Summary
Aims
Cordycepin plays an important role in modulating the function of central nervous system (CNS). However, the modulating mechanism is poorly understood. Excitatory synaptic transmission, the essential process in brain physiology and pathology, is critical in the signal integration activities of the CNS. To further understand the effects of cordycepin on CNS, we investigated the effects of cordycepin on excitatory synaptic transmission in the CA1 region of rat hippocampal slices.
Methods
The effects of cordycepin on excitatory synaptic transmission were investigated by using in vitro field potential electrophysiology and whole‐cell patch clamp techniques.
Results
Cordycepin significantly decreased the amplitudes of field excitatory postsynaptic potentials (fEPSPs) elicited in the CA1 by stimulation of the Schaffer‐commissural fibers. And the reduction in fEPSPs amplitude was associated with an increase in the paired‐pulse facilitation. Cordycepin also suppressed α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazole‐propionic acid (AMPA) and N‐methyl‐d‐aspartic acid (NMDA) receptor‐mediated responses but did not directly affect AMPA receptors and NMDA receptors. Furthermore, quantal analysis revealed that cordycepin decreased the frequency but not amplitude of miniature spontaneous excitatory postsynaptic currents.
Conclusions
These results demonstrate that cordycepin suppresses excitatory synaptic transmission by decreasing the excitatory neurotransmitter release presynaptically, which provides an evidence for the novel potential mechanism of cordycepin in modulating the function of CNS.
Keywords: AMPA, Cordycepin, NMDA, Paired‐pulse facilitation, Synaptic transmission
Introduction
Cordyceps militaris is a rare fungus in traditional Chinese medicine. It has been widely used in China for anti‐aging and nourishing 1 and for the prevention and treatment of a variety of diseases in the circulatory, immune, respiratory, and glandular systems 2, 3, 4. Cordycepin (3‐deoxyadenosine), a major functional component of Cordyceps millitaris 5, has been shown to have antitumor 6, anti‐inflammatory 7, antidiabetic 8, and renoprotective effects 9. Besides, increasing evidences also indicated that cordycepin may play an important role in modulating the function of central nervous system (CNS). It was reported that the extract of C. millitaris prevented neuronal death and ameliorated β‐amyloid peptide‐induced memory deficits in rats 10. Cordycepin has significant neuro‐protective effects against damage resulting from ischemia/reperfusion insult through its free radical scavenging activity 11, 12. These results indicate that cordycepin is an important mediator in the neuroinflammatory responses in brain trauma, ischemic brain injury, and some neurodegenerative disorders including β‐amyloid peptide‐induced‐Alzheimer's disease.
Our previous study has shown that cordycepin can decrease the neuron activity through membrane hyperpolarization 13, which indicated a novel potential mechanism of cordycepin in modulating the responses of CNS by adjusting its electrophysiologic properties. To better understand the modulating function of cordycepin on the CNS, we examined the effects of cordycepin on excitatory synaptic transmission in the CA1 region of rat hippocampal brain slices by using in vitro field potential electrophysiology and whole‐cell patch clamp techniques.
Material and Methods
Chemicals and Regents
Chemicals used for making artificial cerebrospinal fluid (ACSF), tetrodotoxin (TTX), ATP‐Na, 6‐cyano‐7‐nitroquinoxaline‐2,3‐dione (CNQX), DL‐2‐Amino‐5‐phosphonovaleric acid (APV), and bicuculline were purchased from Sigma Co. (St. Louis, MO, USA). Cordycepin with a purity of more than 98% was provided from our university 14. Cordycepin was dissolved in ACSF at concentration of 10, 20, 50, and 100 mg/L, and its effects were tested by bath perfusion (solution exchange was completed in about 30 seconds).
Preparation of Hippocampal Brain Slices
The care and use of animals and the experimental protocol of this study were approved by the Institutional Care and Use Committee of our university. All experiments were performed on the CA1 region of hippocampal brain slices prepared from 15 to 30 days old Sprague‐Dawley rats as described previously 15. Briefly, animals were anesthetized with isoflurane and decapitated. The brains were quickly removed from cranial cavity and placed into an ice‐cold (~4°C) oxygenated ACSF containing (mM): NaCl 117, KCl 4.7, MgCl2 1.2, NaH2PO4 1.2, NaHCO3 25, CaCl2 2.5, d‐glucose 10, and saturated with 95% O2/5% CO2, pH 7.4. The hippocampus was dissected free, and transverse hippocampal slices (400 μm in thickness) were cut using a tissue chopper. Slices were allowed to recover for at least 1 h prior to experiments. Individual slices were transferred to a recording chamber, which was continually perfused with ACSF at a rate of 4 mL/min. All experiments were performed at room temperature (22–25°C).
Field Excitatory Postsynaptic Potentials Recording
Field excitatory postsynaptic potentials (EPSPs) were recorded in CA1 region with recording electrodes (filled with 2 M NaCl) made from borosilicate glass capillaries (World Precision Instruments, Sarasota, FL, USA). Field EPSPs were evoked by electrical stimulation of the Schaffer‐collateral fibers/commissural pathway with a bipolar nichrome‐stimulating electrode. The stimulation intensity was adjusted initially to produce typical value between 1.5–2.5 mV on the amplitude of half‐maximal fEPSPs, and sampling was carried out using pulses delivered once every 20 seconds. In the experiments in which paired‐pulse facilitation (PPF) was elicited, a paired‐pulse stimulus at interpulse intervals of 25 milliseconds was applied once every 20 seconds. Recordings were collected until a stable baseline was present for 10–20 min; if this condition did not occur the slice was not included in the study. After establishing baseline conditions, the effects of cordycepin were tested by bath application for 15 min. Synaptic responses mediated by α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazole‐propionic acid (AMPA) receptors were measured by pretreatment with N‐methyl‐d‐aspartic acid (NMDA) receptor antagonist APV (50 μM), and NMDA receptor‐mediated synaptic responses were measured by changing the ACSF to a low concentration of MgCl2 (50 μM) and infusing the AMPA receptor antagonist CNQX (10 μM) 16. Field EPSPs were digitized and stored, and measures of amplitude were collected online.
Whole Cell‐Current Recording
Recordings were performed in whole‐cell voltage‐clamp configuration. The resistance of the recording electrodes was 3–5 MΩ when filled with intracellular solution. For recording miniature spontaneous excitatory postsynaptic currents (mEPSCs), the intracellular solution was composed of the followings (mM): K‐gluconate 140, MgCl2 2, Na‐ATP 2, Na‐GTP 0.2, EGTA 5, HEPES 10, pH 7.3; osmolarity was adjusted to 320 mOsm with sucrose. mEPSCs were recorded under voltage clamp at −70 mV in the presence of 100 μM APV, 30 μM bicuculline and 1 μM TTX. The recorded neuronal cells were allowed to stabilize for 5–10 min till the stable conditions. The effects of cordycepin were tested by bath perfusion for 3 min.
Data were acquired by Clampex 9.2 (Molecular Devices, Sunnyvale, CA, USA) via a digidata 1322 series A/D board set to a sampling frequency of 10 kHz. After whole‐cell access was achieved, the series resistance was partially compensated by the amplifier. Both input resistance and series resistance were monitored throughout the experiments. Only those recordings with a stable series resistance (≤20 MΩ) and input resistance were accepted.
Data Analysis
To characterize the effects of cordycepin on the evoked field potentials in the hippocampal CA1 region, the mean of the fEPSPs amplitudes recorded under stable conditions at the start of the experiment was taken as the baseline, and the amplitudes following drug application were expressed as a percentage of baseline. In the experiments in which PPF was elicited, the PPF ratio was calculated by dividing the mean peak amplitude of the second fEPSPs by the mean peak amplitude of the first one. When calculating the effects of cordycepin on the AMPA receptor or NMDA receptor‐mediated synaptic responses, the mean of the fEPSPs amplitudes recorded from steady‐state stage under the pretreatment of the NMDA receptor antagonist APV or AMPA receptor antagonist CNQX was taken as the baseline.
Miniature synaptic events of mEPSCs were counted and analyzed offline using Mini Analysis software (version 6.0.3; Synaptosoft, Decatur, GA, USA) and Igor 6.0 (OriginLab, Northampton, MA, USA). The peak amplitude threshold for detection of an event was set at the level five times higher than the root‐mean‐square noise. All deflections from baseline that were greater than the threshold were detected. Selected events were then visually examined, and any spurious events were manually rejected, whereas any missed events were flagged for inclusion in the mean amplitude and frequency calculations. Frequencies were calculated by dividing the total number of mEPSCs events by the total time sampled.
All data were presented as mean ± SEM, unless otherwise indicated. Statistical significance of difference was calculated using Student's paired t‐test or the Kolmogorov–Smirnov test. A level of confidence of P < 0.05 was employed for the statistical significance.
Results
Reduction in fEPSPs by Cordycepin in Hippocampal Slices
The effects of cordycepin on the synaptic responses elicited in the CA1 by stimulation of the Schaffer‐commissural fibers are illustrated in Figure 1. Bath perfusion of hippocampal slices with cordycepin profoundly suppressed the amplitude of fEPSPs in a concentration‐dependent manner. The cordycepin‐mediated decrease in fEPSPs appeared 1–3 min after cordycepin reached the chamber. It peaked at 5–10 min and recovered gradually to the baseline level after washout out in 5–30 min, indicating that the effects of cordycepin are reversible. At concentrations of 10, 20, 50, and 100 mg/L, the resultant percentages of fEPSPs amplitudes were decreased to 90.6 ± 10.68% (8 slices, 5 animals), 50.29 ± 29.22% (12 slices, 6 animals), 32.01 ± 19.92% (10 slices, 6 animals), and 0 (6 slices, 4 animals), respectively (Figure 1); the recovered time to the baseline level after washout out was 3–5, 5–10, 10–30, and 30–60 min, respectively. As the concentration of 20 mg/L was found to significantly decrease fEPSPs (P < 0.01) and recovered quickly after washout, this concentration was adopted for the subsequent tests.
Figure 1.
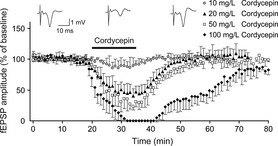
Effects of cordycepin on fEPSPs elicited in the CA1 region by stimulation of the Schaffer‐commissural fibers. Sample traces (top) show fEPSPs recorded before, during, and after bath application of cordycepin (20 mg/L). The bottom graph shows time courses of the effects of different concentrations of cordycepin (10, 20, 50, and 100 mg/L) on the amplitude of fEPSPs. Note that in this and in the following figure, each data point in the graph plots, the average of the peak amplitudes of three concecutive fEPSPs and expressed as a percentage of basal values. Bath application of cordycepin is indicated by a horizontal bar.
Cordycepin Increases Paired‐Pulse Facilitation
PPF is a short‐term presynaptic plasticity, and the change in PPF ratio reflected the alterations in synaptic efficacy, determined by the probability of neurotransmitter release 17. We examined the PPF ratio during application of cordycepin to verify a possible effect of presynaptic release of glutamate. In all 12 fEPSPs recordings (12 slices, 6 animals), cordycepin‐induced reduction in fEPSPs amplitude (to 48 ± 12% of the baseline, P < 0.01) was associated with a significant increase in PPF ratio (2.88 ± 0.41 for cordycepin treatment and 1.22 ± 0.17 for the control, P < 0.01) (Figure 2A,B). These results indicate a decrease in fEPSPs amplitude caused by cordycepin is attributable to a decrease in the amount of neurotransmitter release presynaptically.
Figure 2.
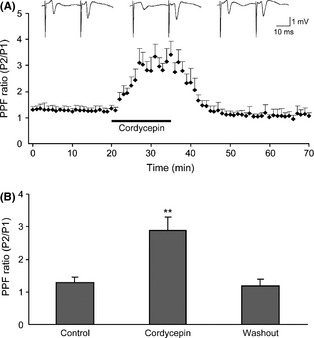
Effects of cordycepin at 20 mg/L on the paired‐pulse facilitation (PPF). (A) Sample traces (top) show fEPSPs recorded by a paired‐pulse stimulus (25‐millisecond interval) before, during, and after bath application of cordycepin. Bottom graph shows that the PPF ratio was increased markedly in the presence of cordycepin, each data point in the graph was calculated by dividing the mean peak amplitude of the second fEPSPs (P2) by the mean peak amplitude of the first one (P1). (B) Statistical data show cordycepin significantly increased PPF ratio. **P < 0.01 as compared with control.
Effect of Cordycepin on AMPA and NMDA Receptor‐Mediated Responses
We examined the involvement of NMDA receptors on the cordycepin effect, by applying cordycepin in the presence of the NMDA receptor antagonist APV. Because AMPA receptors were entirely responsible for the postsynaptic response in the regular ACSF medium, pretreated APV did not induce significant changes in synaptic responses elicited in the CA1 region by stimulation of the Schaffer‐commissural fibers (Figure 3A); this synaptic response can be entirely blocked by CNQX, a potent and selective inhibitor of the AMPA receptor (Figure 3A). In the presence of APV, cordycepin produce an apparent decrease on the AMPA receptor‐mediated responses (Figure 3A,B). The resultant percentage of AMPA receptor‐mediated fEPSPs amplitudes was decreased to 45.34 ± 19.18% (9 slices, 7 animals) (Figure 3B). No significant difference was detected when compared to cordycepin alone (Figures 1 and 3B) (P > 0.05). This blockade of NMDA receptors indicates that NMDA receptors are not involved in the cordycepin‐mediated decrease in the synaptic response recorded in the CA1 region. Additionally, the involvement of AMPA receptors on the effects of cordycepin was also measured following infusion of 10 μM CNQX, in slices maintained in low‐magnesium medium throughout the experiment. CNQX eliminates 40–50% of the amplitude of fEPSPs elicited in the CA1 region by stimulation of the Schaffer‐commissural fibers. The component that remains is magnesium‐sensitive and demonstrated to be blocked by the NMDA receptor antagonist, APV (Figure 3C). Cordycepin produced an apparent decrease on NMDA receptor‐mediated responses (Figure 3C,D). The resultant percentage of NMDA receptor‐mediated fEPSPs amplitudes was decreased to 42.86 ± 13.33% (8 slices, 6 animals) (Figure 3C,D), no significant difference was detected when compared to cordycepin alone (P > 0.05) (Figures 1 and 3D), indicating AMPA receptors are not involved in the cordycepin‐mediated reduction in CA1 fEPSPs.
Figure 3.
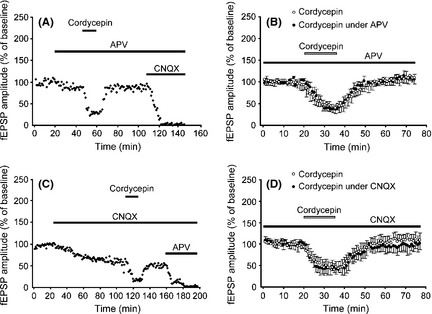
Effects of cordycepin on α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazole‐propionic acid (AMPA) and N‐methyl‐d‐aspartic acid (NMDA) receptor‐mediated responses. (A) A representative experiment to examine the involvement of NMDA receptors in cordycepin functions, by applying cordycepin in the presence of the NMDA receptors antagonist dl‐2‐amino‐5‐phosphonovaleric acid (APV). (B) Time courses of the effects of cordycepin applied alone (open circles) or applied under the pretreatment of APV (black circles). (C) A representative experiment to examine the involvement of AMPA receptors in cordycepin functions, by applying cordycepin in the presence of the AMPA receptor antagonist 6‐cyano‐7‐nitroquinoxaline‐2,3‐dione (CNQX). (D) Time courses of the effects of cordycepin applied alone (open circles) or applied under the pretreatment of CNQX (black circles).
Cordycepin Decreases the Frequency of mEPSCs Without Alteration in Their Amplitude
As suggested by the experiments above, we then examined the effects of cordycepin on the mEPSCs to further determine whether cordycepin acts only on presynaptic terminal or both including postsynaptic component because spontaneous mEPSCs are believed to be the postsynaptic response to a single spontaneously released synaptic vesicle. Changes in the frequency of mEPSCs are thought to result from modification of the presynaptic component of synaptic transmission, while changes in the amplitude of mEPSCs indicate alterations in the postsynaptic component 18, 19. The neuronal cells recorded in the CA1 region exhibited mEPSCs, while the membrane potential was held at −70 mV (Figure 4A). Bath application of cordycepin decreased the frequency of the mEPSCs occurrence in all neurons recorded. Overall, the mEPSCs frequency was decreased significantly to 42.9 ± 13.5% of the control in response to cordycepin (12 slices, 5 animals; P < 0.01) (Figure 4A,B,D). As for the amplitude of mEPSCs, there was no significant change with cordycepin application (93.8 ± 5.36% of control) (Figure 4A,C,D). These results suggest that cordycpin decreases glutamatergic synaptic transmission in the CA1 region of rat hippocampus via a presynaptic mechanism, which is consistent with experiments above, and the mechanism in decreasing neurotransmitter release is strongly recommended.
Figure 4.
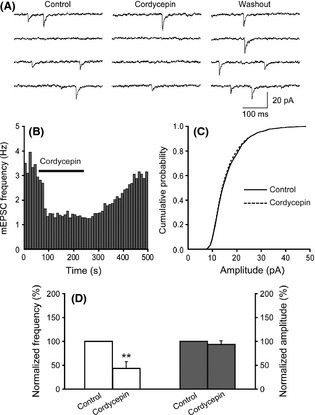
Effects of cordycepin on mEPSCs frequency and amplitude in CA1 pyramidal neurons in hippocampal slice. (A) Sample traces represent mEPSCs recorded in a CA1 pyramidal neuron in the whole process of cordycepin treatment. (B) Graph shows that cordycepin decreased mEPSCs frequency. (C) Cumulative amplitude distribution of mEPSCs shows that cordycepin did not change the amplitude of mEPSCs (Kolmogorov–Smirnov test, P > 0.05). (D) Summary of changes in mEPSCs frequency (left white bars) and amplitude (right gray bars) induced by cordycepin. The frequency and the amplitude of mEPSCs before cordycepin application were defined as control and normalized to 100%. Membrane holding potential −70 mV. **P < 0.01 as compared with control.
Discussion
In the present study, we found that bath application of cordycepin decreased fEPSPs recorded in the Schaffer‐collateral to CA1 synapses of rat hippocampal slices (Figure 1), indicating that the excitatory synaptic transmission is reduced in cordycepin‐treated hippocampal slices. Many cellular mechanisms could contribute to this change of transmission, including presynaptic transmitter release, and postsynaptic receptor quantity and efficacy. PPF is a short‐term presynaptic plasticity, and changes in the PPF ratio reflect alterations in synaptic efficacy which is determined by the probability of neurotransmitter release 17. Interestingly, our results demonstrate that a cordycepin‐induced reduction in fEPSPs amplitude is associated with a significant increase in the PPF ratio. This suggests that the decrease in fEPSPs amplitude caused by cordycepin is attributable to a decrease in the amount of neurotransmitter release presynaptically. Furthermore, cordycepin also decreased AMPA and NMDA receptor‐mediated responses through the use of NMDA and AMPA receptor antagonists APV and CNQX, respectively (Figure 3A,C). However, the resultant percentage decrease in AMPA or NMDA receptor‐mediated fEPSPs amplitudes was not altered when compared with those observed with cordycepin alone (Figure 3B,D), implying that cordycepin does not directly affect NMDA or AMPA receptor‐mediated responses. These results suggest that the cordycepin‐induced decrease in AMPA and NMDA receptor‐mediated responses is simply a consequence in the reduction in presynaptic mechanism. This idea was further supported by observing quantal release. Analysis revealed that cordycepin decreased the frequency but not amplitude of mEPSCs. Supporting this concept, it is believed that the frequency of mEPSCs results from modification of the presynaptic component, and changes in the amplitude of mEPSCs indicate alterations in the postsynaptic component 18, 19. Taken together, our data indicate that cordycepin suppresses synaptic transmission via a presynaptic mechanism, decreasing in neurotransmitter release is strongly recommended.
The biologic significance as well as pathophysiological mechanism of cordycepin‐induced reduction in excitatory synaptic transmission remains to be determined. Previous studies have shown that excessive activation of postsynaptic glutamate receptors, such as AMPA receptors and NMDA receptors, has been implicated in the pathophysiology of stroke, epilepsy, traumatic brain injury, ischemic brain injury, and varieties neurodegenerative disorders including β‐amyloid peptide‐induced‐Alzheimer's disease 20, 21, 22. Additionally, extrasynaptic glutamate receptors like NMDA receptors are also associated to excitotoxic neuronal death, which may be activated by glutamate spillover from synapses or by ectopic release of glutamate from astrocytes 23, 24. Furthermore, astrocytes can not only adjust the extrasynaptic NMDA receptors function but modulate synaptic transmission by affecting presynaptic receptors and regulating presynaptic neurotransmitter release. Astrocyte dysfunction is causative of hyperexcitation and neurotoxicity 23, 25. Therefore, the suppression of excitatory synaptic transmission by reducing presynaptic glutamate release in cordycepin‐treated hippocampal slices detected in our experiments may result from direct action of cordycepin on presynaptic terminals or/and affecting through neuron‐astrocyte signaling pathway. No matter to which, one important common pathophysiological mechanism of excitotoxic neuronal death may result from an extended neuronal depolarization induced by excess/excitotoxic glutamate exposure 21, 22. Interestingly, our previous study showed that cordycepin could reduce neuronal activity by inducing membrane hyperpolarization 13. In combination with our previous study 13, the results in this study poses a strong insight that the suppression effect of cordycepin on the excitatory synaptic transmission is an important mechanism involved in the affections of neuron membrane hyperpolarization. This pathway may in turn contribute to the protective effects of cordycepin on CA1 neurons from glutamate excitotoxicity damage in the pathogenesis of postischemic cell death and other excitotoxic disorders.
In conclusion, the present work demonstrates that cordycepin suppresses excitatory synaptic transmission in rat hippocampal slices by reducing presynaptic transmitter release, which provides an evidence for a novel potential mechanism of cordycepin in modulating the function of CNS. Although the biologic significance of cordycepin‐induced reduction in excitatory synaptic transmission is unclear at present, it may represent a neuromodulatory action for cordycepin in the CNS 11, 12, 13.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
The authors thank Ms. Kelly Varga and Dr. Lei Jiang for critical reading and helpful comments on this manuscript.
The first two authors contributed equally to this study.
References
- 1. He W, Zhang M, Ye J, Jiang T, Fang X, Song Y. Cordycepin induces apoptosis by enhancing JNK and p38 kinase activity and increasing the protein expression of Bcl‐2 pro‐apoptotic molecules. J Zhejiang Univ Sci B 2010;11:654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bueters T, Euler MV, Bendel O, Euler GV. Degeneration of newly formed CA1 neurons following global ischemia in the rat. Exp Neurol 2008;209:114–124. [DOI] [PubMed] [Google Scholar]
- 3. Calabresi P, Pisani A, Mercuri NB, Bernardi G. On the mechanisms underlying hypoxia‐induced membrane depolarization in striatal neurons. Brain 1995;118:1027–1038. [DOI] [PubMed] [Google Scholar]
- 4. Lin M, Hatcher JT, Chen QH, Wurster RD, Li L, Cheng ZJ. Maternal diabetes increases large conductance Ca2+ activated K+ outward currents that alter action potential properties but do not contribute to attenuated excitability of parasympathetic cardiac motoneurons in the nucleus ambiguous of neonatal mice. Am J Physiol Requl Integr Comp Physiol 2011;300:R1070–R1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paterson R, Paterson M. Cordyceps: a traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry 2008;69:1469–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoo H, Shin J, Cho J, et al. Effects of Cordyceps millitaris extract on angiogensis and tumor growth. Acta Pharmacol Sin 2004;25:657–665. [PubMed] [Google Scholar]
- 7. Kim H, Shrestha B, Lim S, et al. Cordycepin inhibits lipopolysaccharide‐induced inflammation by the suppression of NF‐kappaB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur J Pharmacol 2006;545:192–199. [DOI] [PubMed] [Google Scholar]
- 8. Yun Y, Han S, Lee S, et al. Anti‐diabetic effects of CCCA CMESS, and cordycepin from Cordyceps militaris and the immune responses in streptozotocin‐induced diabetic mice. Nat Prod Sci 2003;9:291–298. [Google Scholar]
- 9. Cho M, Lee D, Kim M, Sung J, Ham S. Antimutagenicity and cytotoxicity of cordycepin isolated from Cordyceps militaris . Food Sci Biotechnol 2003;12:472–475. [Google Scholar]
- 10. Jin DQ, Park BC, Lee JS, Choi HD, Lee YS, Yang JH. Mycelial extract of Cordyceps ophioglossoides prevents neuronal cell death and ameliorates beta‐amyloid peptide‐induced memory deficits in rats. Biol Pharm Bull 2004;27:1126–1129. [DOI] [PubMed] [Google Scholar]
- 11. Cheng Z, He W, Zhou X, et al. Cordycepin protects against cerebral ischemia/reperfusion injury in vivo and in vitro. Eur J Pharmacol 2011;664:20–28. [DOI] [PubMed] [Google Scholar]
- 12. Hwang IK, Lim SS, Yoo KY, et al. A phytochemically characterized extract of Cordyceps milittaris and cordycepin protect hippocampal neurons from ischemic injury in gerbils. Planta Med 2008;74:114–119. [DOI] [PubMed] [Google Scholar]
- 13. Yao LH, Li CH, Yan WW, Huang JN, Liu WX, Xiao P. Cordycepin decreases activity of hippocampal CA1 pyramidal neuron through membrane hyperpolarization. Neurosci Lett 2011;479:327–331. [DOI] [PubMed] [Google Scholar]
- 14. Ni H, Zhou XH, Li HH, Huang WF. Column chromatographic extraction and preparation of cordycepin from Cordyceps militaris waster medium. J Chromatogr B 2009;877:2135–2141. [DOI] [PubMed] [Google Scholar]
- 15. Zhou Y, Tang H, Liu J, Dong J, Xiong H. Chemokine CCL2 modulation of neuronal excitability and synaptic transmission in rat hippocampal slices. J Neurochem 2011;116:406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xiao P, Staubli U, Kessler M. Lynch Gary. Selective effects of aniracetam across receptor types and forms of synaptic facilitation in hippocampus. Hippocampus 1991;1:373–380. [DOI] [PubMed] [Google Scholar]
- 17. Chu HY, Wu Q, Zhou S, et al. SKF83959 suppresses excitatory synaptic transmission in rat hippocampus via a dopamine receptor‐independent mechanism. J Neurosci Res 2011;89:1259–1266. [DOI] [PubMed] [Google Scholar]
- 18. Basavarajappa BS, Ninan I, Arancio O. Acute ethanol suppresses glutamatergic neurotransmission through endocannabinoids in hippocampal neurons. J Neurochem 2008;107:1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nelson ED, Kavalali ET, Monteggia LM. Activity‐dependent suppression of miniature neurotransmission through the regulation of DNA methylation. J Neurosci 2008;28:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arundine M, Tymianski M. Molecular mechanisms of calcium‐dependent neurodegeneration in excitotoxicity. Cell Calcium 2003;34:325–337. [DOI] [PubMed] [Google Scholar]
- 21. Wang Y, Qin ZH. Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis 2010;15:1382–1402. [DOI] [PubMed] [Google Scholar]
- 22. Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium 2010;47:122–129. [DOI] [PubMed] [Google Scholar]
- 23. Seifert G, Steinhäuser C. Neuron‐astrocyte signaling and epilepsy. Exp Neurol 2011;doi: 10.1016/j.expneurol.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 24. Petralia RS. Distribution of extrasynaptic NMDA receptors on neurons. ScientificWorld Journal 2012;2012:267120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Go′mez‐Gonzalo M, Losi G, Chiavegato A, et al. An excitatory loop with astrocytes contributes to drive neurons to seizure threshold. PLoS Biol 2010;8:e1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]


