Acute CO inhalation is a leading cause of death relevant to gas poisoning in the world since increasing use of carbon‐based fuels. It has been demonstrated that acute CO poisoning produces severe brain damages, which leads to the high mortality and delayed neurological syndrome (DNS) 1. Numerous studies have indicated that an increase in production of reactive oxygen species (ROS) is of crucial relevance to the pathogenesis of CO poisoning 2, 3.
Recently, it has been suggested that hydrogen, a potent free radical scavenger, selectively reduces the most cytotoxic ROS hydroxyl radical (˙OH) and effectively protects against neonatal cerebral hypoxia–ischemia injury and cerebral ischemia–reperfusion injury 4, 5. Our previous study has demonstrated that hydrogen‐rich saline could reduce delayed neurologic sequelae in experimental carbon monoxide toxicity 6. However, the potential effect of hydrogen on brain damage following acute CO poisoning yet has not been investigated 7.
In this study, acute CO poisoning was induced in Sprague‐Dawley rats by exposing them to 1000 ppm CO in air for 40 min and then 3000 ppm CO for another 20 min until they lost consciousness. Hydrogen‐rich saline (10 mL/kg) or normal saline (NS) (10 mL/kg, peritoneally) was administered immediately and again at 8 h and 16 h after CO insult. As shown in Figure 1A, more Nissl‐stained cells were observed in CO poisoned rats with hydrogen‐rich saline treatment than with NS 24 h after CO poisoning. ROS and its subsequent lipid peroxidation have been demonstrated to play a role in the brain injury following CO exposure with or without reoxygenation, which mimics the accident and the resuscitation with or without hyperbaric oxygen treatment. The increase in the levels of lipid peroxidation products such as MDA is a marker of lipid damage. In this experiment, we observed a significant increase in the levels of brain MDA in rats after CO poisoning (Figure 1B). And hydrogen treatment following CO exposure markedly decreased the levels of MDA in the cortex and hippocampus.
Figure 1.
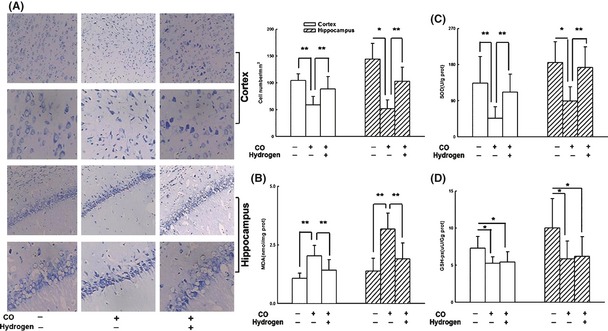
Hydrogen preserved more Nissl staining cells and reduced ROS generation, upregulating endogenous antioxidative enzymes. Hydrogen‐rich saline (10 mL/kg, i.p.) treatment reduced neuronal loss and dead cells in cortex and hippocampus (A), decreased the level of MDA (B), and increased SOD activity (C), but no difference in GSH‐px activity was found either in the cortex or in the hippocampus between CO+ Hydrogen group and CO+ NS group (C). Data are expressed as mean ± SD (n = 6–9 per group). Data were analyzed by one‐way analysis of variance (ANOVA) followed by a Student–Newman–Keuls (SNK) test for multiple comparisons. *P < 0.05, **P < 0.01.
Endogenous antioxidants are a group of substances that can significantly inhibit or delay oxidative process once they are oxidized themselves. Antioxidant enzymes such as SOD and glutathione (GSH) are important in providing protection from CO exposure 8. In the present study, hydrogen‐rich saline induced an increase in the activity of SOD (Figure 1C) but not GSH‐px (Figure 1D) in the brain tissue. The mechanism of protection of SOD is most likely as follows: O2 − can undergo either spontaneous or enzyme‐catalyzed (SOD) dismutation to hydrogen peroxide (H2O2), or can react with nitric oxide (NO·) to form the toxic product ONOO− 9. Hydrogen combats with ONOO− 5 and the reaction between O2 − and NO· is accelerated, which in turn results in inhibition of the enzyme‐catalyzed reaction by SOD. However, no significant difference of GSH‐px activity was observed between NS group and hydrogen group 24 h after CO injury. There are two possibilities accounting for this result. First, GSH‐px enzyme in the brain is insensitive to hydrogen‐rich saline therapy. Second, the level of GSH‐px was not markedly changed at the corresponding time points. The results suggested that hydrogen plays antioxidative action, possibly via reducing ROS formation and promoting ROS elimination.
In addition, apoptosis is another important factor of brain injury after CO poisoning, and hydrogen presents antiapoptotic effect 4. In this study, hydrogen‐rich saline treatment inhibited activation of downstream caspase‐3 (Figure 2A), and caspase‐9 (Figure 2B) after CO insult. Upregulation of Bcl‐2 or downregulation of Bax has been found to attenuate apoptotic cell death previously 10. We have observed in this study that hydrogen enhanced the Bcl‐2 but did not change Bax expression in the penumbra and hippocampus and increasing Bcl‐2/Bax ratio (Figure 2C,D).
Figure 2.
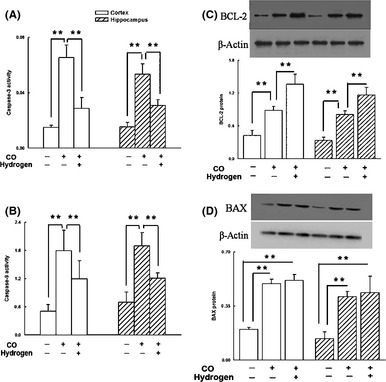
The antiapoptotic effect of hydrogen in the cortex and hippocampus after CO poisoning. Hydrogen‐rich saline decreased the enzymatic activity of caspase‐3 (A) and caspase‐9 (B) and upregulated the expression of Bcl‐2 in the cortex and hippocampus (C), but not the expression of Bax (D). Data are expressed as mean ± SD (n = 6–9 per group). Data were analyzed by one‐way analysis of variance (ANOVA) followed by a Student–Newman–Keuls (SNK) test for multiple comparisons. *P < 0.05, **P < 0.01.
Taken together, hydrogen‐rich saline may be clinically useful against acute CO poisoning, possibly via reducing ROS level, upregulating endogenous antioxidative enzymes, suppressed caspase enzyme activity, upregulated ratio of Bcl‐2 and Bax expression, and abated apoptosis.
Acknowledgments
This study was supported by National Natural Science Foundation of China (81272138).
References
- 1. Thom SR. Hyperbaric‐Oxygen therapy for acute carbon monoxide poisoning. N Engl J Med 2002;347:1105–1106. [DOI] [PubMed] [Google Scholar]
- 2. Hara S, Mukai T, Kurosaki K, Endo T. Characterization of hydroxyl radical generation in the striatum of free‐moving rats due to carbon monoxide poisoning, as determined by in vivo microdialysis. Brain Res 2004;1016:281–284. [DOI] [PubMed] [Google Scholar]
- 3. Thom SR. Carbon‐monoxide‐mediated brain lipid peroxidation in the rat. J Appl Physiol 1990;68:997–1003. [DOI] [PubMed] [Google Scholar]
- 4. Cai JM, Kang ZM, Liu K, et al. Neuroprotective effects of hydrogen saline in neonatal hypoxia‐ischemia rat model. Brain Res 2008;1256:129–137. [DOI] [PubMed] [Google Scholar]
- 5. Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 2007;13:688–694. [DOI] [PubMed] [Google Scholar]
- 6. Sun Q, Cai JM, Zhou JR, et al. Hydrogen‐rich saline reduces delayed neurologic sequelae in experimental carbon monoxide toxicity. Crit Care Med 2011;39:765–769. [DOI] [PubMed] [Google Scholar]
- 7. Shen MH, He J, Cai JM, Sun Q, Sun XJ, Huo ZL. Hydrogen as a novel and effective treatment of acute carbon monoxide poisoning. Med Hypotheses 2010;75:235–237. [DOI] [PubMed] [Google Scholar]
- 8. Thom SR. Leukocytes in carbon monoxide‐mediated brain oxidative injury. Toxicol Appl Pharmacol 1993;123:234–247. [DOI] [PubMed] [Google Scholar]
- 9. Fattman CL, Schaefer LM, Oury TD. Extracellular superoxide dismutase in biology and medicine. Free Radic Biol Med 2003;35:236–256. [DOI] [PubMed] [Google Scholar]
- 10. Adams JM, Cory S. Bcl‐2‐regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol 2007;19:488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]


