SUMMARY
Phylogenetically, acetylcholine is an ancient neurochemical. Therefore, it is not surprising that cholinergic neurons project extensively throughout the central nervous system, innervating a wide range of structures within the brain. In fact, acetylcholine is involved in processes that underpin some of our most basic central functions. Both muscarinic and nicotinic receptor families, which mediate cholinergic transmission, have been implicated in the pathophysiology of psychiatric and neurological disorders. The question that remains to be definitively answered is whether or not these receptors are viable targets for the development of future therapeutic agents.
Keywords: Acetylcholine, Alzheimer's disease, Bipolar disorder, Major depressive disorder, Parkinson's disease, Schizophrenia, Substance abuse
Introduction
This review will focus on central muscarinic receptors, discussing the data implicating them in the pathophysiology of psychiatric disorders, such as schizophrenia, bipolar disorder, major depressive disorder (MDD), and substance abuse, as well as neurological diseases such as Alzheimer's and Parkinson's disease. This information will be coupled with recent advances in the development of compounds that selectively target individual muscarinic receptors and the outcomes of preclinical testing, in order to assess whether or not targeting the implicated receptors can produce the desired physiological effect.
Acetylcholine
The existence of acetylcholine predates the development of the nervous system, with the chemical present in primitive plants and bacteria (see [1]). Thus, it is not surprising that this molecule, normally considered a neurotransmitter, is involved in the regulation of a myriad of functions fundamental to continued existence, such as cell proliferation, differentiation, migration, and maintaining homoeostasis (see [1] regarding nonneuronal roles for acetylcholine). Species that diverged more than 350 million years ago have central cholinergic systems, with acetylcholine underlying aspects of behavior and learning in insects [2] and mammals [3]. In primates, the central cholinergic system has three main components: (1) projections from the basal forebrain, innervating the hippocampus, most cortical regions and some subcortical nuclei, (2) projections from the brainstem, which innervate the thalamus and midbrain as well as other regions of the brainstem, and (3) interneurons, predominantly striatal but also present in the nucleus accumbens (see [4] for a review on central cholinergic function). With this widespread innervation of phylogenetically old and new brain structures, acetylcholine has been implicated as playing vital roles in modulating diverse central functions such as sleep, cognition, motor control, and sensory processing. These actions are mediated by two families of receptors, the nicotinic and muscarinic receptors [5]. While this review focuses on muscarinic receptors, the high degree of integration between the two arms of the cholinergic system makes it unlikely that they function independently of each other [6].
Muscarinic Receptors
The muscarinic receptor family consists of five metabotropic receptors, M1–5; upon activation they trigger second messenger cascades within the neurons that express them [Table 1]. Individual receptors are preferentially coupled to distinct G proteins, with M1, 3, and 5 coupling to Gαq/11 subunits, leading to the activation of phospholipase C. M2 and 4 receptors, on the other hand, couple to Gαi/o subunits, resulting in the inhibition of adenylyl cyclase [7]. However, in addition to their canonical signaling pathways, cell expression systems have revealed that muscarinic receptors are capable of activating multiple signal transduction pathways, often depending on the cell type studied. For example, the M1, 3, and 5 receptors can stimulate pathways involving phospholipase A2, phospholipase D, and tyrosine kinase as well as calcium channels. In addition to inhibiting adenylyl cyclase, M2 and M4 receptors can also use phospholipase A2 as a second messenger [8].While these findings have the potential to make it difficult to determine the physiological consequences of changes in the functionality of different muscarinic receptors, they need to be interpreted with caution since such studies often involve the overexpression of nonnative receptors in a particular cell line and thus the activation of diverse signal transduction systems, which may not occur physiologically.
Table 1.
The properties of the five muscarinic receptors, including the nature of their allosteric ligands
| Receptor | M1 | M2 | M3 | M4 | M5 |
|---|---|---|---|---|---|
| G‐protein α subunit | q/11 | i/o | q/11 | i/o | q/11 |
| Canonical signaling | PLC | Inhibits adenylyl cyclase | PLC | Inhibits adenylyl cyclase | PLC |
| CNS distribution | Cortex, basal ganglia | Nucleus basalis, hippocampus, basal ganglia | Cortex, basal ganglia | Basal ganglia, cortex | Hippocampus, substantia nigra, VTA |
| Ligands (type) | AC‐260584 (ag; [59]) BQCA (PAM; [60]) TBPB (ag; [61]) | THRX‐160209 (dualsteric antagonist; [79]) | LY2033298 (PAM; [62]) VU0152099 (PAM;[64]) VU0152100 (PAM; [64]) | VU0238429 (PAM; [97]) Amiodarone (PAM; [98]) |
PLC, phospholipase C; VTA, ventral tegmental area; ag, agonist; PAM, positive allosteric modulator; AC‐260584, (4‐[3‐(4‐butylpiperidin‐1‐yl)‐propyl]‐7‐fluoro‐4H‐benzo[1,4]oxazin‐3‐one; BQCA, benzylquinolone carboxylic acid; TBPB, [1‐(1′‐2‐methylbenzyl)‐1,4′‐bipiperidin‐4‐yl)‐1H‐benzo[d]imidazol‐2(3H)‐one]; LY2033298, (3‐amino‐5‐chloro‐6‐methoxy‐4‐methyl‐thieno[2,3‐b]pyridine‐ 2‐carboxylic acid cyclopropylamide); VU0152099, [3‐amino‐N‐(benzo[d][1,3]dioxol‐5‐ylmethyl)‐4,6‐dimethylthieno[2,3‐b]pyridine carboxamide]; VU0152100, [3‐amino‐N‐(4‐methoxybenzyl)‐4,6‐dimethylthieno[2,3‐b]pyridine carboxamide]; Dualsteric, targets both orthosteric and allosteric sites; [x] indicates reference for the compound.
Muscarinic M1 receptors are found throughout the brain, with the highest concentrations in cortical regions, including the hippocampus [9]. Cortical M1 receptors are primarily located postsynaptically; there they are predominantly associated with excitatory synapses but are also found at cholinergic synapses [10]. Although they are present in all cortical layers, M1 receptors are most dominant in cortical layers III and V/VI [10], where they are found on pyramidal neurons [11].
M2 receptors are highly expressed in the nucleus basalis and occipital cortex, being present at lower levels in the hippocampus, caudate putamen, and other cortical regions [9]. In the cortex, M2 receptors are located both pre‐ and postsynaptically [10]. The presynaptic M2 receptors are located on the axons of symmetric synapses, in keeping with their role as autoreceptors [12]. However, M2 receptors are also present in a subset of glutamatergic synapses and GABAergic interneurons [13]; nearly a third of cortical GABAergic neurons express M2 receptors [14].
M3 receptors show a similar distribution to M1 receptors, but with a much lower level of expression. Like M1 receptors, M3 receptors are also reported to be present in cortical pyramidal cells and glial cells in the rat [15]. Levels of M4 receptors are highest in the caudate putamen [9], where they are often associated with dopaminergic receptors [16]. Finally, M5 receptors are present at very low levels, being restricted predominantly to the hippocampus, substantia nigra, and ventral tegmental area [17].
Difficulties in developing specific ligands for the individual muscarinic receptors, due to their high degree of homology at the acetylcholine binding site (the orthosteric binding site), means our current knowledge of specific functions of the individual receptors comes from the study of genetically modified animals rather than traditional pharmacological approaches. Thus, we know that mice lacking the M1 receptor (M1−/‐) have high striatal dopamine levels and exhibit increased locomotor activity, both spontaneously and in response to amphetamine [18,19]. M1−/‐ animals appear to have normal learning and memory [19, 20] in paradigms requiring hippocampal processing. However, they show deficits in paradigms thought to require interactions between the hippocampus and cortex [21], which are proposed to be analogous to working memory. Mice that lack M2 receptors have disrupted hippocampal acetylcholine homeostasis [22], impaired thermoregulation, and antinociception [23]. The dominant centrally mediated effect of a lack of M3 receptors results is reduced food intake [24]. Like M1−/‐ mice, animals lacking the M4 receptor also exhibit increased locomotor activity, both basally [25] and in response to dopaminetics [26]. They also have increased basal levels of dopamine in the nucleus accumbens [27] and acetylcholine levels in the hippocampus [22]. Finally, animals without M5 receptors do not develop an opiate dependence and their subsequent withdrawal from the drugs is attenuated [28]. However, the analgesic actions of these drugs are unaffected by the lack of this receptor [29].
Acetylcholine and Disorders of the Central Nervous System
The muscarinic component of the central cholinergic system has been implicated in the pathophysiology of a number of disorders, both psychiatric and neurological in nature. In the interests of cohesion, each disorder will be dealt with individually; data regarding a role of the muscarinic system in the disorder will be accompanied by the most recent advances in targeting relevant muscarinic receptors and the therapeutic potential of such an approach.
Muscarinic Receptors and Schizophrenia
Schizophrenia is a potentially devastating disorder, which can exact a heavy toll on sufferers, their relatives and financially, society as a whole. It is diagnosed following the presentation of a constellation of symptoms [30], and thus, like other disorders diagnosed on this basis, is probably a syndrome that consists of a number of diseases, with similar clinical presentations [31]. Although some of the earliest interventions for schizophrenia involved modulating the cholinergic system in the form of acetylcholine‐induced seizures [32] or atropine‐induced comas [33], most of the evidence for a role of the muscarinic system in the pathophysiology of the syndrome comes from basic research rather than clinical studies. The data regarding alterations in the cholinergic system in schizophrenia have been discussed in detail in another review [34], which reviewed the data implicating the central nicotinic and muscarinic systems in the pathophysiology of schizophrenia and so will only be summarized here.
Association studies have not established a strong relationship between sequence variations in muscarinic receptors and schizophrenia. A specific single nucleotide polymorphism (SNP) in the M1 receptor has been associated with altered performance on measures for executive function in people with schizophrenia [35], however, to date, there have been no associations between any M1 SNPs and the disorder itself [35, 36, 37]. Variations in the M5 receptor have been associated with schizophrenia but only when they occur in combination with changes in the sequence of the alpha 7 nicotinic receptor (CHRNA7) [38] suggesting that changes in both receptors may contribute to the symptoms of the disorder.
Initial investigations into the role of the cholinergic system in the disorder, using postmortem human brain tissue and a radioligand that bound to all muscarinic receptors, were equivocal with reports of binding densities being unchanged or decreased [39]. This was followed by reports of increased levels of binding that were regionally specific [40], which seemed to be due to alterations in the kinetics of the receptor ligand interactions.
With the development of more specific ligands for muscarinic receptors came a number of studies, also using postmortem tissue, reporting decreased binding densities of a ligand that interacted with M1/M4 receptors throughout cortical and subcortical regions from patients with schizophrenia [41, 42]. These studies were supported by a neuroimaging study that reported widespread decreases in muscarinic receptors in patients who were off antipsychotic medication at the time of imaging [43]. More integrative approaches, determining expression at the levels of radioligand binding, mRNA, and protein, lead to the discovery that in the cortex the reduced binding density was due to lower levels of the M1 receptor [44], while in the hippocampus it was the M4 receptor that was expressed at a lower level [45]. Of particular note are the findings that in psychiatric disorders: (1) the decrease in the M1 receptor is specific to schizophrenia [42, 46] and (2) changes in M2 and M3 receptors appear to be limited to subcortical regions [47, 48].
More recently, it has been shown that the decrease in cortical M1 receptors is restricted to a subgroup of patients within the syndrome of schizophrenia [37]. The subgroup comprises approximately 25% of the subjects with schizophrenia, who have, on average, 75% lower M1 receptors than either control subjects or other subjects with schizophrenia (See Figure 1). While the G protein recruitment in response to an orthosteric agonist is impaired in this group, the response to an allosteric partial agonist is not [49], suggesting that the M1 receptors remain a potential drug target in this group. Furthermore, the ability to separate the syndrome of schizophrenia into component diseases, based on biochemical markers, holds the promise of developing more effective treatments that target individual components of the syndrome instead of the current practice of trying to treat a syndrome with a single pharmaceutical entity.
Figure 1.
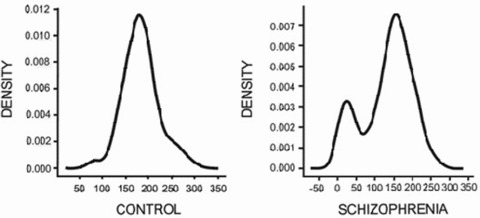
Distribution analyses of [3H]pirenzepine binding (x‐axis) to M1 receptors from control subjects (left) and subjects with schizophrenia (right) [37].
The growing evidence that muscarinic systems are compromised in schizophrenia, combined with a greater understanding of the roles of the individual muscarinic receptors, resulted in the suggestion that stimulating M1 receptors might prove to beneficial in ameliorating the cognitive deficits—a core, enduring characteristic of the disorder [50]. In addition, it was proposed that agonists for both the M1 and M4 receptors could prove to be efficacious in treating the positive symptoms associated with the disorder [50].
A proof of principle study using xanomeline, a partial agonist with higher affinities for M1 and M4 receptors than the remaining members of the muscarinic receptor family [51], supported these hypotheses. Treatment‐resistant patients with schizophrenia, who were off antipsychotic medication during the trial, showed an improvement in a number of cognitive domains and better clinical ratings following 2 weeks of treatment [52]. Subsequent studies have shown that the antipsychotic efficacy of xanomeline is predominantly due to its actions at the M4 rather than the M1 receptor [53].
In spite of these promising findings, attempts to modulate the central muscarinic system have been modest rather than impressive. This is primarily due to the problems associated with specifically activating a single muscarinic receptor, rather than all members of the family, given the high degree of homology between the receptors for the site to which acetylcholine binds, the orthosteric binding site [54]. Inadvertent binding to the peripheral M2 and M3 receptors gives rise to the side‐effect profiles (M2; cardiovascular, themoregulation, M3; gastrointestinal, visual) that limit the use of cholinergic agents [55]. Indeed, despite the promising clinical results seen with xanomeline, the side‐effect profile precluded further development of the drug. The discovery that muscarinic receptors have binding sites in addition to the evolutionary restricted acetylcholine site [56] led to resurgence in muscarinic drug development programs. These sites are referred to as allosteric binding sites and molecules that bind to them can influence the binding and subsequent physiological effects of acetylcholine. Importantly, these sites appear to be less conserved between individual members of the muscarinic receptor family [57], making them ideal targets for the development of more selective muscarinic agents. As with molecules that bind to the orthosteric binding site, there are a number of classes of allosteric ligands. Two classes of allosteric agents produce an “agonist effect”; an allosteric agonist will produce a measurable physiological response in its own right, in addition to enhancing the effect of acetylcholine either by altering the affinity of the orthosteric site for acetylcholine or by modulating the responsiveness of downstream signal transduction pathways. The binding of a positive allosteric modulator (PAM) produces no measurable physiological response itself but, by the same mechanisms employed by the allosteric agonist, facilitates the physiological response to an orthosteric agonist. By contrast, an allosteric antagonist modulates the affinity or efficacy of a receptor for an orthosteric ligand, reducing the physiological response. However, allosteric ligands are much more complex than this brief description implies, for example, a ligand that enhances the effects of acetylcholine may have no effect on the responses produced by carbachol, another orthosteric muscarinic agonist. This level of selectivity has the potential to add a completely new dimension to our ability to modulate central cholinergic function.
The capacity to develop drugs more selective for individual muscarinic receptors (see Table 1) has resulted in a number of promising preclinical reports on the effects of these drugs. There are now a number of M1 activators (agonists or modulators) in production. As predicted, these drugs have been shown to improve cognitive performance, in paradigms such as spatial memory tasks [58], novel object recognition [59], and improving reversal learning in a mouse proposed to model some of the pathophysiology of Alzheimer's disease [60]. In addition, M1 allosteric activators have been shown to be effective in animal models that are used to predict antipsychotic activity; including amphetamine‐induced hyperlocomotion [61], MK‐801‐induced hyperlocomotion, and apomorphine‐induced climbing [58]. M4 allosteric activators have also been shown to be efficacious in a number of models used to predict antipsychotic activity, such as conditioned avoidance response [62], prepulse inhibition [63], and amphetamine‐induced hyperlocomotion [64]. These recent developments have opened up new opportunities for the treatment of the cognitive deficits and psychotic symptoms of schizophrenia, with the only question remaining being how the lead compounds progress to and perform in clinical trials.
Muscarinic Receptors and MDD
MDD affects approximately 5% of the population, with almost 20% of the population suffering some form of depression [65] but not necessarily meeting all of the criteria for MDD [30]. Like schizophrenia, a diagnosis of MDD is based upon the presentation of a number of symptoms and thus, is also probably a syndrome [65]. MDD is epitomized by feelings of helplessness and despair, coupled with a loss of interest or pleasure [30].
Recent attention has focused on the role of the serotonergic and noradrenergic systems in MDD as a result of the efficacy of drugs, which inhibit the reuptake of these neurotransmitters as antidepressants. However, the cholinergic system was proposed to mediate some symptoms of depression in the 1950s [66]. This proposal arose from a study in which volunteers with no history of psychiatric disorders were administered cholinesterase inhibitors, subsequently developing depression‐like symptoms. This initial finding was further supported by reports of increased incidences of prolonged depressive symptoms in workers exposed to cholinesterase inhibitors in the form of insecticides [67]. These and similar studies lead to the hypothesis that during depression, the central cholinergic system dominates over the adrenergic system [68]. Further support for a role of the muscarinic system in MDD comes from numerous psychopharmacological studies, which are reviewed elsewhere [69], concluding that supersensitivity of cholinergic receptors is involved in the pathophysiology of affective disorders.
More recently, a “proof of principle’ study found that treating MDD patients with low doses of the muscarinic antagonist, scopolamine, resulted in lower levels of anxiety and improved clinical ratings of depression [70], which persisted beyond the treatment regimen. This study has since been replicated in a larger cohort of MDD patients [71], with the improvements in anxiety and depression again lasting beyond scopolamine being cleared from the system. Significantly, in both studies these improvements were apparent within 3–5 days of first receiving scopolamine as opposed to the 3–4 weeks required for the majority of antidepressants. More recently, the same group has shown that, despite similar initial responses, the antidepressant effects of scopolamine are more marked in females than in males [72] irrespective of whether they had MDD or bipolar disorder. The outcomes of these small clinical investigations have been so successful that the National Institute of Mental Health (NIMH) has filed a use patent for scopolamine in the treatment of depression. However, it should be noted that the data for the final study came from the same cohorts used in the pilot and follow‐up study, therefore further replication of these results in independent cohorts are desirable. The increased responsiveness to scopolamine in females is probably not due to changes in M2 receptor availability [73, 74] but may be due to changes in M2 receptor gene sequence, with the A/T 1890 SNP being associated with depression in women but not men [75]. The association between variation in the M2 gene and increased risk for affective disorders is supported by some, [76, 77] but not all [78] studies. The latter study specifically investigated the link with MDD in a large cohort, while the others either used a cohort, which encompassed MDD, bipolar disorder, and seasonal affective disorder [77] or smaller cohorts [76, 77]. Either of these factors could contribute to the differences between the studies.
There are relatively few studies that provide direct evidence for a role of muscarinic receptors in MDD. As discussed in the section on schizophrenia, cortical levels of M1/M4 receptors are not altered in MDD [42, 46]. However, the latter study reports a decrease in M2/M4 receptors in the dorsolateral prefrontal cortex but not in other cortical regions [46]. The same study failed to find changes in levels of M3 receptors, indicating that the change in M2/M4 receptors is specific, rather than generic. However, it should be noted that an earlier study reported no differences in level of binding to M2/M4 receptors in the anterior cingulate cortex [48], a region not included in the more recent study. Similarly, neither of the imaging studies conducted using a relatively selective M2 ligand, reported altered M2 receptor distribution volume in the anterior cingulate, amygdala, or hippocampus of people with MDD compared to controls [73, 74]. Together, these findings add weight to the idea that there is a specific, regional decrease in the M2 receptor in the brains from people with MDD.
Although the recent activity in developing allosteric ligands has extended to the M2 receptor [79], the fact that the peripheral side effects seen with cholinergics are mediated, in part, by the M2 receptor [55] will complicate attempts to target this receptor in the central nervous system.
Muscarinic Receptors and Bipolar Disorder
Bipolar disorder used to be referred to as manic depressive disorder. As suggested by this descriptive name, the diagnosis refers to affective disorders where patients experience periods of elevated mood, in some cases these take the form of mania. Thus, patients can experience depression, elevated mood, or a combination of the two [30]. This “cycling” of mood is generally interspersed with periods of “normal” mood, the rate at which sufferers move between these states varies, not just between individuals but also in the same person, making it very difficult to manage clinically. The mania is managed with a mood stabilizer such as lithium or sodium valproate but the depressive symptoms tend not to respond well to antidepressants.
The cholinergic system was initially implicated in bipolar disorder by the same study, which showed cholinesterase inhibitors increased depressive symptoms. In bipolar disorder, cholinesterase inhibitors were reported to generally improve manic symptoms [66]. The initial finding was supported by later studies showing that physostigmine, another cholinesterase inhibitor, reduced the mania in bipolar patients [80]. However, in some patients physostigmine worsened their depression [81]. These findings contributed to the hypothesis that the cholinergic system is underactive during mania, forming part of the cholinergic‐adrenergic hypothesis of mania and depression. This hypothesis combines the findings regarding affective disorders to propose that the cholinergic system is overactive in patients with depression, while the adrenergic system is hyperactive during mania [68].
The proof of principle study using scopolamine as an antidepressant, described in the section related to MDD, included patients with bipolar disorder [70]. They too showed a rapid, significant improvement in their depressive symptoms suggesting that modulating the cholinergic system is effective in ameliorating depression across a spectrum of illness. Patients with bipolar disorder have been reported to be more sensitive to cholinergic agonists than people with psychiatric disorders, with arecoline producing periods of rapid eye movements periods during sleep in patients more rapidly than controls [82].
As with MDD, there is relatively little direct evidence to show perturbed central cholinergic activity. Binding to M1/M4 receptors has been shown not to be altered in the cortex from people with bipolar disorder [42, 46], suggesting that the M1 receptor is not altered in this disorder. However, the latter study reported decreased binding to M2/M4 receptors in the dorsolateral prefrontal cortex and decreased binding to M3 receptors in the frontal pole from people with bipolar disorder [46]. While the finding of decreased binding to M2/M4 receptors was not in agreement with an earlier postmortem study, which found no change in the anterior cortex from patients with bipolar disorder [48], it is in agreement with a neuroimaging study [73]. Using positron emission tomography and a selective M2 ligand, a decrease in M2 receptor distribution volume in the anterior cingulate of patients with bipolar disorder compared to both patients with MDD and controls with no history of psychiatric disorders was reported. Furthermore, the extent of reduced M2 receptor distribution was found to correlate with the severity of depressive symptoms [73]. The most recent finding in this area is that the decrease in M2 receptor distribution volume is associated with an SNP (rs324650) in the M2 receptor; people with bipolar disorder who are homozygous for the minor allele (TT) have lower M2 receptor distribution volume than those who are heterozygous or homozygous for the major allele [74]. This is a different pattern to that seen in healthy nonpsychiatric controls, where the allelic effect on binding is AA < AT < TT, suggesting that there is an interaction with an unidentified factor in people with bipolar disorder. Furthermore, the TT sequence appears to be associated with severity of illness, which might explain the findings between M2 receptor distribution volume and severity of illness seen in the earlier imaging study. Although, the frequencies of the six SNPs genotyped in this study did not alter across diagnoses, suggesting that none of these SNPs constitute a risk factor for either MDD or bipolar disorder [74], people with bipolar disorder, who were homozygous for the minor allele had stronger histories of suicide attempts. Whether this is a consequence of the increased severity of illness associated with this genotype remains to be determined.
Thus, the weight of evidence currently suggests that the M2 receptor may have a role in the pathophysiology of bipolar disorder and more particularly the depressive symptoms associated with the disorder. As already discussed with regard to the viability of the M2 receptor as a therapeutic target in MDD, the greatest obstacle will be targeting central rather than peripheral M2 receptors with the unwanted side effects they mediate [55]. However, the fact that many antipsychotic drugs have some degree of efficacy with respect to controlling the mania associated with bipolar disorder [55], suggests that the selective M1 or M4 agonists being developed, with their promising outcomes in preclinical models that predict antipsychotic efficacy [58, 61, 62, 63, 64], may prove to be useful in controlling this aspect of the disorder.
Muscarinic Receptors and Substance Abuse
Substance abuse is a growing health issue around the world; the World Health Organization estimates there are 2 billion alcohol users, 1.3 billion smokers and 185 million drug users worldwide [83]. In 1995, the United Nations International Drug Control Program reported that alcohol or tobacco contributed to almost 5 million deaths a year globally, with approximately 200,000 additional deaths related to injecting drugs [84].
Although it is well established that the dopaminergic mesocorticolimbic pathway plays a key role in the behaviors associated with reward and addiction, more recently it has been proposed that this pathway plays a role in establishing the reward‐seeking behaviors, which underpin addiction [85]. The mesocorticolimbic pathway arises from the ventral tegmental area and targets limbic structures, such as the amygdala and nucleus accumbens, as well as projecting to the prefrontal cortex. Interactions between the cholinergic and dopaminergic systems have been demonstrated in the ventral tegmental area; the nucleus accumbens and the prefrontal cortex, raising the possibility that the cholinergic system can modulate the reward system at multiple nodes (see [86] on cholinergic function and stimulant addiction). This concept is supported by preclinical studies, which show that infusion of the muscarinic antagonist, scopoloamine, into the basolateral amygdala disrupted the acquisition of cocaine‐seeking behavior in rats [87]. Further support for a role for the cholinergic system in addiction come from studies looking at the association of gene sequence variation, with changes in the M5 receptor gene sequence being associated with increased smoking and increased risk of developing cannabis dependence [88]. Variations in the M2 gene sequence were associated with a combination of alcohol and comorbid drug dependence, but not alcohol dependence alone [89]. More recently, variations in the M2 receptor gene sequence have been associated with an increased risk of smoking/nicotine dependence [90]. Together these data suggest that the muscarinic system may have a role in the pathophysiology of addiction, however, the exact nature of this role requires further elucidation.
There have been a number of studies on the effects of cholinesterase inhibitors on psychostimulants. Thus, in monkeys, physostigmine reduces cocaine self‐administration [91] and galantamine prevents the excitatory behaviors induced by dexamphetamine [92]. To date, there have only been a couple of studies in humans: Donepezil was found to reduce subjective measures of cocaine dependence, but did not affect drug taking itself [93]. Similarly, rivastigmine reduced the positive subjective effects induced by methamphetamine but did not alter drug‐taking behavior [94]. These data suggest that a more targeted approach to modulating the cholinergic influence over the mesocorticolimbic pathway might be necessary in order to have an effect on the act of drug taking.
One option for a more targeted intervention is the M5 receptor, which has a very restricted expression within the central nervous system, being limited to the hippocampus and ventral tegmental area [17], where they are expressed by dopaminergic neurons. Indeed, the M5 receptor has been shown to be vital in mediating morphine reinforcement and withdrawal [29], mice lacking the M5 have an attenuated dopamine release in the nucleus accumbens following exposure to morphine [28], and infusion of M5 antisense mRNA into the ventral tegmental area reduced the reward effects of hypothalamic stimulation [95]. These findings have led to the proposal that M5 antagonists might be beneficial in treating substance dependence. However, the M5 receptors also play a role in mediating the cerebrovascular vasodilatation induced by acetylcholine [28] and impaired cholinergic dilation of cerebral vasculature is implicated in focal cerebral ischemia. Thus, despite its restricted expression pattern, it may be that the M5 receptor also has unacceptable side effects. Furthermore, it would appear, at least as far as cocaine dependence is concerned, that the cholinergic system plays a more complex role in various cognitive aspects of addiction [96], suggesting that modulation of the mesolimbic pathway alone might not be sufficient to prevent the development of addictive behavior.
To date, the only allosteric ligands for the M5 receptor are PAMs [97, 98], the effects of which on substance dependence have yet to be assessed. Therefore, it remains to be seen if the M5 receptor is a viable target for therapies designed to reduce drug dependence.
Muscarinic Receptors and Alzheimer's Disease
It has been estimated that over 25 million people worldwide suffer from dementia (see [99] on strategies for disease modification). Of the dementias, Alzheimer's disease is the most prevalent; patients suffer a progressive memory impairment that eventually impacts on most cognitive domains, often leaving the sufferer requiring custodial care. The pathological identifier of Alzheimer's disease is the presence of senile plaques, formed by extracellular β‐amyloid protein [100] and neurofibrillary tangles in brain regions associated with cognition. Other neuropathological events include shrinkage and death of cholinergic, serotonergic, and glutamatergic neurons (see [101] on transmitters and the pathology of Alzheimer's disease). To date, the only association study that looked at muscarinic receptor genes failed to show a relationship between the C267A polymorphism in the M1 receptor and Alzheimer's disease [102].
There are numerous reports of alterations in cholinergic markers in the brains from people with Alzheimer's disease; these include a loss of choline acetyltransferease in the cortex [103] and hippocampus [104]. There are also reports of a decrease in hemicholinium (high‐affinity choline uptake) binding in frontal cortex and hippocampus but not cerebellum or caudate putamen [105]. Together, these data suggest there is a loss of cholinergic projection neurons to the cortex and hippocampus in Alzheimer's disease, but that projections to the caudate‐putamen are relatively spared. Currently, cholinesterase inhibitors are an approved treatment for the cognitive deficits associated with Alzheimer's disease; increasing the synaptic levels of acetylcholine by inhibiting its breakdown. Disappointingly, the clinical effects of these drugs are modest, possibly because of the degeneration of central cholinergic neurons.
With respect to the muscarinic receptors, cortical and hippocampal M2 receptor levels, but not those in subcortical regions, are reported to be reduced [104]. This is in contrast to a later study, using more specific ligands, which found that binding to M2 receptors is reduced in the striatum and increased in the insular cortex [106], a finding in agreement with a previous report of increased binding to muscarinic receptors, proposed to be presysnaptic, in the cingulate cortex [107]. The picture is further confounded by reports of increased binding to M2/M4 receptors in the basal ganglia (including the caudate putamen) but no differences in the insula or cingulate cortices [108] and increased M2/M4 levels in the temporal cortex of patients with Alzheimer's disease [109]. Overall, the general pattern seems to be that there is an increase in cortical presynaptic muscarinic receptors, although the lack of specific ligands clouds the issue. Similarly, cortical M1 receptor mRNA was found to be increased in tissue from subjects with Alzheimer's disease [11] and levels of M3 receptors reported to be increased in the frontal cortex [109]. However, support for increased postsynaptic receptors is not unequivocal with reports of unchanged binding to M1 receptors in the insula and cingulate cortices compared to controls [108], decreased binding to muscarinic receptors (M1, 3, 4, & 5) in the orbitofrontal cortex from subjects with Alzheimer's disease and a history of psychosis [110] and decreased M1 immunoreactivity in the hippocampus [111]. In addition to the fact that the studies were conducted in various brain regions, the lack of cohesion between these findings could be due to the fact that the cohorts are very diverse in the severity of their pathology. The impact of pathology is highlighted by the recent report of the functional activity of M1 receptors being negatively correlated with neuropathology severity [112]. Despite the lack of agreement between studies, the majority of the data suggest that the central muscarinic system is disrupted in the pathophysiology of Alzheimer's disease.
The major impact of Alzheimer's disease is seen initially on memory and later on other cognitive processes. Thus, M1 receptors, with their potential to modulate cognition, have stimulated a high degree of interest as a therapeutic target. This interest is enhanced by the fact that M1 agonists can modulate the proteolysis of amyloid precursor protein [113], from which β‐amyloid is derived. Amyloid precursor protein is proteolyzed by secretases; in the amyloidergic pathway the precursor is cleaved first by β‐secretase and then γ‐secretase to yield β‐amyloid. However, in the nonamyloidergic pathway, α‐secretase cleaves amyloid prescursor protein in the β‐amyloid sequence, thus preventing generation of this protein (see Figure 2A). In transgenic mice, expressing human amyloid precursor protein, M1 agonists have been shown to significantly reduce cortical and hippocampal β‐amyloid levels as well as improving cognitive performance [113]. This modulation of levels of β‐amyloid appeared to be due to two synchronous events; increased α‐secretase, thereby reducing the amount of substrate available for the formation of β‐amyloid and decreased γ‐secretase activities, resulting in a slower rate of β‐amyloid production [113] (see Figure 2B). This indication that M1 agonists may be able to moderate progression of the disease, coupled with their precognitive effect, has made the M1 receptors a target of significant interest for the development of drugs to treat Alzheimer's disease.
Figure 2.
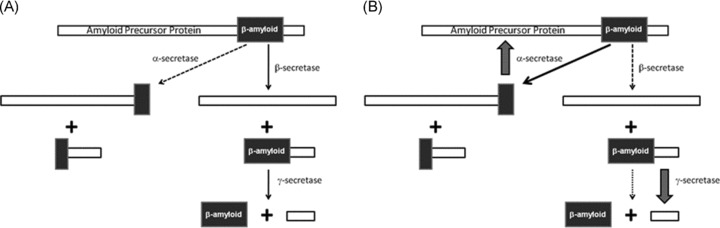
Schematic showing (A) amyloid precursor protein cleavage and (B) the effect of M1 agonists on this pathway.
As previously discussed, advances in targeting the M1 receptor specifically have been hampered by a lack of selectivity of compounds, as demonstrated by the clinical tests of xanomeline [114], which had unacceptable cholinergic side‐effect profiles. The development of more selective allosteric ligands has rejuvenated efforts to determine whether the M1 receptor constitutes a viable drug target for Alzheimers’ disease. Initial reports are promising, with M1 selective activators increasing the nonamyloidergic processing of amyloid precursor protein in cultured cells [61] and a transgenic mouse model [60]. However, there are two caveats to this approach of reducing the amyloidergic burden in Alzheimer's disease. First, although it has yet to be determined, β‐amyloid presumably has a biological function and therefore reducing the production of the protein may have detrimental effects. Second, amyloid precursor protein is not the only substrate for the secretases; altering their activities will therefore have consequences for other, unrelated systems.
Muscarinic Receptors and Parkinson's Disease
Parkinson's disease is a neurodegenerative disorder, the predominant symptoms of which relate to movement; muscular rigidity, a resting tremor and bradykinesia (problems initiating movements and an inability to adjust the body's position), and a lack of balance. These symptoms are the result of the death of dopaminergic neurons projecting from the pars compacta of the substantia nigra to the caudate‐putamen. Within the caudate‐putamen these neurons principally target the cholinergic interneurons. As these interneurons gradually receive less dopaminergic control, striatal levels of acetylcholine increase resulting in the movement disturbances (see [115] on striatal cholinergic interneurons and dopamine depletion).
In addition to the role played by the cholinergic interneurons in the generation of the motor symptoms, there is evidence of other perturbations of the central cholinergic system in Parkinson's disease with dementia. Reduced levels of cortical choline acetyltransferase [103, 116, 117, 118] could be indicative of a loss of innervating neurons. These findings are supported by postmortem [116] and neuroimaging [119, 120] studies using nonselective ligands, which report increased levels of cortical muscarinic receptors in the brains from patients with Parkinson's disease with dementia, despite the radioligand uptake being reduced in frontal regions and temporal lobes [119]. More selective ligands used in postmortem studies, suggest that it is the M1 receptor, which is increased in cortical, but not subcortical regions [117]. However, a more recent study found that M1 receptors were significantly reduced in the caudate from people with Parkinson's disease who also had dementia [108] and there are also reports of unchanged levels of M1 receptors using less selective ligands [121]. Similarly, there is conflicting evidence for changes in the M2/M4 receptors in Parkinson's disease, with reports of decreased [103] and unchanged cortical [108] levels. Unfortunately, some studies used a cohort consisting of tissue from people with Lewy body dementias or Parkinson's disease, making it difficult to determine which disorder is associated with the decrease in thalamic M4 receptors [122] or the decrease in M2 receptors seen in the insular cortex [106].
Overall, combining the cortical data on levels of muscarinic receptors and choline acetyltransferase, it is possible that the receptor upregulation reported by the majority of studies is a compensatory mechanism for the reduced cholinergic input to the cortex. This concept is supported by the finding that hemicholinium binding (to the high‐affinity choline uptake site) is increased in the striatum and hippocampus from people with Parkinson's disease [121], which suggests the cholinergic system is trying to compensate for low levels of acetylcholine.
With the role played by the striatal interneurons in the development of motor symptoms, anticholinergic drugs were one of the original treatments for Parkinson's disease; indeed they are still used as an adjunct treatment to the dopamine precursor, levadopa, because they ameliorate the movement disorder associated with the disease [123]. However, due to their nonspecific nature these drugs have a number of side effects—including exacerbation of cognitive decline [123]. Using cholinesterase inhibitors to treat the cognitive deficits associated with Parkinson's disease with dementia is not a viable option since they exacerbate the motor symptoms [124].
The development of more selective ligands for the muscarinic receptors opens the possibility of more targeted approaches for these two symptom domains. If the M1 allosteric activators currently in development fulfill their preclinical promise of procognitive effects, they would constitute a more direct approach to treating the cognitive symptoms, as discussed for schizophrenia and Alzheimer's disease. In addition, since the M4 receptor is involved in the modulation of dopaminergic activity [27], selective antagonists at this receptor could redress the imbalance in striatal dopamine that underlies the dyskinesia associated with the disease, without the effect on cognition seen with nonselective antagonists. This hypothesis is supported by the success of a study using a relatively selective M4 antagonist in an animal model of resting tremor [125], with less effects on selected memory tasks than other nonselective antagonists. Following the recent report of the synthesis of selective M4 antagonists [126], it will be interesting to discover whether they have the ability to reduce the motor symptoms without further impairing cognition in patients with Parkinson's disease.
In summary, all these disorders of the central nervous system offer the potential of therapeutic applications for selective activators or inhibitors of muscarinic receptors. In schizophrenia, the M1 agonists offer the potential to improve the cognitive deficits associated with the disorder and may prove to have some efficacy in treating psychosis, while the M4 agonists currently show promise as antipsychotic agents. In both MDD and bipolar disorder, there seems to be a potential for therapeutics aimed at the M2 receptor, with an antagonist required for depressive symptoms and an agonist for mania, however, the major hurdle to overcome with this approach will be avoiding the side effects, most of which are due to activity at either M2 or M3 peripheral receptors. There are indications that an M5 antagonist could prove to be beneficial in treating addiction, although these too could have issues with side effects in the form of impaired cerebrovascular vasodilatation. The precognitive effects of M1 agonists make them of interest in the treatment of Alzheimer's disease, particularly since they also modulate amyloid protein precursor processing—reducing the amyloidergic processing pathway. Finally, in addition to the possibility of using M1 agonists to reduce the cognitive deficits seen in Parkinson's disease, the potential exists for a more selective M4 antagonist to be of therapeutic value in the treatment of the dyskinesia associated with the disease. The recent advances that have occurred in developing more selective ligands for individual muscarinic receptors have allowed some of the hypotheses arising from basic research to be tested in animal models, with promising results. However, it remains to be seen whether these encouraging successes in animal models translate to clinically efficacious outcomes.
Conflict of Interest
The authors have no conflict of interest.
Funding
Dr. Scarr is the Royce Abbey Postdoctoral Fellow, funded by Australian Rotary Health. Australian Rotary Health had no role in the data collection, manuscript preparation, and/or publication decisions.
Financial Disclosure
Dr. Scarr has no financial relationships to disclose for the previous 12 months.
References
- 1. Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: The non‐neuronal cholinergic system in humans. Br J Pharmacol 2008;154:1558–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ismail N, Robinson GE, Fahrbach SE. Stimulation of muscarinic receptors mimics experience‐dependent plasticity in the honey bee brain. Proc Natl Acad Scie USA 2006;103:207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sarter M, Bruno JP. Cognitive functions of cortical acetylcholine: Toward a unifying hypothesis. Brain Res Rev 1997;23:28–46. [DOI] [PubMed] [Google Scholar]
- 4. Perry E, Walker M, Grace J, Perry R. Acetylcholine in mind: A neurotransmitter correlate of consciousness Trends Neurosci 1999;22:273–280. [DOI] [PubMed] [Google Scholar]
- 5. Dale HH. The action of certain esters and ethers of choline and their relation to muscarine. J Pharmacol Exp Ther 1914;6:147–190. [Google Scholar]
- 6. Lucas‐Meunier E, Fossier P, Baux G, Amar M. Cholinergic modulation of the cortical neuronal network. Pflugers Archiv Eur J Physiol 2003;446:17–29. [DOI] [PubMed] [Google Scholar]
- 7. Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 1998;50:279–290. [PubMed] [Google Scholar]
- 8. Felder CC. Muscarinic acetylcholine receptors: Signal transduction through multiple effectors. FASEB J 1995;9:619–625. [PubMed] [Google Scholar]
- 9. Flynn DD, Ferrari‐DiLeo G, Mash DC, Levey AI. Differential regulation of molecular subtypes of muscarinic receptors in Alzheimer's disease. J Neurochem 1995;64:1888–1891. [DOI] [PubMed] [Google Scholar]
- 10. Mrzljak L, Levey AI, Goldman‐Rakic PS. Association of m1 and m2 muscarinic receptor proteins with asymmetric synapses in the primate cerebral cortex: Morphological evidence for cholinergic modulation of excitatory neurotransmission. Proc Natl Acad Sci USA 1993;90:5194–5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harrison PJ, Barton AJ, Najlerahim A, McDonald B, Pearson RC. Increased muscarinic receptor messenger RNA in Alzheimer's disease temporal cortex demonstrated by in situ hybridization histochemistry. Brain Res Mol Brain Res 1991;9:15–21. [DOI] [PubMed] [Google Scholar]
- 12. Zhang W, Basile AS, Gomeza J, Volpicelli LA, Levey AI, Wess J. Characterization of central inhibitory muscarinic autoreceptors by the use of muscarinic acetylcholine receptor knock‐out mice. J Neurosci 2002;22:1709–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mrzljak L, Levey AI, Belcher S, Goldman‐Rakic PS. Localization of the m2 muscarinic acetylcholine receptor protein and mRNA in cortical neurons of the normal and cholinergically deafferented rhesus monkey. J Comp Neurol 1998;390:112–132. [PubMed] [Google Scholar]
- 14. Disney AA, Aoki C. Muscarinic acetylcholine receptors in macaque V1 are most frequently expressed by parvalbumin‐immunoreactive neurons. J Comp Neurol 2008;507:1748–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levey AI, Edmunds SM, Heilman CJ, Desmond TJ, Frey KA. Localization of muscarinic M3 receptor protein and M3 receptor binding in rat brain. Neuroscience 1994;63:207–221. [DOI] [PubMed] [Google Scholar]
- 16. Weiner DM, Levey AI, Brann MR. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc Natl Acad Sci USA 1990;87:7050–7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vilaro MT, Palacios JM, Mengod G. Localization of m5 muscarinic receptor mRNA in rat brain examined by in situ hybridization histochemistry. Neurosci Lett 1990;114:154–159. [DOI] [PubMed] [Google Scholar]
- 18. Gerber DJ, Sotnikova TD, Gainetdinov RR, Huang SY, Caron MG, Tonegawa S. Hyperactivity, elevated dopaminergic transmission, and response to amphetamine in M1 muscarinic acetylcholine receptor‐deficient mice. Proc Natl Acad Sci USA 2001;98:15312–15317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miyakawa T, Yamada M, Duttaroy A, Wess J. Hyperactivity and intact hippocampus‐dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J Neurosci 2001;21:5239–5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shinoe T, Matsui M, Taketo MM, Manabe T. Modulation of synaptic plasticity by physiological activation of M1 muscarinic acetylcholine receptors in the mouse hippocampus. J Neurosci 2005;25:11194–11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anagnostaras SG, Murphy GG, Hamilton SE, et al Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci 2003;6:51–58. [DOI] [PubMed] [Google Scholar]
- 22. Tzavara ET, Bymaster FP, Felder CC, et al Dysregulated hippocampal acetylcholine neurotransmission and impaired cognition in M2, M4 and M2/M4 muscarinic receptor knockout mice. Mol Psychiatry 2003;8:673–679. [DOI] [PubMed] [Google Scholar]
- 23. Gomeza J, Shannon H, Kostenis E, et al Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA 1999;96:1692–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamada M, Miyakawa T, Duttaroy A, et al Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature 2001;410:207–212. [DOI] [PubMed] [Google Scholar]
- 25. Salamone JD, Correa M, Carlson BB, et al Neostriatal muscarinic receptor subtypes involved in the generation of tremulous jaw movements in rodents implications for cholinergic involvement in parkinsonism. Life Sci 2001;68:2579–2584. [DOI] [PubMed] [Google Scholar]
- 26. Gomeza J, Zhang L, Kostenis E, et al Enhancement of D1 dopamine receptor‐mediated locomotor stimulation in M(4) muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA 1999;96:10483–10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tzavara ET, Bymaster FP, Davis RJ, et al M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: Relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J 2004;18:1410–1412. [DOI] [PubMed] [Google Scholar]
- 28. Yamada M, Basile AS, Fedorova I, et al Novel insights into M5 muscarinic acetylcholine receptor function by the use of gene targeting technology. Life Sci 2003;74:345–353. [DOI] [PubMed] [Google Scholar]
- 29. Basile AS, Fedorova I, Zapata A, et al Deletion of the M5 muscarinic acetylcholine receptor attenuates morphine reinforcement and withdrawal but not morphine analgesia. Proc Natl Acad Sci USA 2002;99:11452–11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th – text revision ed Washington , DC : American Psychiatric Association, 2000. [Google Scholar]
- 31. Jablensky A. Subtyping schizophrenia: Implications for genetic research. Mol Psychiatry 2006;11:815–836. [DOI] [PubMed] [Google Scholar]
- 32. Cohen LH, Thale T, Tissenbaum MJ. Acetylcholine treatment of schizophrenia. Arch Neurol Psychiatry 1944;51:171–175. [Google Scholar]
- 33. Forrer GR, Miller JJ. Atropine Coma: A somatic therapy in psychiatry. Am J Psychiatry 1958;115:455–458. [DOI] [PubMed] [Google Scholar]
- 34. Scarr E, Dean B. Role of the cholinergic system in the pathology and treatment of schizophrenia. Expert Rev Neurother 2009;9:73–86. [DOI] [PubMed] [Google Scholar]
- 35. Liao DL, Hong CJ, Chen HM, et al Association of muscarinic m1 receptor genetic polymorphisms with psychiatric symptoms and cognitive function in schizophrenic patients. Neuropsychobiology 2003;48:72–76. [DOI] [PubMed] [Google Scholar]
- 36. Mancama D, Arranz MJ, Landau S, Kerwin R. Reduced expression of the muscarinic 1 receptor cortical subtype in schizophrenia. Am J Med Genet B Neuropsychiatr Genet 2003;119:2–6. [DOI] [PubMed] [Google Scholar]
- 37. Scarr E, Cowie TF, Kanellakis S, Sundram S, Pantelis C, Dean B. Decreased cortical muscarinic receptors define a subgroup of subjects with schizophrenia. Mol Psychiatry 2009;14:1017–1023. [DOI] [PubMed] [Google Scholar]
- 38. De Luca V, Wang H, Squassina A, Wong GW, Yeomans J, Kennedy JL. Linkage of M5 muscarinic and alpha7‐nicotinic receptor genes on 15q13 to schizophrenia. Neuropsychobiology 2004;50:124–127. [DOI] [PubMed] [Google Scholar]
- 39. Bennett JP Jr., Enna SJ, Bylund DB, Gillin JC, Wyatt RJ, Snyder SH. Neurotransmitter receptors in frontal cortex of schizophrenics. Arch Gen Psychiatry 1979;36:927–934. [DOI] [PubMed] [Google Scholar]
- 40. Watanabe S, Nishikawa T, Takashima M, Toru M. Increased muscarinic cholinergic receptors in prefrontal cortices of medicated schizophrenics. Life Sci 1983;33:2187–2196. [DOI] [PubMed] [Google Scholar]
- 41. Dean B, Crook JM, Opeskin K, Hill C, Keks N, Copolov DL. The density of muscarinic M1 receptors is decreased in the caudate‐ putamen of subjects with schizophrenia. Mol Psychiatry 1996;1:54–58. [PubMed] [Google Scholar]
- 42. Zavitsanou K, Katsifis A, Mattner F, Xu‐Feng H. Investigation of m1/m4 muscarinic receptors in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression disorder. Neuropsychopharmacology 2004;29:619–625. [DOI] [PubMed] [Google Scholar]
- 43. Raedler TJ, Knable MB, Jones DW, et al In vivo determination of muscarinic acetylcholine receptor availability in schizophrenia. Am J Psychiatry 2003;160:118–127. [DOI] [PubMed] [Google Scholar]
- 44. Dean B, McLeod M, Keriakous D, McKenzie J, Scarr E. Decreased muscarinic(1) receptors in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry 2002;7:1083–1091. [DOI] [PubMed] [Google Scholar]
- 45. Scarr E, Sundram S, Keriakous D, Dean B. Altered hippocampal muscarinic M4, but not M1, receptor expression from subjects with schizophrenia. Biol Psychiatry 2007;61:1161–1170. [DOI] [PubMed] [Google Scholar]
- 46. Gibbons AS, Scarr E, McLean CA, Sundram S, Dean B. Decreased muscarinic receptor binding in the frontal cortex of bipolar disorder and major depressive disorder subjects. J Affect Disord 2009;116:184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Crook JM, Dean B, Pavey G, Copolov D. The binding of [3H]AF‐DX 384 is reduced in the caudate‐putamen of subjects with schizophrenia. Life Sci 1999;64:1761–1771. [DOI] [PubMed] [Google Scholar]
- 48. Zavitsanou K, Katsifis A, Yu Y, Huang XF. M2/M4 muscarinic receptor binding in the anterior cingulate cortex in schizophrenia and mood disorders. Brain Res Bull 2005;65:397–403. [DOI] [PubMed] [Google Scholar]
- 49. Salah‐Uddin H, Scarr E, Pavey G, et al Altered M(1) muscarinic acetylcholine receptor (CHRM1)‐Galpha(q/11) coupling in a schizophrenia endophenotype. Neuropsychopharmacology 2009;34:2156–2166. [DOI] [PubMed] [Google Scholar]
- 50. Raedler TJ, Bymaster FP, Tandon R, Copolov D, Dean B. Towards a muscarinic hypothesis of schizophrenia. Mol Psychiatry 2006;12:232–246. [DOI] [PubMed] [Google Scholar]
- 51. Bymaster FP, Whitesitt CA, Shannon HE, et al Xanomeline: A selective muscarinic agonist for the treatment of Alzheimer's disease. Drug Dev Res 1997;40:158–170. [Google Scholar]
- 52. Shekhar A, Potter WZ, Lightfoot J, et al Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry 2008;165:1033–1039. [DOI] [PubMed] [Google Scholar]
- 53. Woolley ML, Carter HJ, Gartlon JE, Watson JM, Dawson LA. Attenuation of amphetamine‐induced activity by the non‐selective muscarinic receptor agonist, xanomeline, is absent in muscarinic M(4) receptor knockout mice and attenuated in muscarinic M(1) receptor knockout mice. Eur J Pharmacol 2009;603:147–149. [DOI] [PubMed] [Google Scholar]
- 54. Langmead CJ, Watson J, Reavill C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacology and Therapeutics 2008;117:232–243. [DOI] [PubMed] [Google Scholar]
- 55. Bymaster FP, Felder CC. Role of the cholinergic muscarinic system in bipolar disorder and related mechanism of action of antipsychotic agents. Mol Psychiatry 2002;7(Suppl 1):S57–S63. [DOI] [PubMed] [Google Scholar]
- 56. Clark AL, Mitchelson F. The inhibitory effect of gallamine on muscarinic receptors. Br J Pharmacol 1976;58:323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Langmead CJ, Austin NE, Branch CL, et al Characterization of a CNS penetrant, selective M1 muscarinic receptor agonist, 77‐LH‐28–1. Br J Pharmacol 2008;154:1104–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vanover KE, Veinbergs I, Davis RE. Antipsychotic‐like behavioral effects and cognitive enhancement by a potent and selective muscarinic M‐sub‐1 receptor agonist, AC‐260584. Behav Neurosci 2008;122:570–575. [DOI] [PubMed] [Google Scholar]
- 59. Bradley SR, Lameh J, Ohrmund L, et al AC‐260584, an orally bioavailable M1 muscarinic receptor allosteric agonist, improves cognitive performance in an animal model. Neuropharmacology 2010;58:365–373. [DOI] [PubMed] [Google Scholar]
- 60. Shirey JK, Brady AE, Jones PJ, et al A Selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J Neurosci 2009;29:14271–14286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jones CK, Brady AE, Davis AA, et al Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic‐like activity in rats. J Neurosci 2008;28:10422–10433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Leach K, Loiacono RE, Felder CC, et al Molecular mechanisms of action and in vivo validation of an m(4) muscarinic acetylcholine receptor allosteric modulator with potential antipsychotic properties. Neuropsychopharmacology 2010;35:855–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chan WY, McKinzie DL, Bose S, et al Allosteric modulation of the muscarinic M4 receptor as an approach to treating schizophrenia. Proc Natl Acad Sci USA 2008;105:10978–10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Brady AE, Jones CK, Bridges TM, et al Centrally active allosteric potentiators of the M4 muscarinic acetylcholine receptor reverse amphetamine‐induced hyperlocomotor activity in rats. J Pharmacol Exp Ther 2008;327:941–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron 2002;34:13–25. [DOI] [PubMed] [Google Scholar]
- 66. Rowntree DW, Nevin S, Wilson A. The effects of diisopropylfluorophosphonate in schizophrenia and manic depressive psychosis. J Neurol Neurosurg Psychiatry 1950;13:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gershon S, Shaw FH. Psychiatric sequelae of chronic exposure to organophosphorus insecticides. Lancet 1961;1:1371–1374. [DOI] [PubMed] [Google Scholar]
- 68. Janowsky DS, El Yousef MK, Davis JM, Sekerke HJ. A cholinergic‐adrenergic hypothesis of mania and depression. Lancet 1972;2:632–635. [DOI] [PubMed] [Google Scholar]
- 69. Dilsaver SC. Cholinergic mechanisms in depression. Brain Res 1986;396:285–316. [DOI] [PubMed] [Google Scholar]
- 70. Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: A randomized, placebo‐controlled clinical trial. Arch Gen Psychiatry 2006;63:1121–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Drevets WC, Furey ML. Replication of scopolamine's antidepressant efficacy in major depressive disorder: A randomized, placebo‐controlled clinical trial. Biol Psychiatry 2010;67:432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Furey ML, Khanna A, Hoffman EM, Drevets WC. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology 2010;35:2479–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cannon DM, Carson RE, Nugent AC, et al Reduced muscarinic type 2 receptor binding in subjects with bipolar disorder. Arch Gen Psychiatry 2006;63:741–747. [DOI] [PubMed] [Google Scholar]
- 74. Cannon DM, Klaver JK, Gandhi SK, et al Genetic variation in cholinergic muscarinic‐2 receptor gene modulates M(2) receptor binding in vivo and accounts for reduced binding in bipolar disorder. Mol Psychiatry 2010;doi: 10.1038/mp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Comings DE, Wu S, Rostamkhani M, McGue M, Iacono WG, MacMurray JP. Association of the muscarinic cholinergic 2 receptor (CHRM2) gene with major depression in women. Am J Med Genet 2002;114:527–529. [DOI] [PubMed] [Google Scholar]
- 76. Wang JC, Hinrichs AL, Stock H, et al Evidence of common and specific genetic effects: Association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Human Mole Genet 2004;13:1903–1911. [DOI] [PubMed] [Google Scholar]
- 77. Luo X, Kranzler HR, Zuo L, Wang S, Blumberg HP, Gelernter J. CHRM2 gene predisposes to alcohol dependence, drug dependence and affective disorders: Results from an extended case−control structured association study. Human Mole Genet 2005;14:2421–2434. [DOI] [PubMed] [Google Scholar]
- 78. Cohen‐Woods S, Gaysina D, Craddock N, et al Depression Case Control (DeCC) Study fails to support involvement of the muscarinic acetylcholine receptor M2 (CHRM2) gene in recurrent major depressive disorder. Human Mole Genet 2009;18:1504–1509. [DOI] [PubMed] [Google Scholar]
- 79. Steinfeld T, Mammen M, Smith JAM, Wilson RD, Jasper JR. A novel multivalent ligand that bridges the allosteric and orthosteric binding sites of the M2 muscarinic receptor. Mol Pharmacol 2007;72:291–302. [DOI] [PubMed] [Google Scholar]
- 80. Davis KL, Berger PA. Pharmacological investigations of the cholinergic imbalance hypotheses of movement disorders and psychosis. Biol Psychiatry 1978;13:23–49. [PubMed] [Google Scholar]
- 81. Janowsky DS, El Yousef MK, Davis JM, Hubbard B, Sekerke HJ. Cholinergic reversal of manic symptoms. Lancet 1972;1:1236–1237. [DOI] [PubMed] [Google Scholar]
- 82. Nurnberger J Jr., Berrettini W, Mendelson W, Sack D, Gershon ES. Measuring cholinergic sensitivity: I. Arecoline effects in bipolar patients. Biol Psychiatry 1989;25:610–617. [DOI] [PubMed] [Google Scholar]
- 83. World Health Organization . Management of substance abuse. Available from: http://www.who.int/substance_abuse/facts/global_burden/en/index.html [Accessed March 2010].
- 84. Smith JP. The social impact of drug abuse. 2, 1–49. 1995. United Nations International Drug Control Programme. Position paper for the World Summit for Social Development (Copenhagen, 6‐12 March, 1995).
- 85. Spanagel R, Weiss F. The dopamine hypothesis of reward: Past and current status. Trends Neurosci 1999;22:521–527. [DOI] [PubMed] [Google Scholar]
- 86. Sofuoglu M, Mooney M. Cholinergic functioning in stimulant addiction: Implications for medications development. CNS Drugs 2009;23:939–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. See RE, McLaughlin J, Fuchs RA. Muscarinic receptor antagonism in the basolateral amygdala blocks acquisition of cocaine‐stimulus association in a model of relapse to cocaine‐seeking behavior in rats. Neurosci 2003;117:477–483. [DOI] [PubMed] [Google Scholar]
- 88. Anney R, Lotfi‐Miri M, Olsson C, Reid S, Hemphill S, Patton G. Variation in the gene coding for the M5 muscarinic receptor (CHRM5) influences cigarette dose but is not associated with dependence to drugs of addiction: Evidence from a prospective population based cohort study of young adults. BMC Genetics 2007;8:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Dick DM, Agrawal A, Wang JC, et al Alcohol dependence with comorbid drug dependence: Genetic and phenotypic associations suggest a more severe form of the disorder with stronger genetic contribution to risk. Addiction 2007;102:1131–1139. [DOI] [PubMed] [Google Scholar]
- 90. Mobascher A, Rujescu D, Mittelstrass K, et al Association of a variant in the muscarinic acetylcholine receptor 2 gene (CHRM2) with nicotine addiction. Am J Med Genet B Neuropsychiatr Genet 2010;153B:684–690. [DOI] [PubMed] [Google Scholar]
- 91. de la Garza R, Johanson CE. Effects of haloperidol and physostigmine on self‐administration of local anesthetics. Pharmacol Biochem Behav 1982;17:1295–1299. [DOI] [PubMed] [Google Scholar]
- 92. Andersen MB, Werge T, Fink‐Jensen A. The acetylcholinesterase inhibitor galantamine inhibits d‐amphetamine‐induced psychotic‐like behavior in Cebus monkeys. J Pharmacol Exp Ther 2007;321:1179–1182. [DOI] [PubMed] [Google Scholar]
- 93. Winhusen TM, Somoza EC, Harrer JM, et al A placebo‐controlled screening trial of tiagabine, sertraline and donepezil as cocaine dependence treatments. Addiction 2005;100(Suppl 1):68–77. [DOI] [PubMed] [Google Scholar]
- 94. de la Garza R, Mahoney JJ III., Culbertson C, Shoptaw S, Newton TF. The acetylcholinesterase inhibitor rivastigmine does not alter total choices for methamphetamine, but may reduce positive subjective effects, in a laboratory model of intravenous self‐administration in human volunteers. Pharmacol Biochem Behav 2008;89:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yeomans J, Forster G, Blaha C. M5 muscarinic receptors are needed for slow activation of dopamine neurons and for rewarding brain stimulation. Life Sci 2001;68:2449–2456. [DOI] [PubMed] [Google Scholar]
- 96. Williams MJ, Adinoff B. The role of acetylcholine in cocaine addiction. Neuropsychopharmacology 2008;33:1779–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bridges TM, Marlo JE, Niswender CM, et al Discovery of the first highly M5‐preferring muscarinic acetylcholine receptor ligand, an M5 positive allosteric modulator derived from a series of 5‐trifluoromethoxy N‐benzyl isatins. J Med Chem 2009;52:3445–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Stahl E, Ellis J. Novel allosteric effects of amiodarone at the muscarinic M5 receptor. J Pharmacol Exp Ther 2010; doi: 10.1124/jpet.109.165316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Citron M. Alzheimer's disease: Strategies for disease modification. Nat Rev Drug Discov 2010;9:387–398. [DOI] [PubMed] [Google Scholar]
- 100. Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA 1985;82:4245–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nitsch RM. From acetylcholine to amyloid: Neurotransmitters and the pathology of Alzheimer's disease. Neurodegeneration 1996;5:477–482. [DOI] [PubMed] [Google Scholar]
- 102. Liu HC, Hong CJ, Liu TY, Chi CW, Tsai SJ. Association analysis for the muscarinic M1 receptor genetic polymorphisms and Alzheimer's disease. Dement Geriatr Cogn Disord 2005;19:42–45. [DOI] [PubMed] [Google Scholar]
- 103. Quirion R. Cholinergic markers in Alzheimer disease and the autoregulation of acetylcholine release. J Psychiatry Neurosci 1993;18:226–234. [PMC free article] [PubMed] [Google Scholar]
- 104. Araujo DM, Lapchak PA, Robitaille Y, Gauthier S, Quirion R. Differential alteration of various cholinergic markers in cortical and subcortical regions of human brain in Alzheimer's disease. J Neurochem 1988;50:1914–1923. [DOI] [PubMed] [Google Scholar]
- 105. Pascual J, Fontan A, Zarranz JJ, Berciano J, Florez JS, Pazos A. High‐affinity choline uptake carrier in Alzheimer's disease: Implications for the cholinergic hypothesis of dementia. Brain Res 1991;552:170–174. [DOI] [PubMed] [Google Scholar]
- 106. Warren NM, Piggott MA, Lees AJ, Burn DJ. The basal ganglia cholinergic neurochemistry of progressive supranuclear palsy and other neurodegenerative diseases. J Neurol Neurosurg Psychiatry 2007;78:571–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Vogt BA, Crino PB, Vogt LJ. Reorganization of cingulate cortex in Alzheimer's disease: Neuron loss, neuritic plaques, and muscarinic receptor binding. Cerebral Cortex 1992;2:526–535. [DOI] [PubMed] [Google Scholar]
- 108. Piggott MA, Owens J, O’Brien J, et al Muscarinic receptors in basal ganglia in dementia with Lewy bodies, Parkinson's disease and Alzheimer's disease. J Chem Neuroanat 2003;25:161–173. [DOI] [PubMed] [Google Scholar]
- 109. Shiozaki K, Iseki E, Uchiyama H, et al Alterations of muscarinic acetylcholine receptor subtypes in diffuse Lewy body disease: Relation to Alzheimer's disease. J Neurol Neurosurg Psychiatry 1999;67:209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tsang S, Francis P, Esiri M, Wong P, Chen C, Lai M. Loss of [3H]4‐DAMP binding to muscarinic receptors in the orbitofrontal cortex of Alzheimer's disease patients with psychosis. Psychopharmacology 2008;198:251–259. [DOI] [PubMed] [Google Scholar]
- 111. Shiozaki K, Iseki E, Hino H, Kosaka K. Distribution of m1 muscarinic acetylcholine receptors in the hippocampus of patients with Alzheimer's disease and dementia with Lewy bodies–an immunohistochemical study. J Neurol Sci 2001;193:23–28. [DOI] [PubMed] [Google Scholar]
- 112. Overk CR, Felder CC, Tu Y, et al Cortical M1 receptor concentration increases without a concomitant change in function in Alzheimer's disease. J Chem Neuroanat 2010;40:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Caccamo A, Oddo S, Billings LM, et al M1 receptors play a central role in modulating AD‐like pathology in transgenic mice. Neuron 2006;49:671–682. [DOI] [PubMed] [Google Scholar]
- 114. Bodick NC, Offen WW, Levey AI, et al Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol 1997;54:465–473. [DOI] [PubMed] [Google Scholar]
- 115. Ding J, Guzman JN, Tkatch T, et al RGS4‐dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nat Neurosci 2006;9:832–842. [DOI] [PubMed] [Google Scholar]
- 116. Ruberg M, Ploska A, Javoy‐Agid F, Agid Y. Muscarinic binding and choline acetyltransferase activity in Parkinsonian subjects with reference to dementia. Brain Res 1982;232:129–139. [DOI] [PubMed] [Google Scholar]
- 117. Lange KW, Wells FR, Jenner P, Marsden CD. Altered muscarinic and nicotinic receptor densities in cortical and subcortical brain regions in Parkinson's disease. J Neurochem 1993;60:197–203. [DOI] [PubMed] [Google Scholar]
- 118. Tiraboschi PM, Hansen LAM, Alford MB, et al Cholinergic dysfunction in diseases with Lewy bodies. Neurology 2000;54:407–411. [DOI] [PubMed] [Google Scholar]
- 119. Colloby SJ, Pakrasi S, Firbank MJ, et al In vivo SPECT imaging of muscarinic acetylcholine receptors using (R,R) 123I‐QNB in dementia with Lewy bodies and Parkinson's disease dementia. NeuroImage 2006;33:423–429. [DOI] [PubMed] [Google Scholar]
- 120. Asahina M, Suhara T, Shinotoh H, Inoue O, Suzuki K, Hattori T. Brain muscarinic receptors in progressive supranuclear palsy and Parkinson's disease: A positron emission tomographic study. J Neurol Neurosurg Psychiatry 1998;65:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Rodri’guez‐Puertas R, Pazos A, Pascual J. Cholinergic markers in degenerative parkinsonism: Autoradiographic demonstration of high‐affinity choline uptake carrier hyperactivity. Brain Res 1994;636:327–332. [DOI] [PubMed] [Google Scholar]
- 122. Warren NM, Piggott MA, Lees AJ, Burn DJ. Muscarinic receptors in the thalamus in progressive supranuclear palsy and other neurodegenerative disorders. J Neuropathol Exp Neurol 2007;66:399–404. [DOI] [PubMed] [Google Scholar]
- 123. Ehrt U, Broich K, Larsen JP, Ballard C, Aarsland D. Use of drugs with anticholinergic effect and impact on cognition in Parkinson's disease: A cohort study. J Neurol Neurosurg Psychiatry 2010;81:160–165. [DOI] [PubMed] [Google Scholar]
- 124. Ott BR, Lannon MC. Exacerbation of parkinsonism by tacrine. Clin Neuropharmacol 1992;15:322–325. [DOI] [PubMed] [Google Scholar]
- 125. Betz A, McLaughlin P, Burgos M, Weber S, Salamone J. The muscarinic receptor antagonist tropicamide suppresses tremulous jaw movements in a rodent model of parkinsonian tremor: Possible role of M4 receptors. Psychopharmacology 2007;194:347–359. [DOI] [PubMed] [Google Scholar]
- 126. Varoli L, Andreani A, Burnelli S, et al Diphenidol‐related diamines as novel muscarinic M4 receptor antagonists. Bioorg Med Chem Lett 2008;18:2972–2976. [DOI] [PubMed] [Google Scholar]


