Summary
Aims
To conduct a large‐scale analysis on epidemiology, management, and outcomes of spontaneous subarachnoid hemorrhage (SAH), and to investigate the current situation of aneurysm obliteration in China.
Methods
A multicenter prospective cohort study involving 132 hospitals throughout China from September 2007 to August 2008 was conducted. A total of 651 patients with spontaneous SAH were evaluated.
Results
The most frequent type of SAH was aneurysmal SAH (77.4%), followed by uncommon causes (17.5%) and uncertain etiologies (5.1%). For aneurysmal SAH, the cumulative mortality at 28 days, 3 months, 6 months, and 12 months was 16.9%, 21.2%, 23.6%, and 24.6%, respectively. Obliteration of aneurysms, age, Hunt and Hess grade, and history of stroke affected the 12‐month mortality. In multiple regression analysis, the region, type of hospital, patient's age, history of hypertension, and nonintraventricular hemorrhage impacted aneurysm obliteration.
Conclusion
Aneurysmal rupture is the most common cause of spontaneous SAH in China. The percentage of aneurysm obliteration is still low in China that seems to contribute to long‐term mortality. With continued training of specialists, proper allocation of healthcare resources, and establishment of stroke centers, the rate of securing aneurysms is expected to rise.
Keywords: Aneurysm, Death rate, Health policy, Subarachnoid hemorrhage, Surgery
Introduction
Subarachnoid hemorrhage (SAH) accounts for approximately 3–5% of all strokes 1, 2, but it tends to affect young adults and carries a high morbidity and mortality 3, 4. Early treatment of an aneurysm has mostly replaced the traditional “wait and see” approach. Surgical clipping or endovascular coiling can reduce the rate of rebleeding 2 and allow aggressive hemodynamics that may relieve symptomatic vasospasm 5. Guidelines for management of aneurysmal SAH highly recommend that surgical clipping or endovascular coiling be performed to reduce the rate of rebleeding after aneurysmal SAH (aSAH) 2. However, in China, nonsurgical treatment is still largely utilized in managing aSAH. Previous studies on epidemiology of stroke in China showed that SAH accounted for 0.07–1.8% 6, 7, 8, 9, 10 with a 28‐day mortality of 23–33% 6, 7. However, those studies did not provide data on etiology, management, or long‐term clinical outcomes. In addition, the previous studies only covered a few geographical regions that might not represent the overall epidemiological characteristics of SAH in China.
Materials and Methods
Study Population
The China National Stroke Registry (CNSR) is a nationwide government‐funded registry designed to examine the current status of stroke care in China. It offers us a unique opportunity to investigate etiology, risk factors, and outcomes of SAH and to study the current situation of aneurysm obliteration of aSAH in China.
This analysis is a prospective cohort study 11 based on the CNSR. A total of 22,216 patients from 132 participating hospitals were enrolled within 14 days after SAH onset throughout China from September 2007 to August 2008. Among 22,216 patients, 767 patients (3.5%) were diagnosed as SAH according to World Health Organization criteria 12 combined with computed tomography (CT) or magnetic resonance (MR). Six hundred and fifty‐one patients had baseline information. Five hundred and four patients had ruptured saccular aneurysms confirmed by digital subtraction angiography (DSA) or computed tomography angiography (CTA). Of 504 patients with aneurymal SAH, 156 patients received obliteration of aneurysms by neurosurgical clipping or endovascular coiling, while the other 348 patients received medical management (Figure 1). Given that obliteration of aneurysm (clipping or coiling) is the key management for aneurysmal SAH recommended by the Guidelines, we examined whether securing aneurysm reduces long‐term mortality in these patients. In addition, we studied the factors affecting the prognosis after aSAH. The study was approved by the ethics committees of all participating hospitals, and all patients or their designated relatives gave informed consents.
Figure 1.
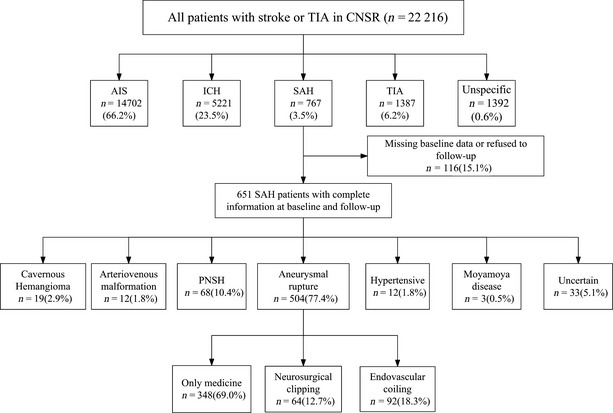
Flow diagram defining the eligible patients with aneurysmal SAH for surgery treatment.
Baseline Data Collection
Baseline information was collected at admission including prehospital care, prestroke modified Rankin Scales (mRS), first symptoms, baseline severity (mainly including Glasgow coma scale and Hunt and Hess grade), and first CT scan (within 24 h). Patient demographics, medical history, and medications were extracted from the medical records. Baseline information also included main vascular risk factors such as hypertension (self‐reported history, taking oral antihypertension drugs, or blood pressures ≥140/90 mmHg on admission), diabetes mellitus (history of diabetes mellitus or taking oral hypoglycemic agents/insulin), dyslipidemia (self‐reported history or taking oral antidyslipdemia drugs), current or previous smoking, moderate or heavy alcohol consumption (≥2 standard alcohol consumption per day), history of stroke (defined as a medical chart–confirmed history of SAH, cerebral infarction, transit ischemic attack, or cerebral hemorrhage), and history of cardio‐peripheral vascular events. Information on the use of oral or intravenous nimodipine and dehydrating agents such as mannitol, glycerol fructose, and hypertonic saline was also collected.
Patients' health insurance plans fell into the following four categories 11, 13, 14: (1) Basic health insurance scheme, provided jointly by the government and the employer, is often used by governmental employees and urban residents. (2) New Cooperative Medical System is provided by the Chinese government to rural residents; (3) commercial insurance; (4) self‐payment (no insurance coverage). Levels of education were grouped into three categories: elementary or below, middle school, and high school or above. Registry hospitals were classified into two categories: region (East vs. central vs. West) and academy (academic vs. nonacademic). The time of symptom onset to admission was divided into two intervals: ≤3 days and more than that. The departments where patients were admitted to were divided into three groups: emergency room (ER), intensive care unit (ICU) or ward, and clinic. Hunt and Hess grade was categorized as 0–3 vs. 4–5, and Glasgow Coma Scale (GCS) included 15–13 vs. 12–3.
Outcome Measures
Patients with SAH were contacted at 28 days, 3 months, 6 months, and 12 months via telephone interview conducted by trained research interviewers at Beijing Tiantan Hospital. The standard scripts were used to collect data. The interviewers were blinded to whether the patient's aneurysm was obliterated or not. Functional and survival outcomes were assessed using the mRS (0: full recovery, 6: death). If a patient was deceased, the death date was confirmed by the death certificate from a local citizen registry or attended hospital 11. If the local citizen registry information could not be obtained or the death was outside of the hospital, the death was deemed reliable if it was reported at two consecutive follow‐up interviews from different proxies.
Statistical Analysis
For descriptive analysis, proportions were used for categorical variables, and mean values with standard deviation were used for continuous variables. Univariate logistic regression analysis was used to identify the relationship between the 12‐month mortality and the demographic or clinical factors (such as age, gender, education, type of health insurance, admission hospital, clinical and radiological grades, prestroke MRS score at admission, hospital arrival time after onset, medical history, and obliteration of aneurysm). Cox survival proportional regression analysis (stepwise logistic model [entry significance level = 0.1 and stay significance level = 0.05]) was performed to analyze the relationship between the demographic or clinical factors and the 12‐month mortality. Factors with statistical significance on independent analysis were included as independent covariates in the Cox regression analysis. Adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) were reported.
Univariate logistic regression analysis was also used to identify the relationship between the demographic or clinical factors and the implementation of obliteration. To identity the association of these factors with the implement of obliteration, multivariate logistic regression analysis was performed using forward selection method, and adjusted odd ratios (ORs) with 95% CIs were also reported.
All data were analyzed with SAS for Windows software, Version 9.2 (SAS Institute Inc., Cary, NC, USA), and a two‐sided significance level of α = 0.05 was assumed.
Results
Demographics and Clinical Characteristics
Spontaneous SAH
In our cohort study, spontaneous SAH accounted for 3.7% of all strokes. The mean age was 57.1 ± 12.8 years, and 56.1% were females; hypertension was the most common risk factor (43.93% of the patients) (see Table S1). Aneurysmal rupture was the most common cause of SAH in China (77.4%), followed by perimesencephalic nonaneurysmal subarachnoid hemorrhage (PNSH) (10.4%), cavernous hemangioma (2.9%), arteriovenous malformation (1.8%), hypertensive intracerebral hemorrhage involving subarachnoid space (1.8%), and moyamoya disease (0.5%). Because of lack of repeated DSA or CTA, the etiology of 33 patients (5.1%) was uncertain (see Table S1 and Figure 1). 79.6% of 651 patients were admitted within 3 days of symptom onset, and 65.7% entered academic hospitals; 76.7% were admitted through emergency room. There was a low frequency of prestroke disability (3.8% of patients had a mRS ≥ 3). In our study, only 25.3% of patients with aSAH underwent neurosurgical clipping or endovascular coiling, while 60.0% received oral or intravenous nimodipine, and 91.3% received dehydrating treatment (see Table S1). Table S1 shows patients' demographic and clinical characteristics according to different SAH etiologies.
Aneurysmal SAH
A total of 504 patients with SAH were found to have ruptured aneurysms. Their mean age was 57.6 ± 12.8 years, and 58.5% were females. Three hundred and sixty‐seven (72.8%) patients were admitted to hospitals located in the eastern region of China, and 325 (64.5%) patients were admitted to academic hospitals. Four hundred and two (79.8%) patients were admitted to the registry hospitals within day 3 after onset, and the proportion of ER admission was 76.4%. The proportion of Hunt and Hess 0–3 grade and GCS 15‐13 scores was 82.7% and 74.2%, respectively. 14.5% of patients had intra‐ventricular blood on CT scans, and 46.0% had history of hypertension. The percentage of patients with history of SAH, cerebral infarction and cerebral hemorrhage was 8.5%, 6.8%, and 3.4%, respectively. Nimodipine was used in 62.9% of cases, while dehydrating medicine used in 93.7% of patients. 31.0% of patients received neurosurgical clipping or endovascular coiling (see Table 1).
Table 1.
Comparison of demographics, clinical characteristics, risk factors, management, and prognosis between Spontaneous subarachnoid hemorrhage patients with aneurysm and without aneurysm
| Aneurysmal SAH | Nonaneurysmal SAHa or Uncertain etiologyb | P‐value | |
|---|---|---|---|
| Number | 504 | 147 | |
| Age | 57.6 ± 12.8 | 55.1 ± 12.5 | 0.033 |
| Female % (n) | 58.5 (295) | 47.6 (70) | 0.019 |
| Health insurance % (n/total) | |||
| Urban employee/Government expense | 36.3 (183) | 43.5 (64) | 0.083 |
| Rural cooperative | 30.4 (155) | 20.4 (30) | |
| Commercial and other | 5.2 (26) | 7.5 (11) | |
| Own expense | 28.2 (142) | 28.6 (42) | |
| Region % (n) | |||
| East | 72.8 (367) | 74.8 (110) | 0.879 |
| Central | 17.3 (87) | 16.3 (24) | |
| West | 9.9 (50) | 8.8 (13) | |
| Academic hospital % (n) | 64.5 (325) | 70.7 (104) | 0.159 |
| Arrival to | |||
| Emergency room | 76.4 (385) | 77.6 (114) | 0.885 |
| ICU or Ward | 13.1 (66) | 11.6 (17) | |
| Clinic | 10.5 (53) | 10.9 (16) | |
| Prestroke modified Rankin Scale | |||
| 0–2 | 95.4 (481) | 98.6 (145) | 0.075 |
| 3–5 | 4.6 (23) | 1.4 (2) | |
| Level of consciousness on admission | |||
| Alert | 66.1 (333) | 80.3 (118) | 0.003 |
| Drowsy | 19.3 (97) | 8.8 (13) | |
| Unconscious | 14.7 (74) | 10.9 (16) | |
| Hunt–Hess grade % (n) | |||
| 0–3 | 82.7 (417) | 96.6 (141) | <0.0001 |
| 4–5 | 17.3 (87) | 3.4 (5) | |
| Glasgow Coma Scale % (n) | |||
| 15–13 | 78.8 (397) | 91.2 (134) | 0.001 |
| 12–3 | 21.2 (107) | 8.8 (13) | |
| Fisher scalec % (n) | |||
| I | 7.1 (30) | 15.5 (16) | <0.0001 |
| II | 45.0 (190) | 74.8 (77) | |
| III | 25.4 (107) | 3.9 (4) | |
| IV | 22.5 (95) | 5.8 (6) | |
| Blood into ventricles % (n) | 14.5 (73) | 5.4 (8) | 0.003 |
| Medical history % (n) | |||
| Hypertension | 46.0 (232) | 36.7 (54) | 0.046 |
| Diabetes mellitus | 7.5 (38) | 9.5 (14) | 0.435 |
| Dyslipidemia | 5.0 (25) | 1.4 (2) | 0.054 |
| Current Smoking | 25.2 (123) | 22.5 (31) | 0.690 |
| Moderate and heavy drink | 8.1 (41) | 6.1 (9) | 0.420 |
| Subarachnoid hemorrhage | 8.5 (43) | 10.2 (15) | 0.531 |
| Cerebral infarction | 6.8 (34) | 5.4 (8) | 0.571 |
| Cerebral hemorrhage | 3.4 (17) | 3.4 (5) | 0.987 |
| Cardio‐peripheral vascular events | 6.7 (34) | 4.8 (7) | 0.384 |
| Dehydration Agents % (n) | 93.7 (463) | 91.0 (132) | 0.261 |
| Nimodipine % (n) | 62.9 (308) | 50.0 (71) | 0.006 |
| Treatment methods % (n) | |||
| Only medicine | 69.0 (348) | 93.9 (138) | <0.0001 |
| Neurosurgery | 12.7 (64) | 3.4 (5) | |
| Endovascular treatment | 18.3 (92) | 2.7 (4) | |
| 28‐Day mortality % (n) | 16.9 (85) | 10.2 (15) | 0.049 |
| 3‐Month mortality % (n) | 21.2 (107) | 12.2 (18) | 0.015 |
| 6‐Month mortality % (n) | 23.6 (119) | 12.9 (19) | 0.005 |
| 12‐Month mortality % (n) | 24.6 (124) | 13.6 (20) | 0.005 |
Nonaneurysmal SAH included perimesencephalic nonaneurysmal subarachnoid hemorrhage (PNSH) (n = 68), cavernous hemangioma (n = 19), arteriovenous malformation (n = 12), hypertensive intracerebral hemorrhage involved subarachnoid space (n = 12), and Moyamoya disease (3).
uncertain etiology (n = 33): because of lack of repeated angiography, we cannot identify the etiology.
Fisher scale: I: no hemorrhage found; II: thickness <1 mm, into whole subarachnoid hemorrhage; III: thickness >1 mm; IV: with intracranial hematoma and ventricular hematoma.
Outcomes
The cumulative mortality at 28 days, 3 months, 6 months, and 12 months was 15.4%, 19.2%, 21.2%, and 22.1%, respectively, for all the patients with spontaneous SAH (see Table S1), and was 16.9%, 21.2%, 23.6%, and 24.6%, respectively for patients with aSAH (see Table 1).
A total of 124 (24.6%) patients with aSAH died within 12 months. In univariate logistic analysis, the following variables were detected to have statistically significant association with death: age, education, academic hospital, time to admission, hospital department of first visit, prestroke mRS, cognitive dysfunction or disturbance of consciousness as first symptom, conscious status on admission, Hunt and Hess grade and GCS at admission, Fisher scale on first CT scan, intraventricular hemorrhage, smoking status, history of hypertension, cerebral infarction and cerebral hemorrhage, and obliteration of aneurysms (see Tables 2 and S2).
Table 2.
Comparison of clinical characteristics between alive and dead patients
| Alive | Dead | P‐value | |
|---|---|---|---|
| Number | 380 | 124 | |
| Age (years, mean ± SD) | 55.6 ± 12.2 | 63.8 ± 12.9 | <0.0001 |
| Gender (female %, n) | 56.8 (216) | 63.7 (79) | 0.178 |
| Region % (n) | |||
| East | 71.6 (272) | 76.6 (95) | 0.274 |
| Central or west | 28.4 (108) | 23.4 (29) | |
| Academic hospital % (n) | 66.8 (254) | 57.3 (71) | 0.053 |
| Prestroke mRS % (n) | |||
| 0–2 | 97.4 (370) | 89.5 (111) | 0.001 |
| 3–5 | 2.6 (10) | 10.5 (13) | |
| Level of consciousness on admission % (n) | |||
| Alert | 73.4 (279) | 43.6 (54) | <0.0001 |
| Drowsy | 16.8 (64) | 26.6 (33) | |
| Unconscious | 9.7 (37) | 29.8 (37) | |
| GCS at admission % (n) | |||
| 15–13 | 80.8 (307) | 54.0 (67) | <0.0001 |
| 12–3 | 19.2 (73) | 46.0 (57) | |
| Hunt and Hess grade % (n) | |||
| 0–3 | 93.1 (353) | 51.6 (64) | <0.0001 |
| 4–5 | 6.9 (26) | 48.4 (60) | |
| Fisher scalea % (n) | |||
| I | 8.9 (28) | 1.9 (2) | <0.0001 |
| II | 50.8 (160) | 28.0 (30) | |
| III | 22.9 (72) | 32.7 (35) | |
| IV | 17.5 (55) | 37.4 (40) | |
| Blood into ventricles % (n) | 11.6 (44) | 23.4 (29) | 0.001 |
| Medical history % (n) | |||
| Hypertension | 41.8 (159) | 58.9 (73) | 0.001 |
| Diabetes mellitus | 6.8 (26) | 9.7 (12) | 0.299 |
| Dyslipidemia | 5.8 (22) | 2.4 (3) | 0.133 |
| Current Smoking | 28.2 (104) | 16.0 (19) | 0.008 |
| Moderate and heavy drink | 9.2 (35) | 4.8 (6) | 0.122 |
| Subarachnoid hemorrhage | 7.6 (29) | 11.3 (14) | 0.205 |
| Cerebral infarction | 5.0 (19) | 12.1 (15) | 0.006 |
| Cerebral hemorrhage | 1.6 (6) | 8.9 (11) | <0.0001 |
| Cardio‐peripheral vascular events | 5.8 (22) | 9.7 (12) | 0.134 |
| Dehydration Agents % (n) | 93.0 (347) | 95.9 (116) | 0.263 |
| Nimodipine % (n) | 64.2 (239) | 58.5 (69) | 0.258 |
| Treatment method % (n) | |||
| Nonsurgery group | 64.0 (243) | 84.7 (105) | <0.0001 |
| Surgery group | 36.1 (137) | 15.3 (19) | |
mRS, modified Rankin Scale; GCS, Glasgow Coma Scale.
Fisher scale: I: no hemorrhage found; II: thickness <1 mm, into whole subarachnoid hemorrhage; III: thickness >1 mm; IV: with intracranial hematoma and ventricular hematoma.
Table 3 shows risk factors associated with mortality within 12 months. Age, gender, treatment, Hunt–Hess grade, GCS, Fisher scale, prestroke mRS, history of hypertension, history of cerebral infarction and hemorrhage, and obliteration of aneurysm were entered into the Cox survival proportional regression model. Age (adjusted HR: 1.028, 95% CI: 1.013–1.043), Hunt and Hess 4–5 grade (adjusted HR: 9.380, 95% CI: 6.332–13.897), history of cerebral infarction (adjusted HR: 1.940, 95% CI: 1.070–3.514), history of cerebral hemorrhage (adjusted HR: 2.095, 95% CI: 1.098–3.998), and obliteration of aneurysms (adjusted HR: 0.451, 95% CI: 0.272–0.747) were associated with 12‐month mortality.
Table 3.
Cox regression analysis for 12‐month mortality in patients with aneurysmal subarachnoid hemorrhagea
| Variables | Unadjusted HR (95%CI) | Adjusted HR (95%CI) |
|---|---|---|
| Age | 1.045 (1.030–1.059) | 1.028 (1.013–1.043) |
| Hunt–Hess grade | ||
| 0–2 | REF | REF |
| 3–5 | 11.189 (7.706–16.246) | 9.380 (6.332–13.897) |
| History of cerebral infarction | 2.208 (1.287–3.789) | 1.940 (1.070–3.514) |
| History of ICH | 4.162 (2.238–7.738) | 2.095 (1.098–3.998) |
| Treatment | ||
| Nonsurgery | REF | REF |
| Surgery | 0.352 (0.216–0.574) | 0.451 (0.272–0.747) |
HR, hazard ratio; CI, confidence interval; REF, reference; ICH, intracerebral hemorrhage.
The following factors were entered into Cox proportional hazards regression model: age, gender, treatment, Hunt–Hess grade, Glasgow Coma Scale, Fisher scale, hypertension, history of cerebral infarction and hemorrhage.
Variables that Affect the Obliteration of Aneurysms
Treatment guidelines for aSAH recommend that the obliteration of aneurysm (clipping or coiling) should be considered and performed as a key treatment element. However, we found that the majority of these patients (69.0%) were not treated with clipping or coiling. Our study also suggested that there was a higher mortality in the nonobliteration group (see Table 3). In univariate logistic analysis, statistically significant differences were found between the obliteration group (n = 348) and the nonobliteration group (n = 156) in the following variables: age, health insurance, region, type of hospital, admission department, prestroke mRS, clinical grade (Hunt and Hess grade and GCS), Fisher scale, intra‐ventricular hemorrhage, and history of hypertension (see Table 4). The similar differences remained statistically significant when multiple regression analysis was used (see Table 5). Younger age (adjusted OR: 0.976, 95% CI: 0.959–0.994), the eastern region (adjusted OR: 6.871, 95% CI: 3.474–13.592), academic hospitals (adjusted OR: 2.602, 95% CI: 1.581–4.283), absence of hypertension (adjusted OR: 0.580, 95% CI: 0.369–0.913), and absence of intraventricular hemorrhage (adjusted OR: 2.066, 95% CI: 1.017–4.197) favored aneurysm obliteration.
Table 4.
Comparison of clinical characteristics between aneurysmal subarachnoid hemorrhage patient without surgery and with surgery treatmenta
| Nonsurgery group | Surgery group | P‐value | |
|---|---|---|---|
| Number | 348 | 156 | |
| Age (years, mean ± SD) | 59.1 ± 13.6 | 54.4 ± 10.5 | <0.0001 |
| Gender (Female %, n) | 59.5 (207) | 56.4 (88) | 0.517 |
| Health insurance % (n) | |||
| Urban employee/Government expense | 36.8 (128) | 35.3 (55) | 0.002 |
| Rural cooperative | 34.5 (120) | 21.2 (33) | |
| Commercial and other | 3.7 (13) | 8.3 (13) | |
| Own expense | 25.0 (87) | 35.3 (55) | |
| Region % (n) | |||
| East | 63.8 (222) | 93.0 (145) | <0.0001 |
| Central or West | 36.2 (126) | 7.0 (11) | |
| Academic hospital % (n) | 56.6 (197) | 82.1 (128) | <0.0001 |
| Arrival to % (n) | |||
| ER | 74.4 (259) | 80.8 (126) | 0.031 |
| ICU or Ward | 12.6 (44) | 14.1 (22) | |
| Clinic | 12.9 (45) | 5.1 (8) | |
| Prestroke mRS % (n) | |||
| 0–2 | 94.0 (327) | 98.7 (154) | 0.019 |
| 3–5 | 6.0 (21) | 1.3 (2) | |
| GCS at admission % (n) | |||
| 15–13 | 71.6 (249) | 80.1 (125) | 0.042 |
| 12–3 | 28.5 (99) | 19.9 (31) | |
| Hunt and Hess grade % (n) | |||
| 0–3 | 75.0 (275) | 91.0 (142) | 0.001 |
| 4–5 | 21.0 (73) | 9.0 (14) | |
| Fisher scaleb % (n) | |||
| I | 8.5 (24) | 4.3 (6) | 0.007 |
| II | 39.2 (111) | 56.8 (79) | |
| III | 27.6 (78) | 20.9 (29) | |
| IV | 24.7 (70) | 18.0 (25) | |
| Blood into ventricles % (n) | 17.2 (60) | 8.3 (13) | 0.009 |
| Medical history % (n) | |||
| Hypertension | 50.9 (177) | 35.3 (55) | 0.001 |
| Diabetes mellitus | 8.6 (30) | 5.1 (8) | 0.17 |
| Dyslipidemia | 4.3 (15) | 6.4 (10) | 0.315 |
| Current Smoking | 22.9 (77) | 30.5 (46) | 0.073 |
| Moderate and heavy drink | 7.8 (27) | 9.0 (14) | 0.644 |
| Subarachnoid hemorrhage | 7.5 (26) | 10.9 (17) | 0.203 |
| Cerebral infarction | 8.1 (28) | 3.9 (6) | 0.082 |
| Cerebral hemorrhage | 3.5 (12) | 3.2 (5) | 0.889 |
| Cardio‐peripheral vascular events | 8.1 (28) | 3.9 (6) | 0.082 |
ER, emergency room; ICU, intensive care unit; mRS, modified Rankin Scale; GCS, Glasgow Coma Scale.
Surgery treatment includes endovascular coiling and neurosurgical clipping.
Fisher scale: I: no hemorrhage found; II: thickness <1 mm, into whole subarachnoid hemorrhage; III: thickness >1 mm; IV: with intracranial hematoma and ventricular hematoma.
Table 5.
Logistic regression for surgery treatment in patients with aneurysmal subarachnoid hemorrhagea
| Variables | Unadjusted OR (95%CI) | Adjusted OR (95%CI) |
|---|---|---|
| Age | 0.971 (0.956–0.986) | 0.976 (0.959–0.994) |
| Region | ||
| West or central | REF | REF |
| East | 7.482 (3.903–14.342) | 6.871 (3.474–13.592) |
| Academic | 3.504 (2.211–5.554) | 2.602 (1.581–4.283) |
| Hypertension | 0.996 (0.983–1.010) | 0.580 (0.369–0.913) |
| Blood into ventricles | ||
| Yes | REF | REF |
| No | 2.292 (1.218–4.312) | 2.066 (1.017–4.197) |
OR, odd ratio; CI, confidence interval; REF, Reference.
The following factors were entered into Logistic regression model: age, gender, health insurance, academic hospital, registry hospital in east of China or not, prestroke mRS, Hunt and Hess grade, GCS, Fisher scale, and history of hypertension.
Discussion
Our cohort study revealed a similar proportion of spontaneous SAH out of all types of stroke between the Chinese and Caucasian patient populations 1. However, it was higher than what was previously reported in the Chinese patient population 1, 6, 7, 8. Several factors may account for this discrepancy. First, 15–25.3% of patients in the previous studies, when compared with 0.6% in the current study, were not conclusively diagnosed because of inadequate diagnostic modalities 6, 7, 15. Second, the previous studies reported that almost one‐third of the patients with SAH died on their way to the hospitals 7, 8, 10. This group of patients was not included in our study. Additionally, patients with acute aSAH should receive nimodipine recommended by treatment guidelines 2. However, in our study, only 62.9% of patients received nimodipine treatment, while most patients were given dehydrating treatment.
The mortality of patients at 28 days in our study was 15.4% (16.9% for aSAH patients) that is lower than those in the previous studies 7, 16. Furthermore, the 12‐month mortality was lower in the previous studies where only short‐term outcomes were recorded 6, 7. The mortality could be a result of improved diagnoses and treatment strategies for aSAH. Early recognition, expedited diagnosis, and adherence to treatment guidelines have improved survival. Dedicated stroke centers with standard diagnostic and treatment protocols in place will certainly play a leading role in reducing morbidity and mortality of aSAH. Despite the fact that significant improvement has been made in the past decades, only a minority (31.0%) of patients have received obliteration of aneurysm in China, which suggests that the majority of patients did not receive the standard treatment as recommended by the guidelines. Our study indicated that unsecuring aneurysm was associated with increased mortality at 12 months after an aSAH, reiterating the importance of early intervention.
Our study demonstrated that the most critical factor for obliteration of aneurysm was the condition of the hospitals. The eastern regions of China are more economically developed, and the hospitals in these regions have better operative capacity. Although the government has increased healthcare investment in the central and western regions of China over the years, the hospitals in these regions are still relatively underdeveloped. It has been suggested that if the expertise to care for these patients is not available at these local or regional hospitals, expedited transfer to more comprehensive medical centers should be considered 2. However, it has been shown that inter‐hospital transfers may have a negative impact on the outcomes 17, as patients may be particularly susceptible to complications such as rebleeding. Thus, urban hospitals in the central and western areas of China should adapt to the ability of managing aSAH including aneurysm obliteration. With the majority of Chinese people living in rural areas, healthcare resources in China need to be more properly allocated to enable local and regional hospitals to treat patients with aSAH better.
Academic hospitals (i.e., teaching hospitals), with higher concentration of medical experts and more advanced medical technology available, also have better operative capacity. They provide a great opportunity for standardized training of medical personnel and promoting treatment protocols. In addition, these hospitals can be a great starting point in China to establish the evaluation and certification system for primary and senior stroke centers, which could in turn help implement the standard of care nationwide in treating patients with SAH.
It is worth noting that, in our study, the type or grade of the hospital did not affect the mortality, unlike patient's age, history of hypertension, and the presence of intraventricular hemorrhage affecting postoperative prognosis. It might be because that the critically ill patients tend to either enter or get transferred to the academic hospitals, skewing the mortality in academic hospitals. In our study, elderly patients with aSAH have a higher mortality and lower percentage of undergoing surgery. This is in concurrence with previous findings 4, 18, 19. High blood pressure hinders obliteration surgery because elevated systolic blood pressure is a risk factor for early rebleeding 20. However, it is well known that monitoring and controlling blood pressure alone is not enough to prevent rebleeding after aSAH without early definitive treatment for patients with aneurysm 2. Furthermore, intraventricular hemorrhage that implies poor radiological grade has a negative impact on obliteration of aneurysm.
There are several limitations in our study. (1) The participating hospitals in the CNSR were selected geographically. These tertiary care hospitals are located in the inner cities. Because nearly 70% of China's population lives in the rural areas, it is likely that patients with aSAH would go to the rural hospitals first. As a result, the percentage of patients receiving surgery is expected to be low because of the lack of availability or other factors. (2) Our data were derived from patients admitted and managed in neurology departments, whereas in reality, SAH patients may be admitted to various different departments such as neurology, neurosurgery, or neuroradiology. (3) The CNSR lacks information on the locations of aneurysm, which may be an important factor affecting long‐term mortality. (4) A relatively high percentage of patients (5.1%) could not be included in the study because of lacking repeated DSA or CTA. The etiologies of the 33 patients in our study therefore were unclear. This may affect our analysis for management and prognosis of aneurysm.
Despite the limitations above, our study was, to our knowledge, the largest hospital‐based multicenter prospective study of spontaneous SAH in China. We obtained valuable information on etiology, clinical characteristics, management, and long‐term mortality following SAH. This information is critical for the evaluation and management of SAH patients in China.
Conclusion
Our study showed that the obliteration of aneurysm (clipping or coiling) decreased the long‐term mortality following aSAH. The implementation of aneurysm obliteration largely depended on the hospitals. Comprehensive medical centers tended to have a higher rate of securing aneurysm. On the basis of our data, we advocate continued training for qualified specialists (neurosurgeons and neuroradiologists) and adhering to the aSAH treatment guidelines.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Table S1. Demographics, clinical presentation, risk factors, management, and prognosis by different subarachnoid hemorrhage etiologies. Table S2. Comparison of demographics, clinical characteristics between alive and dead patients apart from Table 2. Table S3. Comparison of demographics and clinical characteristics between aneurysmal subarachnoid hemorrhage patient without surgery and with surgery apart from Table 4.
Acknowledgments
We thank all participants, participating hospitals, colleagues, nurses, and imaging and laboratory technicians. This study was funded by the Ministry of Science and Technology and the Ministry of Health of China. The Grant No. is National Science and Technology Major Project of China (2008ZX09312 ‐008) and State Key Development Program of (for) Basic Research of China (2009CB521905). The study is also supported by Beijing Public Health System Special Projects of High‐level Training for Medical Technologist (Grant No. 2009‐3‐27).
The first two authors contributed to this work.
References
- 1. Bonita R, Thomson S. Subarachnoid hemorrhage: Epidemiology, diagnosis, management, and outcome. Stroke 1985;16:591–594. [DOI] [PubMed] [Google Scholar]
- 2. Bederson JB, Connolly ES Jr, Batjer HH, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 2009;40:994–1025. [DOI] [PubMed] [Google Scholar]
- 3. de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: A systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry 2007;78:1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: A meta‐analysis. Lancet Neurol 2009;8:635–642. [DOI] [PubMed] [Google Scholar]
- 5. Sen J, Belli A, Albon H, Morgan L, Petzold A, Kitchen N. Triple‐H therapy in the management of aneurysmal subarachnoid haemorrhage. Lancet Neurol 2003;2:614–621. [DOI] [PubMed] [Google Scholar]
- 6. Ingall T, Asplund K, Mahonen M, Bonita R. A multinational comparison of subarachnoid hemorrhage epidemiology in the WHO MONICA stroke study. Stroke 2000;31:1054–1061. [DOI] [PubMed] [Google Scholar]
- 7. Zhang LF, Yang J, Hong Z, et al. Proportion of different subtypes of stroke in China. Stroke 2003;34:2091–2096. [DOI] [PubMed] [Google Scholar]
- 8. Jiang B, Wang WZ, Chen H, et al. Incidence and trends of stroke and its subtypes in China: Results from three large cities. Stroke 2006;37:63–68. [DOI] [PubMed] [Google Scholar]
- 9. Shiue I, Zhang JF, Arima H, et al. Design of the CHina Epidemiology Research in Subarachnoid Haemorrhage (CHERISH) study. Int J Stroke 2010;5:493–498. [DOI] [PubMed] [Google Scholar]
- 10. Wang X, Jiang G, Choi BC, et al. Surveillance of trend and distribution of stroke mortality by subtype, age, gender, and geographic areas in Tianjin, China, 1999–2006. Int J Stroke 2009;4:169–174. [DOI] [PubMed] [Google Scholar]
- 11. Wang Y, Cui L, Ji X, et al. The China National Stroke Registry for patients with acute cerebrovascular events: Design, rationale, and baseline patient characteristics. Int J Stroke 2011;6:355–361. [DOI] [PubMed] [Google Scholar]
- 12. Goldstein M BH, Orgogozo JM, Sartorius N, Symon L, Vereshchagin NV. Stroke . Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke 1989;20:1407–1431. [DOI] [PubMed] [Google Scholar]
- 13. Liu Y. Development of the rural health insurance system in China. Health Policy Plan 2004;19:159–165. [DOI] [PubMed] [Google Scholar]
- 14. Liu Y. Reforming China's urban health insurance system. Health Policy 2002;60:133–150. [DOI] [PubMed] [Google Scholar]
- 15. Wu Z, Yao C, Zhao D, et al. Sino‐MONICA project: A collaborative study on trends and determinants in cardiovascular diseases in China, Part i: Morbidity and mortality monitoring. Circulation 2001;103:462–468. [DOI] [PubMed] [Google Scholar]
- 16. Chen D, Roman GC, Wu GX, et al. Stroke in China (Sino‐MONICA‐Beijing study) 1984–1986. Neuroepidemiology 1992;11:15–23. [DOI] [PubMed] [Google Scholar]
- 17. van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: Diagnosis, causes and management. Brain 2001;124:249–278. [DOI] [PubMed] [Google Scholar]
- 18. Koffijberg H, Buskens E, Algra A, Wermer MJ, Rinkel GJ. Growth rates of intracranial aneurysms: Exploring constancy. J Neurosurg 2008;109:176–185. [DOI] [PubMed] [Google Scholar]
- 19. Zhang Y, Wang T, Zhang JH, Zhang J, Qin X. Subarachnoid hemorrhage in old patients in Chongqing China. Acta Neurochir Suppl 2011;110:245–248. [DOI] [PubMed] [Google Scholar]
- 20. Ohkuma H, Tsurutani H, Suzuki S. Incidence and significance of early aneurysmal rebleeding before neurosurgical or neurological management. Stroke 2001;32:1176–1180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographics, clinical presentation, risk factors, management, and prognosis by different subarachnoid hemorrhage etiologies. Table S2. Comparison of demographics, clinical characteristics between alive and dead patients apart from Table 2. Table S3. Comparison of demographics and clinical characteristics between aneurysmal subarachnoid hemorrhage patient without surgery and with surgery apart from Table 4.


