Summary
Aims
To examine whether transplantation of adipose‐derived stem cells (ADSCs) induces neural differentiation and improves neural function in a rat intracerebral hemorrhage (ICH) model.
Methods
Adipose‐derived stem cells cells were isolated from inguinal fat pad of rat. ICH was induced by injection of collagenase type IV into the right basal ganglia of rat. Forty‐eight hours after ICH, ADSCs cells (10 μL of 2–4 × 107 cells/mL) were injected into the right lateral cerebral ventricle. The differentiation of ADSCs was detected in vitro and in vivo. The neural function was evaluated with Zea Longa 5‐grade scale at day 1, 3, 7, 14, or 28.
Results
Our data demonstrated that ADSCs differentiated into cells that shared the similarities of neurons or astrocytes in vitro. Transplantation of ADSCs decreased cell apoptosis and the transplanted ADSCs were able to differentiate into neuron‐like and astrocyte‐like cells around the hematoma, accompanied with upregulation of vascular endothelial growth factor expression and improvement of neural function.
Conclusions
Our data suggest that transplantation of ADSCs could be a therapeutic approach for ICH stroke.
Keywords: Apoptosis, Hemorrhagic stroke, Progenitor cells, Rat, Transplantation
Introduction
Intracerebral hemorrhage (ICH) is a major cause of mortality and disability. Currently, there is no effective treatment for ICH. Recent studies suggest that transplantation of neural stem cells holds great promise for treatment of ICH 1, 2. However, it is a difficult and injurious procedure to obtain neural stem cells from the brain. An alternative source of stem cells is in need.
Adipose‐derived stem cells (ADSCs) have multilineage differentiation potential and have been shown to differentiate into adipogenic, chondrogenic, myogenic, osteogenic, and hepatic cells in the presence of lineage‐specific induction factors in vitro 3, 4, 5, 6. More recently, ADSCs have been shown to be able to be induced to undergo morphologic and phenotypic changes consisting with neuronal differentiation 7, 8. Following neuronal induction, murine and human ADSCs display immunocytochemical staining for glial fibrillary acidic protein (GFAP), nestin, and neuronal nuclei (NeuN). They are emerging as an alternative source of stem cells. However, in vivo neuronal differentiation of ADSCs and their therapeutic effects on ICH have not been explored.
In this study, we have investigated whether ADSCs can be induced to neuronal differentiation and whether transplantation of ADSCs can improve the neural function of rats with ICH.
Materials and Methods
Animals
Male Sprague‐Dawley (SD) rats (body weight, 120–150 g) were provided by the Animal Center of Huazhong University of Science and Technology (Wuhan, China) and housed under controlled temperature (23–25°C) and lighting (8:00–20:00 light, 20:00–8:00 dark) conditions with free access to standard rat chow and drinking water. The experimental procedures regarding the use of animals were approved by the Institutional Review Board (IRB) of Huazhong University of Science and Technology China and performed in accordance with the guidelines of the Ministry of Science and Technology of the People's Republic of China.
Isolation and Culture of ADSCs
The adipose tissue was aseptically collected from the inguinal fat pads of the rats under anesthesia (6% chloral hydrate, 1 mL/200 g, and intraperitoneal injection). Fat pad tissues were excised, finely minced, and digested with 0.1% collagenase type I (Gibco, Los Angeles, CA, USA) at 37°C for 40 min. An equal volume of basic medium (Dulbecco's Modified Eagle Medium [DMEM; Thermo Scientific Hyclone, Waltham, MA, USA]) supplemented with 10% fetal bovine serum (FBS; Thermo Scientific Hyclone) was added to neutralize the collagenase. Then, samples were centrifuged at 367 × g for 10 min at room temperature to obtain pellets. The pellets were washed with basic medium once, resuspended in basic medium, and filtered through a 0.075‐mm cell strainer to remove undigested debris. The resulting cells were planted in a 25‐cm2 culture flask for growth. Medium was changed every 3 days. Cells were passaged until reaching approximately 70–80% confluence.
Adipogenic and Osteogenic Differentiation of ADSCs
The second passage of cultured ADSCs was used in these experiments. To evaluate ADSCs for adipogenic differentiation capacity, cells were planted on cover slips and cultured in basic medium for 3 days and replaced with adipogenic medium (DMEM medium containing 0.5 mM 3‐Isobutyl‐1‐Methylxanthine [IBMX; Sigma‐Aldrich, Louis, MO, USA], 1 μM dexamethasone [Biosharp, Seoul, Korea], 10 μM insulin [Sigma‐Aldrich], and 200 μM indometacin [Aladdin Chemistry Co., Ltd, Xi'An, China]) for 14 days. The cells were visualized with an inverted phase‐contrast microscope. Adipogenic differentiation was determined by staining with Oil‐Red O (Amresco, Solon, OH, USA). To evaluate ADSCs for osteogenic differentiation capacity, basic medium was replaced with osteogenic medium (DMEM medium supplemented with 0.1 μM dexamethasone, 10 nM VitD3 [Biosharp], 50 μM VitC [Biosharp], and 10 mM beta‐2‐glycerophosphate [Biosharp]) for 14 days. Osteogenic differentiation was analyzed by alkaline phosphatase (AP) staining.
In vitro Analysis of Neural Differentiation from ADSCs
The third passage of cultured ADSCs was evaluated for neural differentiation. ADSCs were cultured in induction medium (neurobasal medium [Gibco] supplemented with 20 ng/mL basic fibroblast growth factor [bFGF; Sigma‐Aldrich], 20 ng/mL epidermal growth factor [EGF; Sigma‐Aldrich], and 2% B27 supplement [Gibco]) for 7 days. Medium was changed every 3 days. The cells were visualized with an inverted phase‐contrast microscope. When the number of neurosphere‐like cell masses reached 15–20, cells were resuspend in neurobasal medium and passaged (1:3).
Fluorescent immunostaining analyses of neural differentiation of ADSCs were performed as previously described 7, 9. Five days after culture, the suspension of neurosphere‐like cell masses was centrifuged at 500 × g for 2 min to obtain cell pellets. The cell pellets were resuspended in 2 mL neurobasal medium and planted on coverslips coated with polylysine in six‐well plates. Cells were cultured in neurobasal medium (1 mL/well) for 24 h to allow cell adherence. For fluorescence immunostaining, cells were fixed with 4% paraformaldehyde for 30 min and blocked with 5% bovine serum albumin (BSA; Thermo Scientific Hyclone) for 1 h at room temperature. After blocking, the cells were incubated with rabbit monoclonal antibodies of nestin (1:150, Sigma‐Aldrich) overnight at 4°C followed by incubation with a FITC‐conjugated goat anti‐rabbit secondary antibody (1:50) (Jackson ImmunoResearch Laboratories, Suffolk, UK) at room temperature for 1 h and 50 μg/mL DAPI (Sigma‐Aldrich) for 5 min. Between each step, cells were washed with phosphate buffer solution (PBS; Sigma‐Aldrich). Reaction products were visualized with a fluorescence microscope (BX51; Olympus, Tokyo, Japan).
Neurosphere‐like cell masses were seeded into six‐well plates and cultured in the neurobasal medium supplemented with B27 for 10 days. 70% of the medium was changed every 4 days. Cells were fixed with 4% paraformaldehyde for 30 min and blocked with 5% BSA for 1 h at room temperature. After blocking, cells were incubated with mouse monoclonal antibodies of NeuN (1:100; Sigma‐Aldrich) or mouse monoclonal antibodies of GFAP (1:100; Millipore, Billerica, MA, USA) overnight at 4°C followed by incubation with a Cy3‐conjugated goat anti‐mouse secondary antibody (1:50; Jackson ImmunoResearch Laboratories) for 1 h and 50 μg/mL DAPI for 5 min. Between each step, cells were washed with PBS. Reaction products were visualized with a fluorescence microscope (BX51; Olympus).
Preparation of Bromodeoxyuridine (BrdU)‐conjugated ADSCs
Cultured ADSCs of the third passage were incubated with 20 μmol/L BrdU (Sigma‐Aldrich) at 37°C and 5% CO2 for 48 h. BrdU‐conjugated ADSCs were centrifuged at 367 × g for 1 min and washed with 0.01 mM PBS. The cells were counted under a microscope. The viability of the cells was assessed by trypan blue exclusion. Cells (2–4 × 107/mL) with 90–95% viability were used for transplantation.
Animal Model of ICH
The ICH model was induced to SD rats by injecting collagenase type IV according to previous report 10. Rats were anesthetized with 6% chloral hydrate (1 mL/200 g, intraperitoneal injection) and positioned in a stereotactic frame (Jangwan I; Zhenghua Bioinstrumentation Co., Ltd., An'Hui, China). A cranial burr hole (1 mm) was drilled on the right coronal suture 3.2 mm lateral to the midline. Collagenase IV (0.5 u/1.5 μL) was injected into the right basal ganglia (coordinates: 1.4 mm posterior, 5.6 mm ventral, 3.2 mm lateral to the bregma) by using a 26‐gauge needle connected to a microinfusion pump (0.3 μL/min). After injection, the needle was removed and the burr hole was filled with bone wax. The skin incision was closed with sutures.
Transplantation of ADSCs
Rats were anesthetized with 6% chloral hydrate (1 mL/200 g, intraperitoneal injection) 48 h after ICH induced by injection of collagenase type IV. Rats were randomly divided into two groups (n = 40/group): ADSCs and control. In the ADSCs group, rats were injected with BrdU‐conjugated ADSCs (10 μL, 2–4 × 104/μL) 11, 12, 13 into the right lateral cerebral ventricle (coordinates: 0.2 mm posterior, 4.5 mm ventral, 1.5 mm lateral to the bregma) by using a 26‐gauge needle connected to a microinfusion pump at a rate of 1 μL/min. Rats in the control group were injected with an equal volume of normal saline solution (NSS) as previously reported 14, 15. After injection, the burr hole was filled with bone wax and the skin incision was closed with sutures. Some rats died of ICH during the experiment were excluded from analysis. There were 15 rats per group that died during the period of ICH induction for 48 h by injecting of collagenase type IV and were excluded from analysis. No rats died after ADSCs or NSS treatment.
Behavioral Tests
The neural function was evaluated with Zea Longa 5‐grade scale at day 1, 3, 7, 14, or 28 after ADSCs or NSS transplantation according to previous report 16. The neurologic findings were scored on a five‐point scale: a score of 0 indicated no neurologic deficit, a score of 1 (failure to extend left forepaw fully) a mild focal neurologic deficit, a score of 2 (circling to the left) a moderate focal neurologic deficit, and a score of 3 (falling to the left) and 4 (no spontaneous walk) indicates severe focal deficits 16. Behavioral assessments were performed by investigators unaware of treatment applied to the animals.
In vivo Analysis of Neural‐like cells Differentiation from ADSCs
At day 7 after ADSCs or NSS transplantation (n = 5/group), brains were immediately snapped frozen in OCT blocks. The brain tissues surrounding the injection needle were sectioned at a thickness of 10 μm in the coronal plane, mounted on slides coated with polylysine, and fixed with 4% paraformaldehyde (15 min). After permeated with 0.5% Triton X‐100 (15 min), the sections were acidified with 2 N hydrochloric acid (30 min) and neutralized with boric acid (10 min). The sections were then blocked with 10% fetal calf serum (1 h) and incubated with primary antibodies (4°C, overnight). After washing three times, the sections were incubated with secondary antibodies (room temperature, 30 min). Antibodies used in this study included mouse monoclonal antibodies of NeuN (Sigma‐Aldrich) at a dilution of 1:100, mouse monoclonal antibodies of GFAP (Millipore) at a dilution of 1:100, rabbit anti‐BrdU polyclonal antibody (Millipore) at a dilution of 1:200, Cy3‐conjugated goat anti‐rabbit secondary antibody (Jackson ImmunoResearch Laboratories) at a dilution of 1:250, and FITC‐conjugated goat anti‐mouse secondary antibody (Jackson ImmunoResearch Laboratories) at a dilution of 1:100. The ADSC‐differentiated neuron‐like (BrdU+NeuN+) and glia‐like cells (BrdU+GFAP+) in the areas surrounding hematoma and the corresponding regions of contralateral side were analyzed under the fluorescence microscopy. The stained cells in five random fields (200×) were counted and averaged for each section and the average of five sections represented the data for each side of the brain. The cells were counted by an investigator who was unaware of animal group information.
Measurement of VEGF expression and Cell Apoptosis
Rat brains were randomly harvested at day 1, 3, 7, 14, or 28 after ADSCs or NSS transplantation (n = 5/group/time point). The frozen rat brain sections (10 μm) were stained using an anti‐vascular endothelial growth factor (VEGF) kit (Zhongshan Co., Ltd., Beijing, China) according to the manufacturer's instructions. VEGF intensity was quantified using an image analysis system linked to an Olympus camera (Olympus BX51). Five randomly chosen fields (400× magnifications) were examined. Data were analyzed and quantified using stereological image processing software Image‐Pro Plus (Version 6.0 for windows). Investigators were blinded to the treatment.
For analysis of cell apoptosis, the adjacent sections (10 μm) of the brains harvested in each group and time point were stained by terminal deoxynucleotidyl transferase‐mediated dUTP nick‐end labeling (TUNEL) using an in situ cell death detection kit (Roche, Basel, Switzerland) according to the manufacturer's instructions. TUNEL‐positive cells were counted in five randomly chosen fields (400× magnifications). The apoptotic rate (%) was calculated as the TUNEL‐positive cells divided by the total number of cells. Investigators who measured apoptotic cells were blinded to the treatment.
Statistical Analysis
Data are presented as mean ± SEM. Continuous variances were analyzed by one‐way analysis of variance (ANOVA), followed by Bonferroni–Dunn post hoc test for comparison of differences between groups were determined. For comparison of the neurological deficit scores among different groups, we used significant difference, the Mann–Whitney U‐test was applied. A P‐value < 0.05 was considered significant.
Results
ADSCs Were Able to Differentiate into Adipogenic and Osteogenic Cells in vitro
Cultured ADSCs grew as a monolayer of spindle‐shaped cells with large nuclei morphologically (Figure 1A). At day 12 after adipogenic induction, lipid droplets accumulated within the cells were revealed by Oil‐Red O staining (Figure 1B). At day 14 after osteogenic induction, mineralized bone nodules were detected within the cells by AP staining (Figure 1C). Meanwhile, the cell morphology changed to a polygonal or cubic shape.
Figure 1.
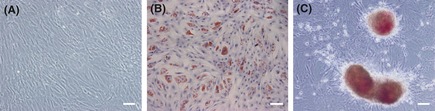
Morphological characteristic of adipogenic and osteogenic differentiation from adipose‐derived stem cells (ADSCs). (A) Morphology of ADSCs. (B) Adipogenic differentiation (Oil‐Red O staining). (C) Osteogenic differentiation (alkaline phosphatase staining). Scale bar: 100 μm.
ADSCs Were Able to Differentiate into Neural‐like and Glia‐like Cells in vitro
Five to 7 days after culturing ADSCs in induction medium, neurosphere‐like cells were detected showing nestin expression (Figure 2A). By day 10, these neurosphere‐like cells started to express neural markers, NeuN (Figure 2B) or GFAP (Figure 2C).
Figure 2.
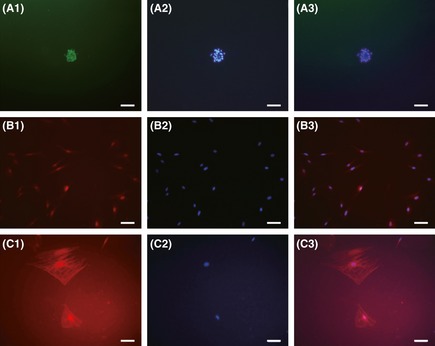
Neural‐like cells differentiations of adipose‐derived stem cells (ADSCs) in vitro. (A) Immunofluorescent staining showed that the neurosphere‐like cells were nestin positive (green: Nestin, blue: cell nuclear). (B) Neurosphere‐like cells were NeuN positive (red: NeuN, blue: cell nuclear). (C) Glia‐like cells were glial fibrillary acidic protein (GFAP) positive (red: GFAP, blue: cell nuclear). Scale bar: 100 μm.
Transplantation of BrdU‐conjugated ADSCs Decreased ICH‐induced Neural Dysfunction
Neural function was evaluated by Zea Longa 5‐grade scale. There was no significant difference in neurological deficit score at day 1 between ADSC transplantation group and control group (vs. control, P > 0.05). However, at day 3, 7, 14, and 28, the scores were significantly lower in ADSC transplantation than those in control group (vs. control, P < 0.05 or 0.01, Figure 3).
Figure 3.
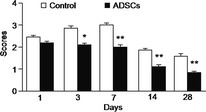
Effect of adipose‐derived stem cells (ADSC) transplantation on neurologic deficit score in intracerebral hemorrhage rats. ADSC transplantation improved neural function at different time points determined by Zea Longa 5‐grade scale. *P < 0.05, **P < 0.01, compared with control group, n = 5/group/time point.
BrdU‐conjugated ADSCs Differentiated into Neural‐like and Glia‐like Cells in vivo after Transplantation
In the coronal section, hematoma appeared in the basal ganglia area of all the rats 24 h after collagenase injection suggesting the success of ICH induction (Figure 4A). Fluorescent immunostaining and immunohistochemical staining showed that BrdU‐conjugated ADSCs concentrated around the tunnel made by injection in day 1 (Figure 4B), and some of them were able to migrate to the area surrounding the hematoma after 7 days (Figure 4C). In the corresponding brain region of the contralateral side, few labeled BrdU‐ADSCs were found. Double‐fluorescence labeling for BrdU and NeuN or GFAP revealed that ADSCs were able to differentiate into neuron‐like (BrdU+/NeuN+) and glia‐like cells (BrdU+/GFAP+), which were predominated in the surrounding area of hematoma (Figure 5A–C). The data suggested that the transplanted ADSCs were able to migrate to the ICH location, and to differentiate into neuron‐like and glia‐like cells.
Figure 4.
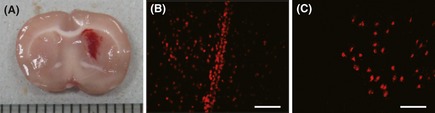
A rat model of intracerebral hemorrhage (ICH) and migration of BrdU‐conjugated adipose‐derived stem cells (ADSCs) after transplantation. (A) Representative coronal brain section showing the success of ICH model in rat. (B) On day 1 after transplantation, BrdU‐conjugated ADSCs concentrated on the tunnel made by injection. (C) On day 7 after transplantation, BrdU‐conjugated ADSCs migrated to the surrounding area of hematoma. Scale bar: 100 μm.
Figure 5.
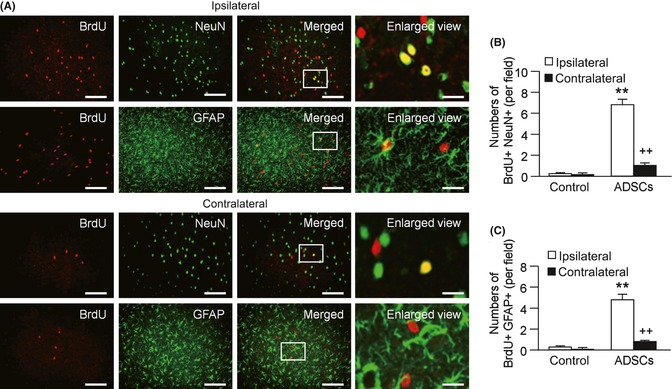
Neural‐like cells differentiations of adipose‐derived stem cells (ADSCs) in vivo. (A) Representative images of neuron‐like cells (red: BrdU, green: NeuN, yellow: merged) or glia‐like cells (red: BrdU, green: glial fibrillary acidic protein, yellow: merged) differentiation on day 7 after BrdU‐conjugated ADSC transplantation. Scale bar: 100 μm. The images in the last column are the enlarged views of the squared areas. Scale bar: 20 μm. (B) Summarized data of neuron‐like cell differentiation. (C) Summarized data of glia‐like cell differentiation. *P < 0.05, **P < 0.01, compared with control group; + P < 0.05, ++ P < 0.01, compared with ipsilateral side, n = 5/group.
Transplantation of BrdU‐conjugated ADSCs upregulated VEGF expression and decreased ICH‐induced cell apoptosis
The levels of VEGF expression were significantly higher in the ADSC transplantation group than in the control group at day 3, 7, 14, and 28 (vs. control, P < 0.05, Figure 6A and B, Table 1). TUNEL staining showed that most of the apoptotic cells were around the hematoma. Apoptotic rate was significantly lower at day 3 after ADSC transplantation (vs. control, P < 0.05, Figure 6C and D, Table 1). The apoptotic rates were slightly lower in the ADSC transplantation group than those in the control group at day 7, 14, and 28 (P > 0.05 for all, Table 1).
Figure 6.
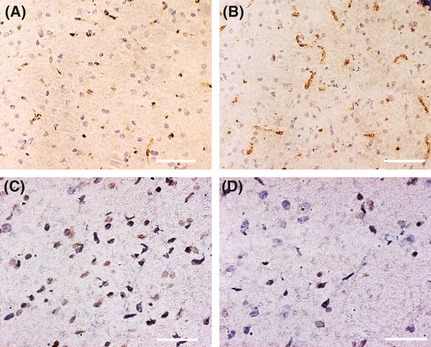
Representative images of vascular endothelial growth factor (VEGF) expression and cell apoptosis in the hematoma surrounding area on day 3 after adipose‐derived stem cells (ADSC) transplantation. (A) VEGF in control group. (B) VEGF in ADSC group. (C) Apoptosis in control group. (D) Apoptosis in ADSC group. Scale bar: 100 μm.
Table 1.
The effects of ADSCs transplantation on VEGF expression and cell apoptosis following intracerebral hemorrhage
| Group | Variants | Days after transplantation | ||||
|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 7 | Day 14 | Day 28 | ||
| Control | VEGF (density) | 0.21 ± 0.02 | 0.20 ± 0.02 | 0.20 ± 0.02 | 0.20 ± 0.01 | 0.18 ± 0.01 |
| Apoptosis (%) | 0.64 ± 0.12 | 0.57 ± 0.11 | 0.51 ± 0.09 | 0.45 ± 0.15 | 0.33 ± 0.08 | |
| ADSCs | VEGF (density) | 0.23 ± 0.01 | 0.24 ± 0.03* | 0.23 ± 0.03* | 0.21 ± 0.01* | 0.20 ± 0.01* |
| Apoptosis (%) | 0.64 ± 0.18 | 0.36 ± 0.04* | 0.42 ± 0.12 | 0.34 ± 0.04 | 0.28 ± 0.07 | |
ADSC, adipose‐derived stem cells; VEGF, vascular endothelial growth factor.
Data are means ± SD. n = 5/group, *P < 0.05 compared to control.
Discussion and Conclusion
Intracerebral hemorrhage causes severe neurological deficits and extensive death rate in patients. Medical therapies against ICH such as mechanical removal of hematoma, prevention of edema formation by drugs, or reduction of intracranial pressure show only limited effectiveness. Alternative approach is required by NINDS ICH Workshop Participants 17, 18. Recent progress in stem cell biology has opened up a new way of therapeutic strategies in brain injury and disease by replacing lost neural cells with transplanted neural stem cells 19, 20, 21. However, obtaining neural stem cells from the brain is a difficult, injurious procedure and painful to patients. A source for easier and higher yield of adult stem cell is needed.
Adipose tissue is an alternative source of pluripotent mesenchymal stromal cells 22. Cells isolated from adipose tissue are self‐renewing and can be induced to differentiate along several mesenchymal tissue lineages, such as adipocytes, osteoblasts, myocytes, and chondrocytes. Our study showed that ADSCs can be induced into neuron‐like cells in vitro, which is consistent with previous reports 7, 23, 24. However, whether these cells can actually differentiate into neurons remains controversial 25, 26, 27. In addition, the functional importance of these cells is poorly understood.
Studies of ADSC transplantation in several disease models start to shed light on their function roles. In an ICH model, transplanted ADSCs are found to densely populate in perihematomal areas at week 6 and significantly reduce both acute cerebral inflammation and chronic brain degeneration promoting long‐term functional recovery 11. However, these cells express endothelial markers (von Willebrand factor and endothelial barrier antigen) rather than neuronal or glial markers arguing against neuronal differentiation of these cells 11. Intracerebral grafting of BDNF‐transduced human adipose tissue stromal cells (hATSCs) significantly improves motor recovery of functional deficits in rats following middle cerebral artery occlusion 28. Some of the engrafted cells express neural markers such as MAP2 and GFAP. However, the functional improvement might be owing to increased neurotrophic factors other than differentiation into neurons because the number of neural marker–positive cells is extremely low 4.
In our study, we found that ADSC transplantation significantly decreased the neurologic deficit scores at day 3, 7, 14, and 28 after ICH, demonstrating that transplantation of ADSCs can improve neuronal function. There are three mechanisms that might be involved in the beneficial effect of ADSC treatment for ICH. Firstly, the ability of transplanted ADSCs to differentiate into neuron‐like and glia‐like cells that compensate for ICH‐induced loss of the nearby neuronal cells can account for the improved functional outcomes. This is supported by the evidence that ADSCs injected into the lateral cerebral ventricle were able to migrate to the surrounding area of hematoma at day 7. Some of these cells were differentiated into neuron‐like cells expressing NeuN or glia‐like cells expressing GFAP. Secondly, VEGF might be another possible mechanism because recent in vivo and in vitro studies showed that VEGF protects neurons from cell death 29, 30, 31. In our study, we found the expression of VEGF in the surrounding area of hematoma was significantly upregulated in the ADSC transplantation group when compared with the control group. Increased VEGF expression was detected as early as on day 3 and remained higher till day 28 after ADSC transplantation. This may be attributed to ADSC transplantation, because ADSCs have been reported to stimulate the release of multiple growth factors including VEGF 32, 33. It should be noted that the data from others 34, 35 showed that ICH itself could induce the upregulation of VEGF in the brain as early as on day 3. These help to interpret the functional improvement in the control group and persistent functional improvement after ADSC transplantation. Finally, the reduction of cell apoptosis in the surrounding area of hematoma could be also involved in the beneficial effects of ADSC transplantation. Our data showed that the number of apoptotic cells around the hematoma was significantly decreased in the transplantation group on day 3.
Our results are not totally agreed with a previous report by Kim et al. 11, which showed that transplanted ADSCs differentiated into endothelial cells rather than neuronal or glial markers 11. We believe that the disagreement could come from the difference of the medium used for culturing ADSCs. In Kim's study, ADSCs were cultured with endothelial growth medium supplemented with 2–5% FBS, which contains various growth factors, including VEGF, fibroblast growth factor, EGF, and insulin‐like growth factor, whereas we cultured ADSCs in DMEM supplemented with 10% FBS. Nevertheless, our in vitro and in vivo findings showing the ability of ADSCs in differentiating into neuron‐like and glial‐like cells were supported by previous studies in ischemic stroke models 4, 28 and in a spinal cord injury model 27.
In summary, our study demonstrated that isolated ADSCs from inguinal fat pads of rats could be induced to neuronal differentiation both in vivo and in vitro. The results suggest that improvements of neurological function after transplantation of ADSCs to rats with ICH might be owing to neuronal differentiation of implanted stem cells, which protect cells against ICH‐induced apoptosis. Taking together, these data reveal that ADSCs could be an alternative source of stem cells for treatment of patients with ICH. However, the safety, time window, and proper dose of ADSC transplantation as well as the functional and characterizing studies on the neurons derived from ADSCs need further investigation.
Disclosures
None.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30770751 & 81171089 to Z.T., 81070878 to B.Z.), Health Commission of Hubei Province of China (JX4A03 to Z.T.), and the National Heart, Lung, and Blood Institute (HL‐098637 to Y.C.).
The first three authors contributed equally to this work.
References
- 1. Lee HJ, Kim KS, Kim EJ, et al. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells 2007;25:1204–1212. [DOI] [PubMed] [Google Scholar]
- 2. Zhang H, Huang Z, Xu Y, Zhang S. Differentiation and neurological benefit of the mesenchymal stem cells transplanted into the rat brain following intracerebral hemorrhage. Neurol Res 2006;28:104–112. [DOI] [PubMed] [Google Scholar]
- 3. Bonora‐Centelles A, Jover R, Mirabet V, et al. Sequential hepatogenic transdifferentiation of adipose tissue‐derived stem cells: relevance of different extracellular signaling molecules, transcription factors involved, and expression of new key marker genes. Cell Transplant 2009;18:1319–1340. [DOI] [PubMed] [Google Scholar]
- 4. Kang SK, Lee DH, Bae YC, Kim HK, Baik SY, Jung JS. Improvement of neurological deficits by intracerebral transplantation of human adipose tissue‐derived stromal cells after cerebral ischemia in rats. Exp Neurol 2003;183:355–366. [DOI] [PubMed] [Google Scholar]
- 5. Lee DH, Kang SK, Lee RH, et al. Effects of peripheral benzodiazepine receptor ligands on proliferation and differentiation of human mesenchymal stem cells. J Cell Physiol 2004;198:91–99. [DOI] [PubMed] [Google Scholar]
- 6. Lee RH, Kim B, Choi I, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem 2004;14:311–324. [DOI] [PubMed] [Google Scholar]
- 7. Fang Z, Yang Q, Xiong W, et al. Neurogenic differentiation of murine adipose derived stem cells transfected with EGFP in vitro . J Huazhong Univ Sci Technolog Med Sci 2010;30:75–80. [DOI] [PubMed] [Google Scholar]
- 8. Safford KM, Hicok KC, Safford SD, et al. Neurogenic differentiation of murine and human adipose‐derived stromal cells. Biochem Biophys Res Commun 2002;294:371–379. [DOI] [PubMed] [Google Scholar]
- 9. Kang SK, Putnam LA, Ylostalo J, et al. Neurogenesis of Rhesus adipose stromal cells. J Cell Sci 2004;117:4289–4299. [DOI] [PubMed] [Google Scholar]
- 10. Rosenberg GA, Mun‐Bryce S, Wesley M, Kornfeld M. Collagenase‐induced intracerebral hemorrhage in rats. Stroke 1990;21:801–807. [DOI] [PubMed] [Google Scholar]
- 11. Kim JM, Lee ST, Chu K, et al. Systemic transplantation of human adipose stem cells attenuated cerebral inflammation and degeneration in a hemorrhagic stroke model. Brain Res 2007;1183:43–50. [DOI] [PubMed] [Google Scholar]
- 12. Wrage PC, Tran T, To K, et al. The neuro‐glial properties of adipose‐derived adult stromal (ADAS) cells are not regulated by Notch 1 and are not derived from neural crest lineage. PLoS One 2008;3:e1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deng W, Obrocka M, Fischer I, Prockop DJ. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun 2001;282:148–152. [DOI] [PubMed] [Google Scholar]
- 14. Li Y, Chopp M, Chen J, et al. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab 2000;20:1311–1319. [DOI] [PubMed] [Google Scholar]
- 15. Yang KL, Chen MF, Liao CH, Pang CY, Lin PY. A simple and efficient method for generating Nurr1‐positive neuronal stem cells from human wisdom teeth (tNSC) and the potential of tNSC for stroke therapy. Cytotherapy 2009;11:606–617. [DOI] [PubMed] [Google Scholar]
- 16. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989;20:84–91. [DOI] [PubMed] [Google Scholar]
- 17. NINDS ICH Workshop Participants . Priorities for clinical research in intracerebral hemorrhage: report from a national institute of neurological disorders and stroke workshop. Stroke 2005;36:e23–e41. [DOI] [PubMed] [Google Scholar]
- 18. Gebel JM, Broderick JP. Intracerebral hemorrhage. Neurol Clin 2000;18:419–438. [DOI] [PubMed] [Google Scholar]
- 19. Kim SU. Human neural stem cells genetically modified for brain repair in neurological disorders. Neuropathology 2004;24:159–171. [DOI] [PubMed] [Google Scholar]
- 20. Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature 2006;441:1094–1096. [DOI] [PubMed] [Google Scholar]
- 21. Muller FJ, Snyder EY, Loring JF. Gene therapy: can neural stem cells deliver? Nat Rev Neurosci 2006;7:75–84. [DOI] [PubMed] [Google Scholar]
- 22. Patrick CW, Jr. Adipose tissue engineering: the future of breast and soft tissue reconstruction following tumor resection. Semin Surg Oncol 2000;19:302–311. [DOI] [PubMed] [Google Scholar]
- 23. Jang S, Cho HH, Cho YB, Park JS, Jeong HS. Functional neural differentiation of human adipose tissue‐derived stem cells using bFGF and forskolin. BMC Cell Biol 2010;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wei Y, Gong K, Zheng Z, et al. Schwann‐like cell differentiation of rat adipose‐derived stem cells by indirect co‐culture with Schwann cells in vitro . Cell Prolif 2010;43:606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qian DX, Zhang HT, Ma X, Jiang XD, Xu RX. Comparison of the efficiencies of three neural induction protocols in human adipose stromal cells. Neurochem Res 2010;35:572–579. [DOI] [PubMed] [Google Scholar]
- 26. Santiago LY, Clavijo‐Alvarez J, Brayfield C, Rubin JP, Marra KG. Delivery of adipose‐derived precursor cells for peripheral nerve repair. Cell Transplant 2009;18:145–158. [DOI] [PubMed] [Google Scholar]
- 27. Zhang HT, Luo J, Sui LS, et al. Effects of differentiated versus undifferentiated adipose tissue‐derived stromal cell grafts on functional recovery after spinal cord contusion. Cell Mol Neurobiol 2009;29:1283–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee TH, Yoon JG. Intracerebral transplantation of human adipose tissue stromal cells after middle cerebral artery occlusion in rats. J Clin Neurosci 2008;15:907–912. [DOI] [PubMed] [Google Scholar]
- 29. Harrigan MR, Ennis SR, Masada T, Keep RF. Intraventricular infusion of vascular endothelial growth factor promotes cerebral angiogenesis with minimal brain edema. Neurosurgery 2002;50:589–598. [DOI] [PubMed] [Google Scholar]
- 30. Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor rescues HN33 neural cells from death induced by serum withdrawal. J Mol Neurosci 2000;14:197–203. [DOI] [PubMed] [Google Scholar]
- 31. Manoonkitiwongsa PS, Schultz RL, McCreery DB, Whitter EF, Lyden PD. Neuroprotection of ischemic brain by vascular endothelial growth factor is critically dependent on proper dosage and may be compromised by angiogenesis. J Cereb Blood Flow Metab 2004;24:693–702. [DOI] [PubMed] [Google Scholar]
- 32. Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004;109:1292–1298. [DOI] [PubMed] [Google Scholar]
- 33. Kondo K, Shintani S, Shibata R, et al. Implantation of adipose‐derived regenerative cells enhances ischemia‐induced angiogenesis. Arterioscler Thromb Vasc Biol 2009;29:61–66. [DOI] [PubMed] [Google Scholar]
- 34. Tang T, Liu XJ, Zhang ZQ, et al. Cerebral angiogenesis after collagenase‐induced intracerebral hemorrhage in rats. Brain Res 2007;1175:134–142. [DOI] [PubMed] [Google Scholar]
- 35. Zhang X, Li H, Hu S, et al. Brain edema after intracerebral hemorrhage in rats: the role of inflammation. Neurol India 2006;54:402–407. [DOI] [PubMed] [Google Scholar]


