Summary
Silymarin, a C25 containing flavonoid from the plant Silybum marianum, has been the gold standard drug to treat liver disorders associated with alcohol consumption, acute and chronic viral hepatitis, and toxin‐induced hepatic failures since its discovery in 1960. Apart from the hepatoprotective nature, which is mainly due to its antioxidant and tissue regenerative properties, Silymarin has recently been reported to be a putative neuroprotective agent against many neurologic diseases including Alzheimer's and Parkinson's diseases, and cerebral ischemia. Although the underlying neuroprotective mechanism of Silymarin is believed to be due to its capacity to inhibit oxidative stress in the brain, it also confers additional advantages by influencing pathways such as β‐amyloid aggregation, inflammatory mechanisms, cellular apoptotic machinery, and estrogenic receptor mediation. In this review, we have elucidated the possible neuroprotective effects of Silymarin and the underlying molecular events, and suggested future courses of action for its acceptance as a CNS drug for the treatment of neurodegenerative diseases.
Keywords: Amyloid‐β aggregation, Antioxidant action, Astrogliosis, Estrogen receptor‐β binding, Neuroinflammation, Oxidative and nitrosative stresses, Silymarin
Introduction
Silymarin, a plant‐derived flavonoid from the plant Silybum marianum 1, 2, is considered the most potential drug to treat almost all kind of liver diseases 3, 4, 5, 6, particularly alcoholic liver disease 7, 8, acute and chronic viral hepatitis 9, 10, 11, and toxins‐mediated liver dysfunctions 12, 13. Silymarin is basically a mixture of lignan‐derived flavonols, containing mainly silybin followed by silydianin, silychristin, and isosilybin 14, 15, 16, 17, 18. It was first isolated as a mixture from the seed extract of Silybum marianum in 1968 19 and all the constituents were purified 20, and their structures elucidated using techniques like X‐ray crystallography and NMR 21. Since then myriads of research were undertaken to understand the mechanisms of action of different constituents or its mixtures in cellular and animal models, as well as in human subjects. Several studies have reported that oral absorption of Silymarin is about 23–47%, and the peak plasma concentration is achieved in 4–6 h 6, 22 while its serum half‐life is approximately 6 h 23, 24. However, the bioavailability of Silymarin in brain is not known yet in spite of the fact that it is shown to be protective against several CNS disorders.
Silymarin is reported to have a good safety profile with no adverse side effects in either humans or animals in high doses 23. The potential benefit of Silymarin in the treatment of liver disease is associated with its antioxidant property 25 and its ability to block hepatotoxicant binding sites, along with tissue regenerative capabilities 26. Besides hepatoprotection, Silymarin has recently been reported to be a putative neuroprotective agent against several neurodegenerative diseases including Alzheimer's disease (AD) 27, Parkinson's disease (PD) 28, and cerebral ischemia (CI) 29. Although, the underlying neuroprotective mechanism of Silymarin is mainly due to its capacity to inhibit oxidative stress in brain 30, it also confers additional neuroprotection by influencing other pathways such as inflammatory pathways 31, 32, inhibition of β‐amyloid (Aβ) aggregation 33, apoptotic mechanisms of cell death 29, and estrogenic receptor‐mediated pathways of neuronal death 34. Additionally, Silymarin also exhibits the potential to recover psychomotor and cognitive abnormalities 35 in animal models. In this review, we have explained the possible pathways of neuroprotective effect of Silymarin and the underlying cellular and molecular events.
Silymarin in Neurodegenerative Disorders
Neuroprotective evidences in support of Silymarin have been documented not only in animal models of neurodegenerative diseases 28, 34, 36, 37, but also in neuronal and non‐neuronal cellular models 33, 38 of AD, CI, and PD. The in vitro and in vivo doses, and the routes of application, the preparations used, and time window of treatment of Silymarin, which are very important for its potential clinical purpose are mentioned in Table 1. Surprisingly, reports on the effect of Silymarin on other central nervous system disorders where oxidative stress plays a pivotal role, such as Huntington's disease, amyotrophic lateral sclerosis, and multiple sclerosis 39, 40 are lacking.
Table 1.
The administration routes, doses, preparation, and time window of treatment of Silymarin
| CNS disorders | Model system | Administration routes | Effective doses | Preparation | Time window of treatment | References |
|---|---|---|---|---|---|---|
| Alzheimer's disease | Mouse | Oral | 200 mg/kg | Suspended in 0.3% carboxymethyl cellulose (CMC) | 8 days | 27, 36 |
| Mouse | Oral | 1% | Silymarin in normal diet | 6 months | 38 | |
| PC12 cells | Media | 100 μm | In dimethyl sulfoxide (DMSO) | 24/96 h | 38 | |
| SH‐SY5Y cells | Media | 50 μm | In DMSO | 3 days | 33 | |
| Parkinson's disease | Mouse | Intraperitoneal | 40 mg/kg | In DMSO | 9 weeks | 28 |
| Neuron‐glia culture | Media | 80 μm | In DMSO | 7/25/49 h | 31 | |
| Rat | Intraperitoneal | 200 mg/kg | In propylene glycol (PEG) | 2 weeks | 34 | |
| Cerebral ischemia | Rat | Oral | 200 mg/kg | Suspended in a 0.3% CMC | 15 days | 29 |
| Rat | Intragastric | 50/100 mg/kg | Silibinin dissolved in 0.9% NaCl | 24/72 h | 32 | |
| Rat | Intravenous | 1–10 μg/kg | Ethanol and normal saline | 24 h | 37 | |
| Rat | Oral | 200 mg/kg | In 1% (w/v) CMC | 7 days | 61 | |
| Ageing | Rat | Oral | 200/400 mg/kg | Suspended in corn oil | 14 days | 30 |
| Mouse | Intramuscular | 50 mg/kg | In PEG | 6 weeks | 86 | |
| Cognitive impairment | Mouse | Oral | 100/200 mg/kg | Suspended in 0.3% CMC solution | 7 days | 35 |
Silymarin and Alzheimer's disease
The cognitive impairment and the deposition of extracellular amyloid‐β (Aβ) fibrils in senile plaques, which are the characteristic features of AD brain 41, 42, 43, have been reported to be attenuated by administration of Silymarin 27, 33, 38. In an Aβ‐induced animal model of AD, the cognitive abnormalities, particularly memory impairment was significantly improved after Silymarin administration 27, 36, which is suggested to be due to reduction in oxidative stress and inflammatory responses 27, 44. Additionally, in amyloid precursor protein (APP)‐based transgenic animal model of AD, chronic Silymarin supplementation was reported to recover the characteristic behavioral abnormalities, without causing toxicity to any organs 38. Reports are also available on the protective effect of Silymarin on inhibition of Aβ fibril formation and aggregation in animal and cellular models of AD [33, 38; Figures 1 and 3], which is discussed in later section of the review.
Figure 1.
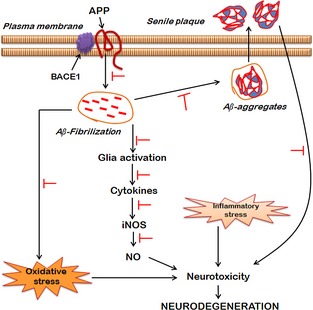
Neuroprotective pathways of Silymarin in Alzheimer's disease (AD). In AD patients' brains, proteolytic cleavage of amyloid precursor proteins (APP) by β‐secretase (BACE1) results in the formation of toxic amyloid‐beta (Aβ) fragments, fragments of which undergo fibrilization as well as oligomerization resulting in the deposition of intracellular Aβ aggregates, and as senile plaques in the extracellular space. Both Aβ fibrils as well as senile plaques mediate neurotoxicity either by enhancing oxidative stress or by exaggerating glial cell‐mediated production of inflammatory markers such as cytokines. The cytokines in turn induce the production of nitric oxide (NO) by activating inducible nitric oxide synthase (iNOS). Silymarin potentially hinders the cleavage of APP and thus impede the fibrilization and oligomerization of Aβ, and reduces Aβ aggregates and senile plaques. The Aβ‐induced oxidative stress and glial cell activation due to overproduction of inflammatory markers has been shown to be inhibited by treatment of Silymarin. Thus, Silymarin, by virtue of its antioxidant and anti‐inflammatory properties, confers neuroprotection in AD pathology (T indicates the inhibitory effect of Silymarin).
Silymarin and Parkinson's Disease
The characteristic features of PD, particularly the loss of dopaminergic neurons in substantia nigra pars compacta and the motor behavioral abnormalities 45, 46, 47, 48, 49, 50, 51 generated by intrastriatal administration of parkinsonian neurotoxin, 6‐hydroxydopamine (6‐OHDA) was considerably attenuated by treatment with Silymarin 34. In maneb‐ and paraquat‐induced animal models of PD, Silymarin was also found to be protective against midbrain dopaminergic neuronal loss and associated behavioral impairments 28. In different toxin‐induced animal models of PD 28, 35, and even in naive animals 52, Silymarin administration showed substantial increase in dopamine and serotonin levels in hippocampus and cortical regions of brain. Interestingly, Silymarin is reported to inhibit monoamine oxidase‐B 53, suggesting additional neuroprotective mechanism of Silymarin to counter the loss of dopamine in PD [54, 55; Figure 3]. However, reports on the effect of Silymarin against parkinsonian hallmark pathology, α‐synuclin aggregation and Lewy body formation 56, 57 are not available. Nevertheless, the molecular mechanism of neuroprotective potential of Silymarin in PD has been mainly attributed to amelioration of oxidative stress 28, 34.
Silymarin and Cerebral Ischemia
The neuroprotective effect of Silymarin on CI‐induced neurochemical alterations including elevated levels of free radicals, nitrite content and inflammatory mediators 58, 59, 60, and behavioral abnormalities have been convincingly established in the literature 29, 61. Silymarin showed considerable reduction in cerebral infarct volume and neuronal cell loss in CI 61. In comparison with commonly used anti‐ischemic drugs such as piracetam and protocatechuic acid, Silymarin significantly improved the brain histochemical changes and psychomotor behavior in animal model of CI 61. Additionally, Silymarin is found to be anti‐apoptotic in CI by means of downregulating apoptosis inducing molecules such as p53, apoptotic protease‐activating factor 1 (apaf‐1), and caspase‐9 in an animal model [29; Figure 3]. Silybinin, which is one of the active constituents of Silymarin, has recently been reported to activate Akt/mTOR signaling pathway, and to downregulate the inflammatory marker, NF‐κB and to upregulate the anti‐apoptotic marker, Bcl‐2 in CI brain 32, thereby suggesting a novel mode of neuroprotection. The underlying molecular mechanism of Silymarin in CI‐induced neurotoxicity is mainly due to downregulation of inflammatory mediators such as inducible nitric oxide synthase (iNOS), myeloperoxidase, cyclooxygenases, NF‐κB and tumor necrosis factor‐beta (TNF‐β) 37, and upregulation of antioxidant enzymes [29; Figure 2].
Figure 2.
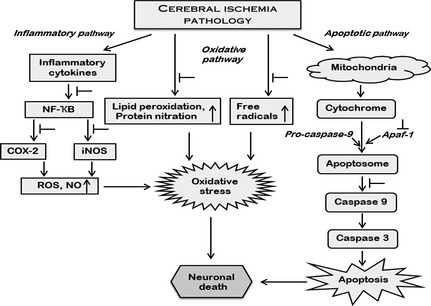
Neuroprotective pathways of Silymarin in cerebral ischemia (CI). The major pathological pathways that are involved in CI include inflammation, oxidative stress, and apoptosis. Silymarin elicits anti‐inflammatory property in CI injury by preventing the activation of NF‐κB‐mediated production of cyclooxygenase‐2 (COX‐2) and inducible nitric oxide synthase (iNOS). Silymarin thus by modulating NF‐κB reduces both oxidative stress as well as nitrosative stress by inhibiting the generation of reactive oxygen species (ROS) and nitric oxide (NO). In addition, Silymarin also suppresses the generation of free radical‐mediated lipid and protein oxidation, having the potential to change the redox state of the cell and hence ameliorate oxidative stress. The activation of intrinsic pathway of apoptosis, which is evident in CI brain, is inhibited by Silymarin, by preventing the formation of apoptosome by inhibiting apoptotic protease‐activating factor 1 (apaf‐1) that regulates the activation of caspases. Thus, Silymarin has the potential to prevent neuronal loss by inhibiting the oxidative stress and apoptotic mode of cell death (T indicates the inhibitory effect of Silymarin).
Molecular Mechanisms of Neuroprotection by Silymarin
Oxidative Stress and Silymarin
Silymarin has been implicated in protecting neurons against oxidative stress 27, 34, 44 and nitrosative stress [36; Figure 3]. Silymarin, being a mixture of flavonoids, is reported to exert direct effect on neuronal oxidant status 1, 30, 44. Silymarin offsets acetaminophen and manganese‐mediated oxidative stress and neurotoxicity in animal models by elevating the activities of both enzymatic and nonenzymatic antioxidant markers 44, 62. Silymarin elicits its neuroprotective effects in manganese‐induced neurotoxicity by reducing both lipid and protein oxidation, as well as by activating acetylcholinesterase activity, and inducible nitric oxide synthase gene expression 63. In animal model of sepsis induced by cecal ligation and perforation, decreased glutathione levels and increase in malondialdehyde content, as well as myeloperoxidase activity in the brain, were reverted by administration of Silymarin 64. In the hippocampi and the cortices of elderly rodent brain, Silymarin is reported to be neuroprotective against oxidative insults by potentially inhibiting formation of oxygen and peroxyl radicals along with protein oxidation products 30. Silymarin administration in an encephalopathy animal model produced by 4‐pentenoic acid, elevated the respiratory activity in brain mitochondria and inhibited lipid peroxidation 65.
Figure 3.
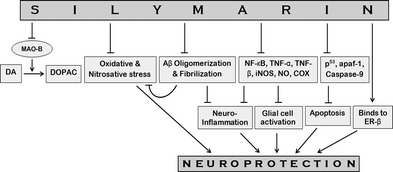
Schematic representation of neuroprotective pathways of Silymarin. The potent herbal antioxidant Silymarin prevents conversion of dopamine (DA) to 3,4‐dihydroxyphenylacetic acid (DOPAC) by inhibiting the DA oxidizing enzyme, monoamine oxidase‐B (MAO‐B). The MAO‐B inhibitory action of Silymarin thus leads to lesser degradation of DA and therefore would increase the extracellular concentration of this catecholamine neurotransmitter. Silymarin prevents the formation of amyloid‐β (Aβ) aggregates and fibrils; as a consequence, it attenuates Aβ‐induced neuroinflammation and cellular stresses. By inhibiting the production of inflammatory agents such as NF‐κB, TNF‐α, TNF‐β, iNOS, NO, COX, Silymarin impedes neuroinflammation and glial activation resulting in increased secretions of trophic factors, leading to neuroprotection. Silymarin also showed anti‐apoptotic property as revealed by inhibition of the production of apoptotic proteins, p53 and apaf‐1, and reduced caspase‐9 activity. Due to estrogen‐like activity and binding ability to ER‐β, Silymarin may provide additional neuroprotection (T indicates the inhibitory effect of Silymarin). TNF‐α, tumor necrosis factors‐α; TNF‐β, tumor necrosis factors‐β; iNOS, inducible nitric oxide synthase; NO, nitric oxide; COX, cyclooxygenase; apaf‐1, apoptotic protease‐activating factor 1; ER‐β, estrogen receptor‐β.
Several studies have established the involvement of oxidative stress in Aβ‐induced neurotoxicity [66, 67; Figure 3]. Silymarin was found to alleviate the cognitive impairment induced by Aβ by preventing the oxidative damage in the hippocampus in terms of lipid peroxidation and glutathione levels 27. The level of nitrotyrosine has been used as a marker of nitrosative stress 68, 69, and Silymarin significantly attenuated the elevation of nitrotyrosine induced by Aβ in the hippocampus and amygdala 36.
β‐Amyloid and Silymarin
The potential role of Silymarin against Aβ pathology has been well reported in both in vitro and in vivo systems. In transgenic mouse model of AD, oligomerization of Aβ induced by over‐expression of APP was potentially inhibited by Silymarin 38. Additionally, administration of Silymarin in animals is also reported to clear the fibrillar Aβ deposits 38. In the in vitro system, Aβ fibrilization and aggregation were reduced significantly after incubation of Aβ peptides with Silymarin [33, 38; Figure 3]. It is also shown that Silymarin has the potential to revert Aβ‐induced oxidative stress 27, 33 and cell viability 38. The attenuation of Aβ toxicity by Silymarin has been reported to be due to its antioxidative property (Figure 1), but without effecting β‐secretase (BACE) 38, which is known to be involved in production of toxic Aβ 70, 71. Aβ‐induced over‐expression of inflammatory mediators such as tumor necrosis factor‐α (TNF‐α) and iNOS mRNA in the hippocampus and amygdala of mouse brain was attenuated by administration of Silymarin 36. Silymarin by reducing nitrotyrosine level in the hippocampus and amygdala also attenuates Aβ‐induced nitrosative stress in animals 36.
Glia and Silymarin
Few reports are also available on the inhibition and prevention of proliferation of glia by Silymarin 31, 37, 72. Wang et al. 31 reported that Silymarin administration in a lipopolysaccharide‐induced animal model of PD, prevented the dopaminergic neurodegeneration by inhibiting activation of microglia, while other studies reported the inhibition of glial cell activation by Silymarin in cellular models possibly by inhibiting iNOS production 37, 72. Meanwhile, Silybinin has been reported to downregulate the CI‐induced inflammation by activating of Akt/mTOR pathway via upregulation of anti‐inflammatory markers 32. Silymarin is also reported to protect both microglia and astroglia from oxidative insults induced by peroxide in ex vivo system 72. However, Silymarin‐mediated inhibition of gliosis is suggested to be due to inhibition of NF‐κB activation as well as other inflammatory mediators [31, 72; Figure 3], but the exact molecular mechanism is yet unclear.
Involvement of Estrogen Receptor
Estrogen receptor‐β (ER‐β) is distributed predominantly in hippocampus and cortical regions of rodents brain 73, 74, and is known to possess neuroprotective potential when it is activated or upregulated 34, 75, 76. Apart from the involvement of ER‐β in cognitive processes such as learning and memory 77, 78, 79, 80, blockade of ER‐β by antagonists has been reported to cause neurotoxicity leading to many diseases, including PD 34, epidemiologically supported by the fact that incidence of this disease in females is significantly low worldwide 81. Estrogen‐mediated neuroprotective effect has been reported to be due to its ability to bind to ER‐β 82. Silymarin administration has been reported to reduce 6‐OHDA‐induced rotational behavior and nigral neuronal loss in parkinsonian rodents partly by modulating ER‐β 34. One of the underlying mechanisms of Silymarin‐induced neuroprotective effect may be due its estrogen‐like activity 75, 83, as well as its potential to bind and activate the ER‐β [34, 75, 84, 85; Figure 3].
Silymarin: Unknown Terrains
Although Silymarin has shown promising neuroprotective potential, still there are some lacunae in understanding the science of this mixture of flavonoids. The reported inhibitory potential of Silymarin on protein deposits formation in AD is not clearly understood yet. It will be exciting to know how Silymarin modulate Aβ fibrilization without effecting BACE that cleaves APP in AD. Similarly, it may be expected that Silymarin might have the potential to inhibit α‐synuclin aggregation and resultant Lewy body formation in PD. Therefore, extensive research needs to be initiated to understand the mechanisms of macromolecular crowding in neurons and the effects of Silymarin. Another avenue that needs to be looked into is how Silymarin confer neuroprotection by interacting with ER‐β receptor. Although Silymarin is a flavonoid and generally flavonoids can traverse the blood–brain barrier, yet no confirmed reports are available on the mode of transport and bioavailability of Silymarin in brain. Hence, there is a greater need to search into these avenues to understand the true picture of Silymarin‐mediated neuroprotection.
Conclusions
The present article concisely reviews the antioxidant, anti‐apoptotic, anti‐inflammatory and enzyme inhibitory activities of Silymarin and shows how use of this molecule could provide protection of neurons against oxidative insults in the brain under distress. The neuroprotective nature of Silymarin seems to be unique as its mode of action is diverse ranging from a general antioxidant nature to specific anti‐amyloidogenic, anti‐inflammatory, and pro‐estrogenic properties. These diverse neuroprotective actions of Silymarin on brain hold great promise to be a “wonder drug” for the treatment of neurodegenerative disorders. The nontoxic nature of this molecule warrants its urgent clinical evaluation for its potential use as an antineurodegenerative molecule in humans. However, its bioavailability in brain, including its ability to penetrate blood–brain barrier is to be established in preclinical studies, prior to any clinical trials.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
R.P. and A.C. are currently Junior Research Fellows in Department of Biotechnology (DBT), Govt. of India, funded research Project (Sanction Order No. BT/230/NE/TBP/2011 dated April 23, 2012). We acknowledge the funding and support provided by DBT and Council for Scientific and Industrial Research (CSIR) organizations under the Govt. of India.
References
- 1. Youdim KA, Shukitt‐Hale B, Joseph JA. Flavonoids and the brain: Interactions at the blood‐brain barrier and their physiological effects on the central nervous system. Free Radic Biol Med 2004;37:1683–1693. [DOI] [PubMed] [Google Scholar]
- 2. Pepping J. Milk thistle: Silybum marianum . Am J Health Syst Pharm 1999;56:1195–1197. [DOI] [PubMed] [Google Scholar]
- 3. Flora K, Hahn M, Rosen H, Benner K. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol 1998;93:139–143. [DOI] [PubMed] [Google Scholar]
- 4. Luper S. A review of plants used in the treatment of liver diseases: Part 1. Altern Med Rev 1998;3:410–421. [PubMed] [Google Scholar]
- 5. Thakur SK. Silymarin – A hepatoprotective agent. Gastroenterol Today 2002;6:78–82. [Google Scholar]
- 6. Pradhan SC, Girish C. Hepatoprotective herbal drug, Silymarin from experimental pharmacology to clinical medicine. Indian J Med Res 2006;124:491–504. [PubMed] [Google Scholar]
- 7. Das SK, Vasudevan DM. Protective effects of Silymarin, a milk thistle (Silybium marianum) derivative on ethanol‐induced oxidative stress in liver. Indian J Biochem Biophys 2006;43:306–311. [PubMed] [Google Scholar]
- 8. Rambaldi A, Jacobs BP, Iaquinto G, Gluud C. Milk thistle for alcoholic and/or hepatitis B or C liver diseases – a systematic cochrane hepato‐biliary group review with meta‐analyses of randomized clinical trials. Am J Gastroenterol 2005;100:2583–2589. [DOI] [PubMed] [Google Scholar]
- 9. Fraschini F, Dermartini G, Esposti D. Pharmacology of Silymarin. Clin Drug Invest 2002;22:51–65. [Google Scholar]
- 10. Mayer KE, Myers RP, Lee SS. Silymarin treatment of viral hepatitis: A systematic review. J Viral Hepat 2005;12:559–567. [DOI] [PubMed] [Google Scholar]
- 11. Ghaffari AR, Noshad H, Ostadi A, Ghojazadeh M, Asadi P. The effects of milk thistle on hepatic fibrosis due to methotrexate in rat. Hepat Mon 2011;11:464–468. [PMC free article] [PubMed] [Google Scholar]
- 12. Lettéron P, Labbe G, Degott C, et al. Mechanism for the protective effects of silymarin against carbon tetrachloride‐induced lipid peroxidation and hepatotoxicity in mice: Evidence that silymarin acts both as an inhibitor of metabolic activation and as a chain‐breaking antioxidant. Biochem Pharmacol 1990;39:2027–2034. [DOI] [PubMed] [Google Scholar]
- 13. Abenavoli L, Capasso R, Milic N, Capasso F. Milk thistle in liver diseases: Past, present, future. Phytother Res 2010;24:1423–1432. [DOI] [PubMed] [Google Scholar]
- 14. Wagner H, Diesel P, Seitz M. The chemistry and analysis of silymarin from Silybum marianum Gaertn. Arzneimittelforschung 1974;24:466–471. [PubMed] [Google Scholar]
- 15. Rainone F. Milk thistle. Am Fam Physician 2005;72:1285–1288. [PubMed] [Google Scholar]
- 16. Kroll DJ, Shaw HS, Oberlies NH. Milk thistle nomenclature: Why it matters in cancer research and pharmacokinetic studies. Integr Cancer Ther 2007;6:110–119. [DOI] [PubMed] [Google Scholar]
- 17. Gazak R, Walterova D, Kren V. Silybin and silymarin – new and emerging applications in medicine. Curr Med Chem 2007;14:315–338. [DOI] [PubMed] [Google Scholar]
- 18. Ghosh A, Ghosh T, Jain S. Silymarin – a review on the pharmacodynamics and bioavailability enhancement approaches. J Pharm Sci Technol 2010;2:348–355. [Google Scholar]
- 19. Wagner H, Horhammer L, Munster R. On the chemistry of Silymarin (silybin) the active principle of the fruits from Silybum marianum (L.) Gaertn. (Carduus marianus L.). Arzneimittelforschung 1968;18:688–696. [PubMed] [Google Scholar]
- 20. Kim NC, Graf TN, Sparacino CM, Wani MC, Wall ME. Complete isolation and characterization of silybins and isosilybins from milk thistle (Silybum marianum). Org Biomol Chem 2003;1:1684–1689. [DOI] [PubMed] [Google Scholar]
- 21. Lee DY, Liu Y. Molecular structure and stereochemistry of silybin A, silybin B, isosilybin A, and isosilybin B, isolated from Silybum marianum (milk thistle). J Nat Prod 2003;66:1171–1114. [DOI] [PubMed] [Google Scholar]
- 22. Dixit N, Baboota S, Kohli K, Ahmed S, Ali J. Silymarin: A review of pharmacological aspects and bioavailability enhancement approach. Indian J Pharmacol 2007;39:172–179. [Google Scholar]
- 23. Saller R, Meier R, Brignoli R. The use of Silymarin in the treatment of liver diseases. Drugs 2001;61:2035–2063. [DOI] [PubMed] [Google Scholar]
- 24. Javed S, Kohli K, Ali M. Reassessing bioavailability of Silymarin. Altern Med Rev 2011;16:239–249. [PubMed] [Google Scholar]
- 25. Song Z, Deaciuc I, Song M, et al. Silymarin protects against acute ethanol‐induced hepatotoxicity in mice. Alcohol Clin Exp Res 2006;30:407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Srivastava S, Srivastava AK, Srivastava S, Patnaik GK, Dhawan BN. Effect of picroliv and Silymarin on liver regeneration in rats. Indian J Pharmacol 1994;26:19–22. [Google Scholar]
- 27. Lu P, Mamiya T, Lu LL, et al. Silibinin prevents amyloid beta peptide‐induced memory impairment and oxidative stress in mice. Br J Pharmacol 2009;157:1270–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singhal NK, Srivastava G, Patel DK, Jain SK, Singh MP. Melatonin or Silymarin reduces maneb‐ and paraquat‐induced Parkinson's disease phenotype in the mouse. J Pineal Res 2011;50:97–109. [DOI] [PubMed] [Google Scholar]
- 29. Raza SS, Khan MM, Ashafaq M, et al. Silymarin protects neurons from oxidative stress associated damages in focal cerebral ischemia: A behavioral, biochemical and immunohistological study in Wistar rats. J Neurol Sci 2011;309:45–54. [DOI] [PubMed] [Google Scholar]
- 30. Galhardi F, Mesquita K, Monserrat JM, Barros DM. Effect of Silymarin on biochemical parameters of oxidative stress in aged and young rat brain. Food Chem Toxicol 2009;47:2655–2660. [DOI] [PubMed] [Google Scholar]
- 31. Wang MJ, Lin WW, Chen HL, et al. Silymarin protects dopaminergic neurons against lipopolysaccharide‐induced neurotoxicity by inhibiting microglia activation. Eur J Neurosci 2002;16:2103–2112. [DOI] [PubMed] [Google Scholar]
- 32. Wang C, Wang Z, Zhang X, et al. Protection by silibinin against experimental ischemic stroke: Up‐regulated pAkt, pmTOR, HIF‐1α and Bcl‐2, down‐regulated Bax, NF‐κB expression. Neurosci Lett 2012;529:45–50. [DOI] [PubMed] [Google Scholar]
- 33. Yin F, Liu J, Ji X, Wang Y, Zidichouski J, Zhang J. Silibinin: A novel inhibitor of Aβ aggregation. Neurochem Int 2011;58:399–403. [DOI] [PubMed] [Google Scholar]
- 34. Baluchnejadmojarad T, Roghani M, Mafakheri M. Neuroprotective effect of Silymarin in 6‐hydroxydopamine hemi‐parkinsonian rat: Involvement of estrogen receptors and oxidative stress. Neurosci Lett 2010;480:206–210. [DOI] [PubMed] [Google Scholar]
- 35. Lu P, Mamiya T, Lu L, et al. Silibinin attenuates cognitive deficits and decreases of dopamine and serotonin induced by repeated methamphetamine treatment. Behav Brain Res 2010;207:387–393. [DOI] [PubMed] [Google Scholar]
- 36. Lu P, Mamiya T, Lu LL, et al. Silibinin attenuates amyloid beta (25–35) peptide‐induced memory impairments: Implication of inducible nitric‐oxide synthase and tumor necrosis factor‐alpha in mice. J Pharmacol Exp Ther 2009;33:319–326. [DOI] [PubMed] [Google Scholar]
- 37. Hou YC, Liou KT, Chern CM, et al. Preventive effect of Silymarin in cerebral ischemia‐reperfusion‐induced brain injury in rats possibly through impairing NF‐κB and STAT‐1 activation. Phytomedicine 2010;17:963–973. [DOI] [PubMed] [Google Scholar]
- 38. Murata N, Murakami K, Ozawa Y, et al. Silymarin attenuated the amyloid‐β plaque burden and improved behavioral abnormalities in an Alzheimer's disease mouse model. Biosci Biotechnol Biochem 2010;74:2299–2306. [DOI] [PubMed] [Google Scholar]
- 39. Gilgun‐Sherki Y, Melamed E, Offen D. Oxidative stress induced‐neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001;40:959–975. [DOI] [PubMed] [Google Scholar]
- 40. Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 2009;7:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mayeux R. Epidemiology of neurodegeneration. Annu Rev Neurosci 2003;26:81–104. [DOI] [PubMed] [Google Scholar]
- 42. Jucker M, Walker LC. Pathogenic protein seeding in Alzheimer's disease and other neurodegenerative disorders. Ann Neurol 2011;70:532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schnabel J. Amyloid: Little proteins, big clues. Nature 2011;475:S12–S14. [DOI] [PubMed] [Google Scholar]
- 44. Nencini C, Giorgi G, Micheli L. Protective effect of Silymarin on oxidative stress in rat brain. Phytomedicine 2007;14:129–135. [DOI] [PubMed] [Google Scholar]
- 45. Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci 2005;28:57–87. [DOI] [PubMed] [Google Scholar]
- 46. Borah A, Mohanakumar KP. Long‐term L‐DOPA treatment causes indiscriminate increase in dopamine levels at the cost of serotonin synthesis in discrete brain regions of rats. Cell Mol Neurobiol 2007;27:985–996. [DOI] [PubMed] [Google Scholar]
- 47. Borah A, Mohanakumar KP. Melatonin inhibits 6‐hydroxydopamine production in the brain to protect against experimental parkinsonism in rodents. J Pineal Res 2009;47:293–300. [DOI] [PubMed] [Google Scholar]
- 48. Borah A, Mohanakumar KP. Long term L‐DOPA treatment causes production of 6‐OHDA in the mouse striatum: Involvement of hydroxyl radical. Ann Neurosci 2009;16:160–165. [Google Scholar]
- 49. Borah A, Mohanakumar KP. L‐DOPA‐induced 6‐hydroxydopamine production in the striata of rodents is sensitive to the degree of denervation. Neurochem Int 2010;56:352–362. [DOI] [PubMed] [Google Scholar]
- 50. Borah A, Mohanakumar KP. Salicylic acid protects against chronic L‐DOPA‐induced 6‐OHDA generation in experimental model of parkinsonism. Brain Res 2010;16:192–199. [DOI] [PubMed] [Google Scholar]
- 51. Borah A, Mohanakumar KP. L‐DOPA induced‐endogenous 6‐hydroxydopamine is the cause of aggravated dopaminergic neurodegeneration in Parkinson's disease patients. Med Hypotheses 2012;79:271–273. [DOI] [PubMed] [Google Scholar]
- 52. Osuchowski MF, Johnson VJ, He Q, Sharma RP. Alterations in regional brain neurotransmitters by silymarin, a natural antioxidant flavonoid mixture, in BALB/c mice. Pharmac Biol 2004;42:384–389. [Google Scholar]
- 53. Mazzio EA, Harris N, Soliman KF. Food constituents attenuate monoamine oxidase activity and peroxide levels in C6 astrocyte cells. Planta Med 1998;64:603–606. [DOI] [PubMed] [Google Scholar]
- 54. Robottom BJ. Efficacy, safety, and patient preference of monoamine oxidase B inhibitors in the treatment of Parkinson's disease. Patient Prefer Adherence 2011;5:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Riederer P, Laux G. MAO‐inhibitors in Parkinson's disease. Exp Neurol 2011;20:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee VM‐Y, Trojanowski JQ. Mechanisms of Parkinson's disease linked to pathological α‐synuclein: New targets for drug discovery. Neuron 2006;52:33–38. [DOI] [PubMed] [Google Scholar]
- 57. Phani S, Loike JD, Przedborski S. Neurodegeneration and inflammation in Parkinson's disease. Parkinsonism Relat Disord 2012;18:S207–S209. [DOI] [PubMed] [Google Scholar]
- 58. Bemeur C, Ste‐Marie L, Montgomery J. Increased oxidative stress during hyperglycemic cerebral ischemia. Neurochem Int 2007;50:890–904. [DOI] [PubMed] [Google Scholar]
- 59. Rodrigo J, Fernández AP, Serrano J, Peinado MA, Martínez A. The role of free radicals in cerebral hypoxia and ischemia. Free Radic Biol Med 2005;39:26–50. [DOI] [PubMed] [Google Scholar]
- 60. Ozkul A, Akyol A, Yenisey C, Arpaci E, Kiylioglu N, Tataroglu C. Oxidative stress in acute ischemic stroke. J Clin Neurosci 2007;14:1062–1066. [DOI] [PubMed] [Google Scholar]
- 61. Muley MM, Thakare VN, Patil RR, Kshirsagar AD, Naik SR. Silymarin improves the behavioural, biochemical and histoarchitecture alterations in focal ischemic rats: A comparative evaluation with piracetam and protocatachuic acid. Pharmacol Biochem Behav 2012;102:286–293. [DOI] [PubMed] [Google Scholar]
- 62. Chtourou Y, Fetoui H, Garoui el M, Boudawara T, Zeghal N. Improvement of cerebellum redox states and cholinergic functions contribute to the beneficial effects of Silymarin against manganese‐induced neurotoxicity. Neurochem Res 2012;37:469–479. [DOI] [PubMed] [Google Scholar]
- 63. Chtourou Y, Fetoui H, Sefi M, et al. Silymarin, a natural antioxidant, protects cerebral cortex against manganese‐induced neurotoxicity in adult rats. Biometals 2010;23:985–996. [DOI] [PubMed] [Google Scholar]
- 64. Toklu HZ, Tunali Akbay T, Velioglu‐Ogunc A, et al. Silymarin, the antioxidant component of Silybum marianum, prevents sepsis‐induced acute lung and brain injury. J Surg Res 2008;145:214–222. [DOI] [PubMed] [Google Scholar]
- 65. Vengerovskii AI, Khazanov VA, Eskina KA, Vasilyev KY. Effects of Silymarin (hepatoprotector) and succinic acid (bioenergy regulator) on metabolic disorders in experimental diabetes mellitus. Bull Exp Biol Med 2007;144:53–56. [DOI] [PubMed] [Google Scholar]
- 66. Schubert D, Behl C, Lesley R, et al. Amyloid peptides are toxic via a common oxidative mechanism. Proc Natl Acad Sci U S A 1995;92:1989–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pappolla MA, Chyan YJ, Omar RA, et al. Evidence of oxidative stress and in vivo neurotoxicity of beta‐amyloid in a transgenic mouse model of Alzheimer's disease: A chronic oxidative paradigm for testing antioxidant therapies in vivo . Am J Pathol 1998;152:871–877. [PMC free article] [PubMed] [Google Scholar]
- 68. Ishii K, Muelhauser F, Liebl U, et al. Sub‐acute NO generation induced by Alzheimer's beta‐amyloid in the living brain: Reversal by inhibition of the inducible NO synthase. FASEB J 2000;14:1485–1489. [DOI] [PubMed] [Google Scholar]
- 69. Tran MH, Yamada K, Nakajima A, et al. Tyrosine nitration of a synaptic protein synaptophysin contributes to amyloid beta‐peptide‐induced cholinergic dysfunction. Mol Psychiatry 2003;8:407–412. [DOI] [PubMed] [Google Scholar]
- 70. Vassar R, Bennett BD, Babu‐Khan S, et al. Beta‐secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science 1999;286:735–741. [DOI] [PubMed] [Google Scholar]
- 71. Hong L, Koelsch G, Lin X, et al. Structure of the protease domain of memapsin 2 (beta‐secretase) complexed with inhibitor. Science 2000;290:150–153. [DOI] [PubMed] [Google Scholar]
- 72. Tsai MJ, Liao JF, Lin DY, et al. Silymarin protects spinal cord and cortical cells against oxidative stress and lipopolysaccharide stimulation. Neurochem Int 2010;57:867–875. [DOI] [PubMed] [Google Scholar]
- 73. Posner MI, Petersen SE, Fox PT, Raichle ME. Localization of cognitive operations in the human brain. Science 1988;240:1627–1631. [DOI] [PubMed] [Google Scholar]
- 74. Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor‐alpha and ‐beta mRNA in the rat central nervous system. J Comp Neurol 1997;388:507–525. [DOI] [PubMed] [Google Scholar]
- 75. Seidlova‐Wuttke D, Becker T, Christoffel V, Jarry H, Wuttke W. Silymarin is a selective estrogen receptor beta (ERbeta) agonist and has estrogenic effects in the metaphysis of the femur but no or antiestrogenic effects in the uterus of ovariectomized (ovx) rats. J Steroid Biochem Mol Biol 2003;86:179–188. [DOI] [PubMed] [Google Scholar]
- 76. El‐Shitany NA, Hegazy S, El‐desoky K. Evidences for antiosteoporotic and selective estrogen receptor modulator activity of Silymarin compared with ethinylestradiol in ovariectomized rats. Phytomedicine 2010;17:116–125. [DOI] [PubMed] [Google Scholar]
- 77. Rissman EF, Heck AL, Leonard JE, Shupnik MA, Gustafsson JA. Disruption of estrogen receptor beta gene impairs spatial learning in female mice. Proc Natl Acad Sci U S A 2002;99:3996–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Swedenborg E, Power KA, Cai W, Pongratz I, Ruegg J. Regulation of estrogen receptor beta activity and implications in health and disease. Cell Mol Life Sci 2009;66:3873–3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hughes ZA, Liu F, Marquis K, et al. Estrogen receptor neurobiology and its potential for translation into broad spectrum therapeutics for CNS disorders. Curr Mol Pharmacol 2009;2:215–236. [DOI] [PubMed] [Google Scholar]
- 80. Hill RA, Boon WC. Estrogens, brain, and behavior: Lessons from knockout mouse models. Semin Reprod Med 2009;27:218–228. [DOI] [PubMed] [Google Scholar]
- 81. Chung SJ, Armasu SM, Biernacka JM, et al. Variants in estrogen‐related genes and risk of Parkinson's disease. Mov Disord 2011;26:1234–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhao L, Wu TW, Brinton RD. Estrogen receptor subtypes alpha and beta contribute to neuroprotection and increased Bcl‐2 expression in primary hippocampal neurons. Brain Res 2004;1010:22–34. [DOI] [PubMed] [Google Scholar]
- 83. Kummer V, Maskova J, Canderle J, Zraly Z, Neca J, Machala NM. Estrogenic effects of Silymarin in ovariectomized rats. Vet Med (Praha) 2001;46:17–23. [Google Scholar]
- 84. Kansra S, Yamagata S, Sneade L, Foster L, Ben‐Jonathan N. Differential effects of estrogen receptor antagonists on pituitary lactotroph proliferation and prolactin release. Mol Cell Endocrinol 2005;239:27–36. [DOI] [PubMed] [Google Scholar]
- 85. Pliskova M, Vondrácek J, Kren V, et al. Effects of Silymarin flavonolignans and synthetic silybin derivatives on estrogen and aryl hydrocarbon receptor activation. Toxicology 2005;215:80–89. [DOI] [PubMed] [Google Scholar]
- 86. Wang Q, Zou L, Liu W, et al. Inhibiting NF‐κB activation and ROS production are involved in the mechanism of silibinin's protection against D‐galactose‐induced senescence. Pharmacol Biochem Behav 2011;98:140–149. [DOI] [PubMed] [Google Scholar]


