851. [DOI] [PubMed] [Google Scholar]
- 26. Varga EV, Yamamura HI, Rubenzik MK, Stropova D, Navratilova E, Roeske WR. Molecular mechanisms of excitatory signaling upon chronic opioid agonist treatment. Life Sci 2003;74: 299–311. [DOI] [PubMed] [Google Scholar]
- 27. Cao CM, Xia Q, Tu J, Chen M, Wu S, Wong TM. Cardioprotection of interleukin‐2 is mediated via kappa‐opioid receptors. J Pharmacol Exp Ther 2004;309: 560–567. [DOI] [PubMed] [Google Scholar]
- 28. Acosta CG, Lopez HS. Delta opioid receptor modulation of several voltage‐dependent Ca (2+) currents in rat sensory neurons. J Neurosci 1999;19: 8337–8348. [DOI] [PMC free article] [PubMed] [Google Scholar]


