Summary
Aims
To study whether adiponectin (APN) could improve neurological outcomes in aged mice after ischemic stroke.
Methods
Adeno‐associated virus carrying APN gene was injected into aged and young adult mice 7 days before transient middle cerebral artery occlusion (tMCAO). Atrophic volumes and neurobehavioral deficiencies were determined up to 28 days after tMCAO. Focal angiogenesis was determined based on blood vessel number in the ischemic regions.
Results
Increased atrophic volume and more sever neurobehavioral deficits were found in the aged mice compared with young adult mice (P < 0.05). AAV‐APN gene transfer attenuated atrophic volume and improved neurobehavioral outcomes, along with increased focal angiogenesis in both aged and young adult mice, compared with control animals (P < 0.05). In addition, the attenuation of atrophic volume and the improvement in neurobehavioral outcomes were much more significant in aged mice than in young adult mice after AAV‐APN administration (P < 0.05). The number of microvessels in aged AAV‐APN mouse ischemic brain was higher than in young adult AAV‐APN treated mouse brain (P < 0.05).
Conclusions
Our results demonstrate that APN overexpression reduces ischemic brain injury and improves neurobehavioral function recovery in aged mice than in young mice, suggesting APN is more beneficial in aged animals after ischemic stroke.
Keywords: Adiponectin, aged mice, angiogenesis, cerebral ischemia, neurobehavioral recovery
Introduction
Ischemic stroke is one of the most vital disorders with high mortality and morbidity in China and worldwide 1. Over the last two decades, numerous neuroprotective drugs were proven to be effective for treating acute stroke in animal stroke models 2, 3. However, none of these drugs were effective in subsequent clinical trials 4. Studies showed that the efficacy of a drug varied in different experimental stroke models. The relationship between the efficacy of drugs and its mechanism remains inconsistent 5. In addition, aging is one of the most important factors in influencing the result of drug because the efficacy of drug is totally different in young adult and aged human or experimental animals 6. Moreover, the aging process is related to cellular functions, and aging attenuated ischemia‐induced angiogenesis 7, 8. Consistent with these observations, in models of heart disease and both global and focal cerebral ischemia, the prominence of ischemic changes advances with age 9, 10, 11, as are postischemic behavioral abnormalities 12. Therefore, using aged animal models of ischemic stroke to assess drugs is essential for the cerebral ischemia research and for clinical translation.
Adiponectin (APN), an adipose‐specific plasma protein, plays a protective role in the development of cardiovascular morbidity 13, 14. APN ameliorated endothelial function and modulated inflammation 15. High level of APN in peripheral blood is associated with a reduced risk of cardiovascular diseases such as the coronary artery disease and the myocardial infarction, while low plasma APN was related to an increased risk of 5‐year mortality after first‐ever ischemic stroke 16, 17. APN suppressed the development of atherosclerosis by inhibiting smooth muscle cell proliferation and migration, which could be related to the vascular protective activity 18, 19. APN also promotes angiogenesis by up‐regulating AMPK and Akt signaling in endothelial cells or through endothelial nitric‐oxide‐synthase‐dependent mechanism 20, 21, 22. Our previous study demonstrated that APN overexpression attenuated brain atrophic volume, improved neurobehavioral recovery, and promoted cerebral angiogenesis 14 days after tMCAO in young adult mice. The effect of APN was mediated by activating AMPK signaling pathway 23. However, whether the similar effects of APN occur in aged brain remains to be further investigated.
Recent studies documented that angiogenesis could be induced after focal cerebral ischemia in animal and human brains 24, 25, 26. Stroke patients with a higher density of microvessels are associated with less morbidity and longer survival 27, 28, 29. Cerebral ischemia‐induced angiogenesis showed benefits for the recovery of motor function 30, 31, 32. These observational studies indicate that focal angiogenesis and neovascularization play important roles for ischemic brain repairing and remodeling. However, whether APN promotes angiogenesis in aged brain remains unknown.
In this study, we aim to explore whether APN is able to overexpress in aged brain via AAV‐APN gene transfer, and whether APN overexpression in aged mice has the similar salutary effects as does in young adult mice following cerebral ischemia. In addition, we also ask whether the role of APN in aged mice is different from young adult mice in cerebral ischemia.
Methods
Experimental Groups
The animal protocol was approved by the Institutional Animal Care and Use Committee (IACUC), Shanghai Jiao Tong University, Shanghai, China. To compare endogenous APN expression in aged and young adult mice brain after stroke, tMCAO was performed in aged male CD‐1 mice (22–24 month‐old, Ship BK, Shanghai, China, n = 6, 3 for Western blot and three for immunostaining; n = 6 in sham group, three mice for Western blot and three mice for immunostaining) and young adult male CD‐1 mice (3‐month‐old, grouped the same way as aged mice). To evaluate the efficiency of AAV‐APN gene transfer in normal mice brain, aged mice group and young mice group were received AAV‐APN transfer (n = 6 per group, three mice for Western blot and three mice for immunostaining) and AAV‐GFP was injected as the control group (grouped the same way as AAV‐APN injected mice). To test the therapeutic efficiency of APN in ischemic mice brain, AAV‐APN was injected into aged and young adult mice brain (n = 10 per group) and AAV‐GFP was injected as a control group (n = 10 per group).
AAV‐APN Gene Transfer in the Mouse Brain
Aged male CD‐1 mice and young adult male CD‐1 mice were anesthetized intraperitoneally using ketamine/xylazine (100/10 mg/kg, Sigma) and then placed in a stereotactic frame (David Kopf Instruments, Tujunga, CA, USA). Five‐microliter viral suspensions containing 4 × 109 genome copies of AAV‐APN were injected into the striatum (AP: −1.0 mm; L −2.0 mm; V −2.5 mm) at a rate of 0.2 μL/min based on our previous study 33. The needle was withdrawn 25 min after injection, and animals were allowed to return to the home cage after mice wakened. A group of mice underwent AAV‐GFP gene transfer as a viral vector control.
Transient Middle Cerebral Artery Occlusion (tMCAO) in Mice
tMCAO was performed 7 days after AAV‐APN gene administration as previously described 23, 34. Briefly, the left common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) were carefully isolated by a surgical microscope (Leica, Wetzlar, Germany). A silicone‐coated 6‐0 suture was gently inserted from ECA stump to ICA to occlude the opening of MCA. The success of occlusion was determined by monitoring the decrease in surface cerebral blood flow (CBF) to 10% of baseline CBF using a laser Doppler flowmetry (Moor Instruments, Devon, England). Reperfusion was performed by the suture withdrawal after 90 min of tMCAO. PH, partial pressure of carbon dioxide (pCO2), and partial pressure of oxygen (pO2) were measured using i‐STAT® System (Abbott Point of Care Inc. Princeton, NJ, USA), and blood pressure was determined by Softron® Sphygmomanometer (Softron BP‐98A, Softron Beijing Inc. Beijing, China). The mice in which CBF dropped to less than 90% of baseline immediately after MCAO and those died during surgery were excluded in the stroke cohorts.
Brain Atrophy Measurement
Brains were removed and frozen immediately in −40°C isopentane. Twenty‐micrometer‐thick section was cut from the frontal pole to hippocampus and stained with 0.1% cresyl violet (Sinopharm Chemical Reagent Co., Shanghai, China). Brain atrophic volume was analyzed using NIH Image J software as previously described 23 and calculated by the following formula: contralateral hemisphere minus normal region of ipsilateral hemisphere, then multiplied by the section interval thickness.
Neurobehavioral Tests
Mice were trained for three consecutive days prior to surgery. Neurobehavioral tests were performed before and 1, 3, 7, 14, and 28 days after tMCAO by an investigator who was blinded to the experimental groups. Modified neurological severity scores (mNSS) of the animals were graded on a scale of 0–14, which is a composite of motor, reflex, and balance tests 35.
For rotarod test, mice were placed on an accelerating rotarod cylinder (Zhenghua, Anhui, China); the speed was increased from 20 to 40 rpm within 5 min. The trial ended if the animal fell off the rungs or gripped the device and spun around for two consecutive revolutions without attempting to walk on the rungs. The time that animals remained on the rotarod was recorded for further analysis 24.
For beam‐walking test, mice were trained to traverse a horizontally elevated square beam with 7 mm in diameter to reach an escape platform placed one meter away. Mice were placed on one end of the beam, and the latency to traverse 80% of the beam toward the escape platform was recorded from three independent trials.
Asymmetric motor behavior was also performed using the corner test. Mice were placed between two boards with dimensions 30 × 20 × 1 cm3 for each in home cage 36. Normal animals turn back randomly from either left or right. However, ischemic animals preferentially turn toward the impaired side. The number of turns taken on each side was recorded from 10 trials of each test.
Western Blot Analysis
Tissue sample was collected from the ipsilateral hemisphere, including injured cortex and striatum, and quantified with BCA protein assay (Pierce, Rockford, IL, USA). Protein (30 μg) was separated by 10% SDS‐PAGE electrophoresis and transferred to a nitrocellulose membrane (Whatman, Piscataway, NJ, USA). After blocking with 5% skim milk, the membrane was probed with anti‐APN antibody (1:500 dilution; R&D, Minneapolis, USA) and visualized using an ECL system (Thermo, Rockford, CA, USA). Image was taken and calculated by Quantity One software (Bio‐Rad, Hercules, CA, USA).
Immunohistochemistry
Frozen brain sections were fixed in 4% paraformaldehyde for 10 min and then blocked with 10% BSA. Sections were incubated overnight at 4°C with CD31 (1:200 dilution, R&D), NeuN (1:100 dilution, Millipore. Rockland, Massachusetts, USA), GFAP (1:300 dilution, Millipore), alpha smooth muscle actin (1:300 dilution, R&D), and PCNA (1:200 dilution, Abcam, Cambridge, MA, USA). After washing, sections were further stained by 488‐conjugated and Cy3‐conjugated antibody (1:1000 dilution, Jackson Immuno Research, West Grove, PA, USA), as previously described 37. Sections were examined under Leica TCS‐SP5 microscope (Leica, Solms, Germany). Images were acquired with LAS AF Software (Leica) using 488 nm or 594 nm excitation laser wavelength, and the exposure time was about 735 ms.
Microvessel Counts
The brain regions that located at left, right, and bottom areas of the needle track from each mouse were chosen. Two investigators blinded to the experimental group assessed blood vessel number separately. Only microvessels with a clearly defined lumen or a well‐defined linear vessel shape were taken into account. Single endothelial cells were ignored. The number of blood vessels was calculated as the mean of the blood vessel counts obtained from the six pictures as previously described 38. The number of small arteries was calculated in the same way.
Statistical Analysis
Data were presented as mean ± SD. Comparison of two groups was analyzed by an unpaired Student's t‐test. Three group comparison data were analyzed by one‐way ANOVA with Dunnett's test. Mortality rates were compared by the chi‐square test. A probability value of less than 5% was accepted as statistical significance.
Results
Increased APN Expression in Aged Mouse Brain After tMCAO
Western blotting and immunohistochemistry were performed to determine the expression profiles of APN in aged and young adult mouse brains after tMCAO. We found that APN was low in normal mouse brain, while the expression was increased in the ischemic mouse brain, which was mainly located near small vessels of ischemic brain. APN expression was also increased in aged mouse brain as early as 1 day after tMCAO and persisted up to 7 days. It was noted that APN expression in the ischemic brain of young adult mouse was significantly higher than that in the aged mouse brain at 1, 3, and 7 days after ischemia (Figure 1A–C).
Figure 1.
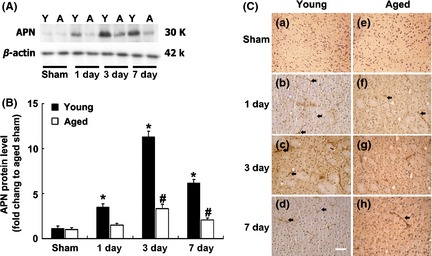
APN was increased in ischemic brain of aged mice. (A) Western blot analysis showed APN expression in normal and ischemic aged and young adult mouse brain at different durations after tMCAO. Y: young mice; A: aged mice. (B) Bar graph showed semi‐quantitative APN expression from (A). Data are presented as mean ± SD, N = 3 per group. *P < 0.05, APN‐young adult versus APN‐aged group; # P < 0.05, APN‐aged versus APN‐aged sham group. (C) Photomicrographs showed the expression of APN in both sham (a, e) and ischemic aged and young adult mouse brain at 1 (b, f), 3 (c, g) and 7 days (d, h) after tMCAO. N = 3 per group. Arrows indicate the APN signal. Bar = 50 μm.
APN Overexpression in Aged Mouse Brain After AAV‐APN Injection
To determine the success of gene transfer, we examined the extent of GFP expression after AAV‐GFP gene transfer. We revealed that the GFP expression could be detected in aged mouse brain for at least 3 weeks (Figure 2A,B). Western blot analysis showed that APN expression was significantly increased in the ipsilateral hemisphere in AAV‐APN‐treated aged mice after tMCAO (P < 0.05). The APN level reached plateau at day 7 and sustained for at least 21 days (Figure 2C,D). Expression pattern of APN in aged mouse brain is similar to that in young adult mouse brain. Double immunostaining demonstrated that APN was expressed in endothelial cells, neurons, and astrocytes after AAV‐APN transfer (Figure 2E).
Figure 2.
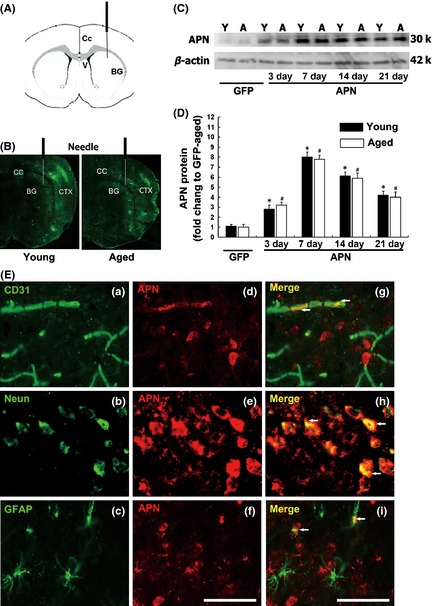
APN overexpression in aged mouse brain after AAV‐APN gene transfer. (A) Graphic illustration indicated injection point in a mouse brain coronal section. (B) The distribution of GFP expression in aged (a) and young adult (b) mouse brain three weeks after AAV‐GFP gene transfer. (C) Western blot analysis showed APN expression in aged and young adult mouse brain after 3, 7, 14, and 21 days of AAV‐APN transduction. (D) Bar graph showed semi‐quantitative APN expression. Data are presented as mean ± SD, N = 3 per group. *P < 0.05, APN‐young adult versus GFP‐young adult group; # P < 0.05, APN‐aged versus GFP‐aged group. (E) Photomicrographs showed that APN was expressed in endothelial cells (a, d, g), neurons (b, e, h), and astrocytes (c, f, i) after AAV‐APN transduction. Bar = 50 μm.
APN Overexpression Attenuated Atrophy in Aged Mice
To explore the effect of APN on the histological outcome after ischemic injury, whole‐brain atrophic volume was examined 2 weeks after tMCAO (Figure 3A). We demonstrated that the atrophic volume was significantly increased in the aged mouse brain compared with that in the young adult mouse brain. In addition, atrophic volume 2 weeks after tMCAO was greatly attenuated in aged mice after AAV‐APN gene transfer compared with the control group (Figure 3B, P < 0.05). Interestingly, the extent of attenuated brain atrophy in aged mice was greater than in young adult mice (30 vs. 20%, Figure 3C, ∆t atrophic volume, P < 0.05), suggesting that APN exerts its protective effect more efficiently in aged ischemic mice.
Figure 3.
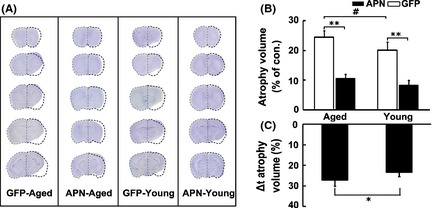
APN overexpression attenuated brain atrophy in aged mice after tMCAO. (A) Photographs represented cresyl violet staining of coronal sections from AAV‐APN‐transduced aged and young adult mice following 14 days of tMCAO. Dash lines illustrated the atrophic areas compared with the contralateral hemisphere. Bar graph showed that total atrophic volume in the AAV‐APN transduced aged and young adult mice (B, C). Data are mean ± SD. N = 10 in each group. # P < 0.05, GFP‐aged versus GFP‐young adult groups; **P < 0.01, APN versus GFP groups; *P < 0.05, APN‐aged versus APN‐young adult groups.
APN Improved Neurobehavioral Recovery After tMCAO in Aged Mice, While Did Not Affect Mice Mortality
To determine whether APN overexpression could improve neurobehavioral outcomes as it does in young adult mice, neurobehavioral tests were performed in aged mice with AAV‐APN gene or vehicle injection. We proved that motor function based on the neurological score, beam walk test, rotarod test, and corner test was greatly improved at 7, 14, and 28 days after AAV‐APN administration following tMCAO, compared with the control group (Figure 4, P < 0.05). More severe neurobehavioral impairments were detected in aged mice after tMCAO. Remarkably, the magnitude of neurobehavioral recovery was greater in aged mice than in young adult ischemic mice (Figure 4E–H, ∆t neurobehavioral tests, P < 0.05).
Figure 4.
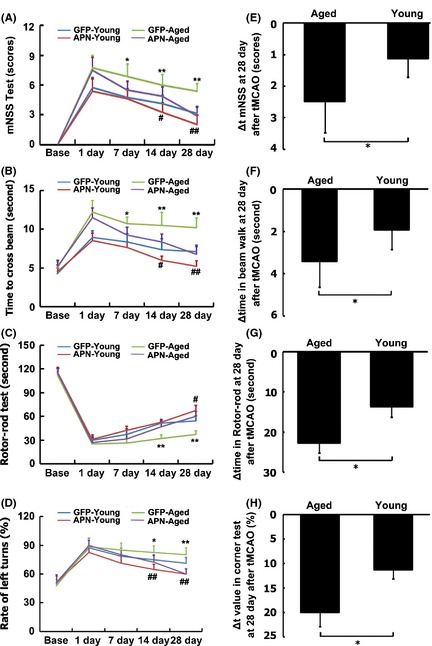
APN overexpression improved neurobehavioral recovery in aged mice after tMCAO. Neurobehavioral tests were evaluated using neurological score (A), beam walk test (B), rotarod test (C), and corner test (D). The behavior tests were performed at 1 day before tMCAO, 1, 3, 7, 14, and 28 days after tMCAO. Data are mean ± SD, n = 10 per group. *or **P < 0.05 or P < 0.01, aged APN versus aged GFP groups, # or # # P < 0.05 or P < 0.01, young adult APN versus young adult GFP groups. Δ of neurological score (E), beam walk test (F), rotarod test (G), and corner test (H) was analyzed between aged APN and young adult APN group. *P < 0.05, aged APN versus young adult APN group.
Cerebral blood flow, mean arterial blood pressure (MABP), PH, pCO2, and pO2 were recorded, and they were similar between the groups (Table S1). In addition, APN injection did not affect the mortality rate after stroke (Table S2).
APN Stimulating Focal Angiogenesis in Aged Mice After tMCAO
To determine whether APN promoted focal angiogenesis after tMCAO, we counted the number of microvessels in ischemic perifocal region (Figure 5A). The number of microvessels was increased in aged ischemic mice injected with AAV‐APN gene compared with the control (Figure 5B, P < 0.05) and the young adult mice (Figure 5C, P < 0.05). PCNA and CD31 double staining demonstrated that the number of proliferating endothelial cells was increased in ischemic perifocal region in aged mice with AAV‐APN gene transfer (Figure 5D, P < 0.05), suggesting that aged brain retained the capacity of angiogenesis in response to ischemic injury. Similarly, angiogenesis was increased in aged mice more than in the young adult mice 4 weeks after APN‐AAV treatment following tMCAO (Figure 5E, ∆ number of newly formed microvessels, P < 0.05).
Figure 5.
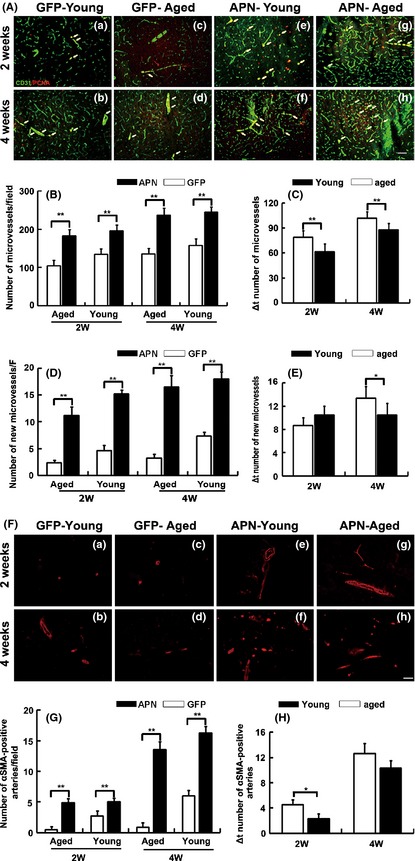
Angiogenesis was increased in aged mice with AAV‐APN gene transfer after tMCAO. (A) Photomicrographs showed the CD‐31 and PCNA double immunostaining in perifocal region in AAV‐APN transduced aged mouse brain 2 and 4 weeks after tMCAO. AAV‐APN transduced young adult mice and AAV‐GFP transduced aged mice were as control. Bar = 20 μm. (B) Bar graph showed the number of microvessels in AAV transduced aged and young adult mice. Values are mean ± SD, N = 6 in each group. **P < 0.01, APN versus GFP groups. (C) Bar graph showed that the number of microvessels between aged APN and young adult APN groups. **P < 0.01, aged APN versus young adult APN group. (D) Bar graphs showing the number of newly formed microvessels in the AAV‐APN transduced aged mice. Data are mean ± SD, n = 6 per group. **P < 0.01, APN versus GFP groups. (E) Bar graph showed that the number of microvessels between aged APN and young adult APN groups. Data are mean ± SD, n = 6 per group. *P < 0.05, aged APN versus young adult APN group. (F) Photomicrographs showed SMA‐positive cells in AAV‐APN transduced aged mouse brain 2 weeks and 4 weeks after tMCAO. (G) Bar graph showed the number of small arteries in the AAV‐APN transduced aged mice after 2 weeks and 4 weeks of tMCAO. Data are mean ± SD, n = 6 per group. **P < 0.01, APN versus GFP groups. (H) Bar graph showed that the number of small arteries between aged APN and young adult APN groups. Values are mean ± SD, N = 6 in each group. *P < 0.05, aged APN versus young adult APN group.
To determine whether small arteries in the ischemic brain were also increased after APN overexpression, we further examined the number of smooth muscle cells in perifocal region. We found that the number of αSMA‐positive cells was greatly increased in aged ischemic brain after AAV‐APN gene transfer, compared with the control (Figure 5F,G, P < 0.05). Similarly, the increased number of small arteries in aged mice was greater in young adult mice 2 weeks after tMCAO (Figure 5H, ∆number of small arteries, P < 0.05), suggesting APN not only promotes angiogenesis, but also improves focal neovascularization.
Discussion
In this study, we demonstrated that APN could be overexpressed in the aged mouse brain under both normal and ischemic conditions. APN overexpression not only reduced the ischemic brain injury and promoted neurobehavioral outcomes in aged mice, but also displayed even better therapeutic effects compared with those in the young adult mice. In addition, focal angiogenesis was significantly increased in the aged ischemic brains after AAV‐APN gene transfer. Our findings suggest that APN is a potential therapy target for ischemic brain injury, especially in the aged mice.
We reported that APN overexpression via AAV‐APN gene transfer could greatly reduce ischemic brain injury and promote neurobehavioral recovery in young adult mice 23. However, whether APN has similar functions in the aged recipients remains unknown. It is possible that aged brain is less responsive to APN treatment because aging adversely influences stroke outcomes due to age‐related changes in the brain microenvironment 39, 40. For example, several growth factors, such as VEGF and IGF‐1, which stimulate angiogenesis and neurogenesis, reduce with aging 41, 42. Besides, aging reduces capillary density after hindlimb ischemia in New Zealand white rabbits 43. Angiogenesis is also reduced wound‐healing process in aged rats 44. If the down‐stream signals of APN are these neurotrophic factors, the APN treatment in the aged brain would be futile. Nevertheless, our present results demonstrated that the effects of APN in aged mice were even better than those in young adult mice, suggesting APN treatment could be used for the treatment of aged‐related diseases.
Our previous studies demonstrated that when injecting AAV vector into the lateral caudate putamen, overexpression of target genes could be achieved in both the parenchyma and ependymal tissues in the young adult mice 23. Whether AAV vector induced target gene overexpression in aged mice was unknown. In the present study, we found that APN overexpression was similar in aged mice and young adults, which reached the maximum in both young adult and aged mice 7 days after AAV‐vector injection and sustained for at least 3 weeks, demonstrating that AAV vector was capable of maintaining a high level of APN in aged mouse brain. Aging is not a barrier for the gene therapy.
Gene therapy has some limitations for its clinical translation. For instance, injecting targeted gene directly into the brain is invasive and repeated injection is not allowed. These problems hamper its translation from bench to bedside. For better translation into clinic, several strategies could be developed. For example, intravenous injection of TAT fusion protein sufficiently permeates the blood brain barrier 45, 46; thus, APN protein fused to TAT could be injected intravenously into the ischemic patients. In addition, with a combination of stem cell and gene therapy, APN could be delivered into the brain via stem cells. APN could be overexpressed in stem cells, and then stem cells could be injected into the ischemic patients through various administration routes 47, 48, 49, 50.
Our major finding is that APN overexpression confers benefits not only in young adult mice, but also in aged ischemic mouse brain. More importantly, the magnitude of reduction in atrophic volume and the extent of neurobehavioral recovery afforded by APN gene transfer in aged ischemic mice were better than those in young adult mice. This effect was correlated with the increase in focal angiogenesis in aged mouse brain after ischemic brain injury. It is unclear why aged mice overexpressing APN have better outcomes compared with the young adult mice. One reason could be aged mouse brain response more sensitively to APN. Nevertheless, the benefits of APN in aged mouse brain needs to be further identified.
Concerted actions of angiogenic molecules are needed during angiogenesis, in which VEGF is the most important factor 51, 52. AAV‐APN gene transfer could further promote focal VEGF release in young adult mice. Ischemic stress activates AMPK signaling pathway in the HUVEC culture and in the mouse ischemic hind limb model 53, 54. These results suggest that VEGF stimulated angiogenesis in ischemic tissue through AMPK signaling pathway. Indeed, we confirmed that APN promoted AMPK phosphorylation in young adult ischemic mice. Inhibiting AMPK phosphorylation by compound C significantly attenuated VEGF expression and angiogenesis 23, suggesting that the effect of APN on angiogenesis is related to the AMPK signaling during cerebral ischemia.
In conclusion, we demonstrated that APN overexpression in young adult and aged ischemic mice reduced brain atrophy, improved neurobehavioral recovery, and increased angiogenesis, suggesting that APN is a potential therapy target in aged rodents for ischemic brain injury.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Table S1. Summary of physiological parameters in mice.
Table S2. Mortality rates of mice after tMCAO within 4 weeks.
Acknowledgments
This work is supported by the National Natural Science Foundation of China Project #81100633 (LHS), #81070939 (GYY), and #81100868 (YW); Major State Basic Research Development Program of China (973 Program) #2011CB504s405 (GYY, YW); and Shanghai Municipal Health Bureau #2010090 (LHS).
The first two authors contributed equally to this work.
References
- 1. Wang YJ, Zhang SM, Zhang L, et al. Chinese guidelines for the secondary prevention of ischemic stroke and transient ischemic attack 2010. CNS Neurosci Ther 2012;18:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fisher M. New approaches to neuroprotective drug development. Stroke 2011;42:S24–S27. [DOI] [PubMed] [Google Scholar]
- 3. Mathai S, Gunn AJ, Backhaus RA, Guan J. Window of opportunity for neuroprotection with an antioxidant, allene oxide synthase, after hypoxia‐ischemia in adult male rats. CNS Neurosci Ther 2012;18:887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sena E, van der Worp HB, Howells D, Macleod M. How can we improve the pre‐clinical development of drugs for stroke? Trends Neurosci 2007;30:433–439. [DOI] [PubMed] [Google Scholar]
- 5. O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol 2006;59:467–477. [DOI] [PubMed] [Google Scholar]
- 6. Deng YX, Wang YL, Gao BQ, et al. Age differences in clinical characteristics, health care, and outcomes after ischemic stroke in China. CNS Neurosci Ther 2012;18:819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kang DH, Anderson S, Kim YG, Mazzalli M. Impaired angiogenesis in the aging kidney: vascular endothelial growth factor and thrombospondin‐1 in renal disease. Am J Kidney Dis 2001;37:601–611. [DOI] [PubMed] [Google Scholar]
- 8. Gao P, Shen F, Gabriel RA, et al. Attenuation of brain response to vascular endothelial growth factor‐mediated angiogenesis and neurogenesis in aged mice. Stroke 2009;40:3596–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Di Cesare F, D'Ilario D, Fioravanti M. Differential characteristics of the aging process and the vascular cognitive impairment in the organization of memory retrieval. J Neurol Sci 2012;322:148–151. [DOI] [PubMed] [Google Scholar]
- 10. Bao L, Taskin E, Foster M, et al. Alterations in ventricular K(ATP) channel properties during aging. Aging Cell 2013;12:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jin K, Minami M, Xie L, et al. Ischemia‐induced neurogenesis is preserved but reduced in the aged rodent brain. Aging Cell 2004;3:373–377. [DOI] [PubMed] [Google Scholar]
- 12. Won SJ, Xie L, Kim SH, et al. Influence of age on the response to fibroblast growth factor‐2 treatment in a rat model of stroke. Brain Res 2006;1123:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Luis DA, Soto GD, Conde R, Izaola O, de la Fuente B. Relation of leptin and adiponectin with cardiovascular risk factors, intact parathormone, and vitamin D levels in patients with primary hyperparathyroidism. J Clin Lab Anal 2012;26:398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oliveira CS, Saddi‐Rosa P, Crispim F, et al. Association of ADIPOQ variants, total and high molecular weight adiponectin levels with coronary artery disease in diabetic and non‐diabetic Brazilian subjects. J Diabetes Complications 2012;26:94–98. [DOI] [PubMed] [Google Scholar]
- 15. Das SK, Patel VB, Oudit GY. Beneficial effects of grape resveratrol on serum adiponectin and inflammation: clinical trial in patients with stable coronary artery disease: editorial to: “Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: a triple‐blind, placebo‐controlled, one‐year clinical trial in patients with stable coronary artery disease” by J. Tome‐Carneiro et al. Cardiovasc Drugs Ther 2013;27:1–4. [DOI] [PubMed] [Google Scholar]
- 16. Hashimoto N, Kanda J, Nakamura T, et al. Association of hypoadiponectinemia in men with early onset of coronary heart disease and multiple coronary artery stenoses. Metabolism 2006;55:1653–1657. [DOI] [PubMed] [Google Scholar]
- 17. Efstathiou SP, Tsioulos DI, Tsiakou AG, Gratsias YE, Pefanis AV, Mountokalakis TD. Plasma adiponectin levels and five‐year survival after first‐ever ischemic stroke. Stroke 2005;36:1915–1919. [DOI] [PubMed] [Google Scholar]
- 18. Arita Y, Kihara S, Ouchi N, et al. Adipocyte‐derived plasma protein adiponectin acts as a platelet‐derived growth factor‐BB‐binding protein and regulates growth factor‐induced common postreceptor signal in vascular smooth muscle cell. Circulation 2002;105:2893–2898. [DOI] [PubMed] [Google Scholar]
- 19. Okamoto Y, Kihara S, Ouchi N, et al. Adiponectin reduces atherosclerosis in apolipoprotein E‐deficient mice. Circulation 2002;106:2767–2770. [DOI] [PubMed] [Google Scholar]
- 20. Ouchi N, Kobayashi H, Kihara S, et al. Adiponectin stimulates angiogenesis by promoting cross‐talk between AMP‐activated protein kinase and Akt signaling in endothelial cells. J Biol Chem 2004;279:1304–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp‐activated protein kinase signaling. J Biol Chem 2004;279:28670–28674. [DOI] [PubMed] [Google Scholar]
- 22. Nishimura M, Izumiya Y, Higuchi A, et al. Adiponectin prevents cerebral ischemic injury through endothelial nitric oxide synthase dependent mechanisms. Circulation 2008;117:216–223. [DOI] [PubMed] [Google Scholar]
- 23. Shen L, Miao J, Yuan F, et al. Overexpression of adiponectin promotes focal angiogenesis in the mouse brain following middle cerebral artery occlusion. Gene Ther 2013;20:93–101. [DOI] [PubMed] [Google Scholar]
- 24. Lu H, Wang Y, Yuan F, Liu J, Zeng L, Yang GY. Overexpression of netrin‐1 improves neurological outcomes in mice following transient middle cerebral artery occlusion. Front Med 2011;5:86–93. [DOI] [PubMed] [Google Scholar]
- 25. Su H, Yang GY. Treatment of focal brain ischemia with viral vector‐mediated gene transfer. Methods Mol Biol 2011;686:429–446. [DOI] [PubMed] [Google Scholar]
- 26. Chen YC, Wu JS, Yang ST, et al. Stroke, angiogenesis and phytochemicals. Front Biosci (Schol Ed) 2012;4:599–610. [DOI] [PubMed] [Google Scholar]
- 27. Lapchak PA, Araujo DM. Advances in ischemic stroke treatment: neuroprotective and combination therapies. Expert Opin Emerg Drugs 2007;12:97–112. [DOI] [PubMed] [Google Scholar]
- 28. Gursoy‐Ozdemir Y, Yemisci M, Dalkara T. Microvascular protection is essential for successful neuroprotection in stroke. J Neurochem 2012;123(Suppl 2):2–11. [DOI] [PubMed] [Google Scholar]
- 29. Terpolilli NA, Kim SW, Thal SC, et al. Inhalation of nitric oxide prevents ischemic brain damage in experimental stroke by selective dilatation of collateral arterioles. Circ Res 2012;110:727–738. [DOI] [PubMed] [Google Scholar]
- 30. Zhu W, Fan Y, Frenzel T, et al. Insulin growth factor‐1 gene transfer enhances neurovascular remodeling and improves long‐term stroke outcome in mice. Stroke 2008;39:1254–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang JP, Liu HJ, Liu XF. VEGF promotes angiogenesis and functional recovery in stroke rats. J Invest Surg 2010;23:149–155. [DOI] [PubMed] [Google Scholar]
- 32. Lu H, Wang Y, He X, et al. Netrin‐1 hyperexpression in mouse brain promotes angiogenesis and long‐term neurological recovery after transient focal ischemia. Stroke 2012;43:838–843. [DOI] [PubMed] [Google Scholar]
- 33. Shen F, Fan Y, Su H, et al. Adeno‐associated viral vector‐mediated hypoxia‐regulated VEGF gene transfer promotes angiogenesis following focal cerebral ischemia in mice. Gene Ther 2008;15:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeng XN, Xie LL, Liang R, Sun XL, Fan Y, Hu G. AQP4 knockout aggravates ischemia/reperfusion injury in mice. CNS Neurosci Ther 2012;18:388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li Y, Chopp M, Chen J, et al. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab 2000;20:1311–1319. [DOI] [PubMed] [Google Scholar]
- 36. Zhang L, Schallert T, Zhang ZG, et al. A test for detecting long‐term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods 2002;117:207–214. [DOI] [PubMed] [Google Scholar]
- 37. Ma J, Xiong JY, Hou WW, et al. Protective effect of carnosine on subcortical ischemic vascular dementia in mice. CNS Neurosci Ther 2012;18:745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shen F, Su H, Liu W, Kan YW, Young WL, Yang GY. Recombinant adeno‐associated viral vector encoding human VEGF165 induces neomicrovessel formation in the adult mouse brain. Front Biosci 2006;11:3190–3198. [DOI] [PubMed] [Google Scholar]
- 39. Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann Neurol 2010;67:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang L, Zhang RL, Wang Y, et al. Functional recovery in aged and young rats after embolic stroke: treatment with a phosphodiesterase type 5 inhibitor. Stroke 2005;36:847–852. [DOI] [PubMed] [Google Scholar]
- 41. Heeschen C, Lehmann R, Honold J, et al. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation 2004;109:1615–1622. [DOI] [PubMed] [Google Scholar]
- 42. Shimada T, Takeshita Y, Murohara T, et al. Angiogenesis and vasculogenesis are impaired in the precocious‐aging klotho mouse. Circulation 2004;110:1148–1155. [DOI] [PubMed] [Google Scholar]
- 43. Rivard A, Fabre JE, Silver M, et al. Age‐dependent impairment of angiogenesis. Circulation 1999;99:111–120. [DOI] [PubMed] [Google Scholar]
- 44. Yamaura H, Matsuzawa T. Decrease in capillary growth during aging. Exp Gerontol 1980;15:145–150. [DOI] [PubMed] [Google Scholar]
- 45. Deng B, Gou X, Chen H, et al. Targeted delivery of Neurogenin‐2 protein in the treatment for cerebral ischemia‐reperfusion injury. Biomaterials 2013;34:8786–8797. [DOI] [PubMed] [Google Scholar]
- 46. Doeppner TR, Nagel F, Dietz GP, et al. TAT‐Hsp70‐mediated neuroprotection and increased survival of neuronal precursor cells after focal cerebral ischemia in mice. J Cereb Blood Flow Metab 2009;29:1187–1196. [DOI] [PubMed] [Google Scholar]
- 47. Chen C, Wang Y, Yang GY. Stem cell‐mediated gene delivering for the treatment of cerebral ischemia: progress and prospectives. Curr Drug Targets 2013;14:81–89. [DOI] [PubMed] [Google Scholar]
- 48. Zhu J, Zhou L, XingWu F. Tracking neural stem cells in patients with brain trauma. N Engl J Med 2006;355:2376–2378. [DOI] [PubMed] [Google Scholar]
- 49. Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol 2005;57:874–882. [DOI] [PubMed] [Google Scholar]
- 50. Barbosa da Fonseca LM, Gutfilen B, Rosado de Castro PH, et al. Migration and homing of bone‐marrow mononuclear cells in chronic ischemic stroke after intra‐arterial injection. Exp Neurol 2010;221:122–128. [DOI] [PubMed] [Google Scholar]
- 51. Sun Y, Jin K, Xie L, et al. VEGF‐induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest 2003;111:1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature 2005;438:954–959. [DOI] [PubMed] [Google Scholar]
- 53. Nagata D, Mogi M, Walsh K. AMP‐activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem 2003;278:31000–31006. [DOI] [PubMed] [Google Scholar]
- 54. Ouchi N, Shibata R, Walsh K. AMP‐activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res 2005;96:838–846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of physiological parameters in mice.
Table S2. Mortality rates of mice after tMCAO within 4 weeks.


