Summary
Aim
The discovery of new natural compounds with pharmacological properties is a field of interest widely growing. Recent literature shows that Brassica vegetables (Cruciferae) possess therapeutic effects particularly ascribed due to their content in glucosinolates, which upon myrosinase hydrolysis release the corresponding isothiocyanates. This study examines the potential neuroprotective and immunomodulatory effects of (RS)‐glucoraphanin from Tuscan black kale (Brassica oleracea L. var. acephala sabellica) bioactivated with myrosinase (bioactive RS‐GRA) (10 mg/kg/day intraperitoneally), in an experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis.
Methods
EAE was induced by immunization with myelin oligodendroglial glycoprotein peptide (MOG 35–55) in mice. After immunization, mice were observed daily for signs of EAE and weight loss. Clinical score was evaluated using a standardized scoring system.
Results
By Western blot analysis of spinal cord tissues, we have demonstrated that treatment with bioactive R S‐GRA significantly decreased nuclear factor (NF)‐kB translocation, pro‐inflammatory cytokine production such as interleukin‐1β (IL‐1β), and apoptosis (Bax and caspase 3 expression).
Conclusion
Our results clearly demonstrate that bioactive RS‐GRA treatment may represent a useful therapeutic perspective in the treatment of this disease.
Keywords: Apoptosis, Bioactive (RS)‐glucoraphanin, Experimental autoimmune encephalomyelitis, Inflammation, Multiple sclerosis
Introduction
Multiple sclerosis (MS) is a progressive inflammatory and demyelinating disease of the central nervous system (CNS) 1, 2 caused by malfunction of the immune system, which affects more than 2.5 million people worldwide every year 3, typically in adults between 20 and 45 years of age; more women than men and Northern European people are shown to be at highest risk for MS 4, 5. MS attacks the myelinated axons in the CNS, destroying the myelin and the axons to varying degrees 6. According to the traditional literature, MS is believed an inflammatory disease primarily affecting brain and spinal cord white matter 7, 8, 9. The clinical signs of MS are highly variable. Patients with MS often have symptoms of upper motor neuron disease that include hyperreflexia, ataxia, spasticity, and visual defects. In some cases, there is evidence of lower motor neuron disease such as sensory defects and partial or complete paralysis 10.
The etiology of MS is yet unknown, but it appears to involve a combination of genetic susceptibility and a nongenetic trigger, such as a virus, metabolism, or environmental factors, that together result in a self‐sustaining autoimmune disorder that leads to recurrent immune attacks against CNS 11, 12, 13. To better understand the etiopathogenesis of MS, researchers use some experimental models and, among these, one of the most used is the experimental autoimmune encephalomyelitis (EAE) 14, 15. EAE is a well‐characterized animal model, showing many clinical and pathological features of human MS, such as paralysis, weight loss, demyelination, and CNS inflammation 16.
However, there are great differences between EAE and MS. The first and most obvious is that MS is a spontaneous disease, while EAE is induced by active sensitization with brain tissue antigens. Recently, spontaneous EAE models were developed, but these are dependent on the use of transgenic approaches to ignore the intrinsic mechanisms of regulation that normally suppress tissue‐specific autoaggression 17, 18. Furthermore, for inducing the disease, the use of strong immune adjuvants is provided, and it seems unlikely that the same intense “immunological boosts” occur in physiological conditions such as infectious diseases. Also, the adoptive transfer of myelin‐specific T cells can induce demyelination, although the extent of primary myelin loss is minimal in comparison with that seen in patients with MS 19. Moreover, for the sake of reproducibility, EAE has been studied mainly in inbred animals or in genetically homogeneous populations. Thus, genetic heterogeneity, which is so critical in the multiple sclerosis population, can be considered only when several different models of EAE are studied in parallel.
Although this model shows limitations, it provides a very reproducible disease model to study the main mechanisms involved in the pathogenesis of T‐cell‐mediated inflammation in the CNS and has offered many insights that have been of great importance for the design of antiinflammatory therapies.
Convincing evidences, supported by both animal and cell line studies, have demonstrated that regular consumption of Brassicaceae vegetables (broccoli, cabbage, cauliflower, Brussels sprouts, etc.) can contribute to reduced risk of neurodegenerative disorders and cancer. It is believed that the benefits of these vegetables are due to their high content of specific phytochemicals, known as glucosinolates (GLs) 20.
Their structures share a common core of a β‐D‐glucopyranose moiety linked via a sulfur atom to a (Z)‐N‐hydroximinosulfate ester and a variable aglycon side chain derived from the α‐amino acid biosynthetic precursor. Several methionine‐derived GLs, which constitute the largest group of GLs, bear in their side chain an additional sulfur atom at different oxidation states (sulfide, sulfoxide, or sulfone functions). Among them, one of the most studied is R S‐(‐)‐glucoraphanin [R S‐GRA; 4(R S)‐methylsulfinylbutyl glucosinolate] a thiosaccharidic compound found in Brassicaceae, notably in Tuscan black kale (Brassica oleracea L. var. acephala sabellica).
GLs coexist in the same plant, but in separate cells, with the enzyme β‐thioglucoside glucohydrolase, usually known as myrosinase (Myr; EC 3.2.1.147), which have also been found within human bowel microflora 21, 22. After mechanical damage of cells, for example, predation/mastication by humans or animals, freeze–thaw injury, or plant pathogens, GLs are hydrolyzed releasing, apart from glucose and sulfate, several biologically active compounds such as isothiocyanates (ITCs), thiocyanates, and nitriles, depending on the hydrolytic conditions 23, 24, 25. At neutral pH condition, Myr catalyzes the GL hydrolysis (>99%) producing the corresponding ITCs, such as R S‐sulforaphane (R S‐SFN) from R S‐GRA 26.
The mechanism underlying the chemopreventive R S‐SFN effects was interpreted as multipotent and ascribed to its ability to influence carcinogen metabolism, both inhibiting phase I enzyme‐mediated activation, by inducing phase‐2 enzymes like glutathione‐S‐transferase (GST) and quinone reductase (QR), and triggering the nuclear factor erythroid 2‐related factor 2 (Nrf‐2) pathway 27, 28.
Recently, CRA‐CIN of Bologna (Italy) developed a gram‐scale production of natural R S‐GRA starting from Tuscan black kale. The aim of this study was to investigate the possible neuroprotective role of the bioactive R S‐GRA on MS, according to an experimental mouse model of EAE.
Additionally, to gain a better insight into the mechanisms of action of R S‐GRA, we have also investigated its effects on NF‐kB translocation and IkB‐α degradation, as NF‐kB is a transcription factor, which is kept inactive by IkB‐α, that plays a central role in the regulation of many genes responsible for the generation of mediators or proteins in inflammation, like tumor necrosis factor‐α (TNF‐α), IL‐1β, inducible NO synthase (iNOS), c‐Jun N‐terminal kinase (JNK), and others.
Moreover, we evaluated the expression of caspase 3 and Bax as marker of apoptosis, as the sequential activation of caspase 3 plays a key role in the execution phase of cell apoptosis, as well as it is known that Bax gene plays an important role in cell death and CNS injury and that neurons lacking Bax are protected against apoptosis 29, 30.
All this, to verify whether any therapeutic change due to the bioactive R S‐GRA treatment, is linked to a mechanism involving the modulation of the inflammatory or apoptotic pathways and the subsequent physiological responses.
Materials and Methods
Animals
Male adult C57BL/6 mice (20–25 g; Harlan Nossan, Milan, Italy) were used for all studies. Mice were housed in stainless steel cages and maintained under 12‐h/12‐h light/dark cycle at 21 ± 1°C and 50 ± 5% humidity. The animals were acclimated to their environment for 1 week, and food and water were given ad libitum. Animal care was in compliance with Italian regulations on protection of animals used for experimental and other scientific purposes (D.M. 116/92) as well as with the EEC regulations (O.J: of E.C.L 358/1 12/18/1986).
Experimental procedures did not cause any significant animal suffering.
Reagents
The myelin oligodendrocyte glycoprotein peptide (MOG)35–55 (MEVGWYRSPFSRVVHLYRNGK; Auspep) was synthesized and purified by Cambridge Research Biochemicals (Billingham, UK).
Complete Freund's adjuvant (CFA) containing Mycobacterium tubercolosis H37Ra was purchased from Difco Laboratories (Sparks, MD, USA), while Bordetella pertussis toxin was from Sigma‐Aldrich Company Ltd. (Milan, Italy).
Unless otherwise stated, all compounds were obtained from Sigma‐Aldrich Company Ltd. All other chemicals were of the highest commercial grade available.
Induction of EAE
After anesthesia, mice were immunized subcutaneously with 300 μL/flank of the emulsion consisting of 300 μg of MOG35–55 in phosphate‐buffered saline (PBS) combined with an equal volume of CFA containing 300 μg heat‐killed M. Tubercolosis H37Ra. Afterwards, animals have been injected with 100 μL of B. Pertussis toxin (500 ng/100 μL, i.p.), that has been also repeated 48 h later. The disease follows a course of progressive degeneration, with visible signs of pathology consisting of flaccidity of the tail and loss of motion of the hind legs.
GLs and Myrosinase Purification, Enzyme Bioactivation of Rs‐GRA
R S‐GRA was isolated according to a procedure developed at CRA‐CIN of Bologna 31. Seeds of Tuscan black kale, supplied by Suba Seeds (Longiano, Italy), were first ground to a fine powder and defatted in hexane. The solvent was removed and the defatted meal was then treated with boiling 70% ethanol (1:8 w/v) to produce a quick deactivation of the endogenous enzyme Myr and to prevent GL hydrolysis. The solid residue was removed by centrifugation and re‐extracted using the same w/v ratio. The two solutions were collected, and the isolation of R S‐GRA from the extract was conducted by one‐step anion exchange chromatography, and the purity was further improved by gel filtration performed using a XK 26/100 column packed with Sephadex G10 chromatography media (GE Healthcare), connected to an AKTA‐FPLC system (GE Healthcare). Individual fractions were analyzed by HPLC, and those containing pure R S‐GRA were pooled and freeze‐dried 32. R S‐GRA was characterized by 1H and 13C NMR spectroscopy, and the absolute purity estimated by HPLC analysis of the desulfoderivative, according to the ISO 9167‐1 method 33, was 99% (peak purity HPLC) and >95% weight basis (hydrated salt containing 1–2 equivalents of water. UV spectra and the molar extinction coefficient value of 6634 M1/cm1 at 225 nm were determined using a Varian Cary 300 Bio UV/vis spectrophotometer).
The enzyme Myr was isolated from seeds of Sinapis alba L. as described by Pessina et al. 34, with some modification. The specific activity of the stock solution used in this study was about 60 U/mg of soluble protein. The enzymatic activity was 32 U/mL, and the solution was stored at 4°C in sterile saline solution at neutral pH until use. One myrosinase unit was defined as the amount of enzyme able to hydrolyze 1 μmol sinigrin per minute at pH 6.5 and 37°C.
R S‐GRA (10 mg/kg) was dissolved in PBS solution pH 7.2, and mouse treatment required the enzyme bioactivation of the phytochemical. The in situ action of the myrosinase enzyme (5 μL/mouse) for 15 min allowed to have a bioactive R S‐GRA quickly, before the i.p treatment (Figure 1).
Figure 1.
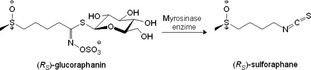
(RS)‐glucoraphanin purified from Tuscan black kale seed, bioactivated with myrosinase enzyme: in situ release of (RS)‐sulforaphane.
Experimental Design
Mice were randomly allocated into the following groups (N = 50 total animals):
EAE + bioactive R S‐GRA group (N = 20): mice subjected to EAE were treated with bioactive R S‐GRA (10 mg/kg + 5 μL/mouse Myr, i.p). Bioactive R S‐GRA was daily administrated 1 week before EAE induction, and after immunization, the treatment was daily protracted until the sacrifice;
EAE group (N = 20): mice subjected to EAE that did not receive bioactive R S‐GRA;
Sham group (N = 5): mice that received vehicle (saline) in place of MOG35–55;
Bioactive R S‐GRA control group (N = 5): mice that received vehicle (saline) in place of MOG35–55 and treated with bioactive R S‐GRA (10 mg/kg + 5 μL/mouse Myr, i.p.);
The experiment provided a housing period of duration of 7 days followed by a period of pretreatment with bioactive R S‐GRA via i.p. injection once a day for 7 days. On the 15th day, the disease was induced according to the following experimental procedure. In the experimental group EAE + bioactive R S‐GRA, the postdrug treatment was continued for a further 7 days after induction of the disease until the 21st day.
At the end of the experiment, the animals were sacrificed, and spinal cord tissues were harvested and processed, to evaluate parameters of disease.
Body Weight and Clinical Score
Mice were observed daily for signs of EAE. Clinical score was evaluated using a standardized scoring system 35. Briefly, clinical signs were scored as follows: 0 = no signs; 1 = partial flaccid tail; 2 = complete flaccid tail; 3 = hind limb hypotonia; 4 = partial hind limb paralysis; 5 = complete hind limb paralysis; 6 = moribund or dead animal.
In addition, the measure of the body weight was daily assessed, and any loss was evaluated as marker of pathology. The graph represents Δ value for each group obtained calculating the difference in body weight between the measure taken the day of sacrifice and that one taken the day of the disease induction (Figure 2).
Figure 2.
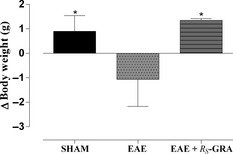
Δ Body weight. C57BL/6 mice were immunized with MOG 35–55 and CFA and then treated daily with bioactive RS‐GRA at the dose of 10 mg/kg. Mice were monitored daily for weight gain/loss. After EAE induction, significative body weight loss was observed in EAE mice. Also, a significative body weight gain was found in EAE+ bioactive RS‐GRA group and in bioactive RS‐GRA control group mice compared with EAE group and sham group. Graph represents for each experimental group, the Δ body weight calculated by subtracting measures taken the day of animal sacrifice and measures taken the day of immunization. Data are mean value ± SEM. *P < 0.05 versus EAE.
Western Blot Analysis for IL‐1β, JNK, IkB‐α, NF‐kB p65, Bax, and Caspase 3
All the extraction procedures were performed on ice using ice‐cold reagents. In brief, spinal cord tissues were suspended in extraction buffer containing 0.32 M sucrose, 10 mM Tris–HCl, pH 7.4, 1 mM EGTA, 2 mM EDTA, 5 mM NaN3, 10 mM 2‐mercaptoethanol, 50 mM NaF, and protease inhibitor tablets (Roche Applied Science, Monza, Italy), and they were homogenized at the highest setting for 2 min. The homogenates were chilled on ice for 15 min and then centrifuged at 1000 g for 10 min at 4°C, and the supernatant (cytosol + membrane extract from spinal cord tissue) was collected to evaluate content of IL‐1β, JNK, IkB‐α, and Bax.
The pellets were suspended in the supplied complete lysis buffer containing 1% Triton X‐100, 150 mM NaCl, 10 mM Tris–HCl, pH 7.4, 1 mM EGTA, and 1 mM EDTA protease inhibitor tablets (Roche Applied Science), and then they were centrifuged for 30 min at 15,000 g at 4°C, and the supernatant (nuclear extract) was collected to evaluate the content of NF‐kB p65 and caspase 3.
Supernatants were stored at −80°C until use. Protein concentration in homogenate was estimated by the Bio‐Rad Protein Assay (Bio‐Rad, Segrate, Milan, Italy) using BSA as standard, and 50 μg of cytosol and nuclear extract from each sample was analyzed.
Proteins were separated on sodium dodecyl sulfate–polyacrylamide minigels and transferred onto nitrocellulose membranes (Protran nitrocellulose transfer membrane; Whatman Schleicher and Schuell, Dassel, Germany), blocked with PBS containing 5% nonfat dried milk for 45 min at room temperature, and subsequently probed at 4°C overnight with specific antibodies for IL‐1β (1:500; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), JNK (1:500; Santa Cruz Biotechnology, Inc.), IkB‐α (1:1000; Cell Signaling Technology, Inc., Boston, MA, USA), Bax (1:500; Santa Cruz Biotechnology, Inc.), NF‐kB p65 (1:1000; Santa Cruz Biotechnology, Inc.), and caspase 3 (1:1000; Cell Signaling), in 1× PBS, 5% (w/v) nonfat dried milk, and 0.1% Tween‐20 (PMT).
Membranes were incubated with peroxidase‐conjugated bovine anti‐mouse IgG secondary antibody or peroxidase‐conjugated goat anti‐rabbit IgG (1:2000; Jackson ImmunoResearch, West Grove, PA, USA) for 1 h at room temperature. To ascertain that blots were loaded with equal amounts of protein lysates, they were also incubated with antibody for β‐actin protein (1:1000; Santa Cruz Biotechnology, Inc.), α‐tubulin (1:250; Santa Cruz Biotechnology, Inc.), and GAPDH HRP conjugated (1:1000; Cell Signaling).
The relative expression of the protein bands of IL‐1β (~17 kDa), JNK (~46 kDa), Bax (~23 kDa), IkB‐α (~37 kDa), NF‐kB p65 (~65 kDa), caspase 3 (~35 kDa) was visualized using an enhanced chemiluminescence system (SuperSignal West Pico Chemiluminescent). The protein bands were scanned and quantitated with ChemiDoc™ MP System (Bio‐Rad) and a computer program (ImageJ).
Statistical Evaluation
Prism software (GraphPad Software, Inc., La Jolla, CA, USA) was used to run all the tests. The results were analyzed by one‐way ANOVA followed by a Bonferroni post hoc test for multiple comparisons. A P value of <0.05 was considered to be statistically significant. Results are expressed as the mean ± SEM of n experiments.
Results
Body Weight Loss as Sign of Disease
Studies on animal models of EAE have demonstrated that the acute phase of the disease coincides with weight loss, probably due to anorexia and deficient fluid uptake. Weight measurement of immunized mice correlated with the severity of the clinical score and showed a significantly reduced weight loss in EAE mice compared with sham mice. A consistent difference between EAE group (Δ = −1.066 g during all the experimental period) and the other groups was observed. After EAE induction, significative body weight gain was found in EAE + bioactive R S‐GRA group (Δ = +1.35 g), compared with EAE group and sham group (Δ = +0.9 g) (Figure 2).
Bioactive R S‐GRA Modulates IL‐1β Levels
To test whether pretreatment with bioactive R S‐GRA modulates the inflammatory process through the regulation of secretion of pro‐inflammatory cytokines, we analyzed spinal cord tissue levels of IL‐1β by Western blot analysis. A basal level of IL‐1β production was found in spinal cord samples collected from EAE mice 7 days after EAE induction (Figure 3), while spinal cord levels of IL‐1β were attenuated by administration of bioactive R S‐GRA (Figure 3).
Figure 3.
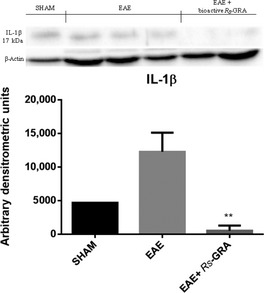
Western blot for IL‐1β. By western blot analysis, a basal level of IL‐1β was detected in spinal cord samples collected from EAE mice 7 days after EAE, while spinal cord levels of IL‐1β were attenuated by the administration of bioactive RS‐GRA. β‐actin was used as internal control. **P < 0.0072 versus EAE.
Effect of Bioactive R S‐GRA on JNK, IkB‐α Degradation, and NF‐kB p65
To investigate the cellular mechanisms whereby pretreatment with bioactive R S‐GRA attenuates the development of EAE, c‐Jun N‐terminal protein kinase (JNK) and nuclear NF‐kB expression were evaluated by Western blot analysis in spinal cord tissue. JNK levels were substantially increased in EAE mice (Figure 4A), while bioactive R S‐GRA pretreatment prevented the EAE‐induced JNK expression (Figure 4A). In addition, NF‐kB p65 levels in the nuclear fractions from spinal cord tissue were significantly increased at 7 days after EAE compared with the sham mice (Figure 4B). Bioactive R S‐GRA treatment reduced the levels of NF‐kB p65 (Figure 4B).
Figure 4.
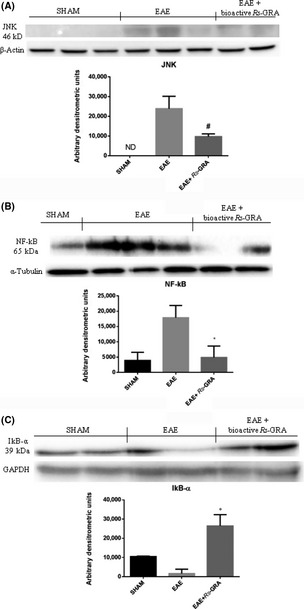
Western blot for JNK, NF‐kB p65, and IkB‐α. By Western blot analysis, JNK levels were found to be substantially increased in EAE mice (A), while bioactive R S‐GRA pretreatment prevented the EAE‐induced JNK expression (A) In addition, EAE caused a significant increase in nuclear NF‐kB p65 compared with the sham mice (B). Bioactive R S‐GRA pretreatment reduced the levels of NF‐kB p65 (B). IkB‐α detection following EAE induction shows that EAE mice present a lower IkB‐α level (sign of IkB‐α degradation) than sham animals. Bioactive R S‐GRA administration prevents the EAE‐induced IkB‐α degradation. The relative expression of the protein bands was standardized for densitometric analysis to β‐actin (A), α‐tubulin (B), and GAPDH (C) levels. *P < 0.008 versus EAE, # P < 0.0021 versus EAE, °P < 0.0142 versus EAE. ND: not detectable.
Also, we evaluated IkB‐α expression following EAE induction. In spinal cord samples from EAE mice, an IkB‐α level lower than sham animals was detected (sign of IkB‐α degradation, Figure 4C). Bioactive RS‐GRA administration prevents the EAE‐induced IkB‐α degradation (Figure 4C).
Effect of Bioactive R S‐GRA on Bax and Caspase 3 Expression
At 7 days after EAE, the appearance of pro‐apoptic protein, Bax, in spinal cord homogenates was investigated by Western blot. Bax levels were appreciably increased in spinal cord samples taken from mice subjected to EAE (Figure 5A). Conversely, bioactive R S‐GRA pretreatment prevented the EAE‐induced Bax expression (Figure 5A). Sequential activation of caspase plays a central role in the execution phase of cell apoptosis, so that using Western blot analysis, we evaluated the activation of caspase 3. Caspase 3 levels were appreciably increased in the spinal cord from mice subjected to EAE (Figure 5B). On the contrary, pretreatment with bioactive R S‐GRA attenuated the EAE‐induced caspase 3 expression (Figure 5B).
Figure 5.
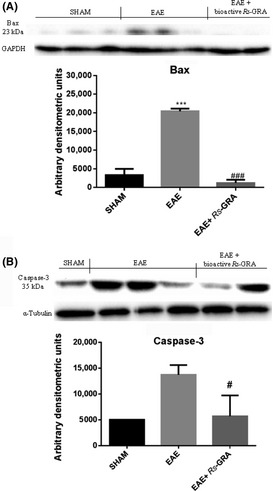
Western blot for Bax and caspase 3. Representative Western blots showing no significant Bax expression in spinal cord tissues from sham mice (A). Bax levels were appreciably increased in spinal cord from EAE mice (A). Also, spinal cord levels of Bax were attenuated by administration of bioactive RS‐GRA (A). ***P < 0.002 versus SHAM, ### P < 0.0020 versus EAE. Moreover, we also demonstrated caspase 3 activation by Western blot, and EAE caused a significant increase in caspase 3 expression (B) compared with the sham mice. Bioactive RS‐GRA reduced caspase 3 levels as shown in figure (B). GAPDH (A) and α‐tubulin (B) were used as internal control. # P < 0.0269 versus EAE. ND: not detectable.
Discussion
MS is an inflammatory demyelinating disease of the CNS, culminating in progressive neurological deterioration. Like many other autoimmune diseases, it is initiated by an uncontrolled T‐cell response to autoantigens presented in the context of class II major histocompatibility complex (MHC) molecules of antigen‐presenting cells (APCs) 3. The most widely accepted hypothesis suggests a dialogue mediated by T‐cell receptors on CD4+ T lymphocytes. These interactions between active CD4+ T cells and myelin antigens apparently provoke a massive destructive inflammatory response and promote continuing proliferation of T and B cells and macrophage activation, which sustains secretion of inflammatory mediators, for example, cytokines/chemokines 11, 36, 37.
Synthetic sulforaphane (R S ,S S‐SFN) has been extensively studied in recent years, both as chemopreventive agent and as potential novel chemotherapeutic compound 38, 39, 40, 41, 42, but this compound currently used is unstable and slightly soluble in water, so the objective of this work was to provide a therapeutic agent of easier availability.
Starting from the idea that R S‐GRA is easily available at CRA‐CIN of Bologna (Italy) together with homogeneous Myr, our goal was to demonstrate the therapeutic effects of bioactive R S‐GRA (Italian patent pending MI2012A001774), as a novel important field of action potentially applicable in inflammatory/autoimmune disease, as EAE.
Astrocytes are the major glial cell within the CNS and have a number of important physiological properties related to CNS homeostasis 43.
Bioactive R S‐GRA can mediate the astroglial response during EAE by modulation of pro‐inflammatory cytokines 44, such as IL‐1β, that, as many other inflammatory mediators, are controlled by NF‐kB. Here, we have demonstrated that therapeutic effects of bioactive R S‐GRA can modulate inflammatory pathway during EAE. It is well known that under normal conditions, NF‐kB is present within the cytoplasm in an inactive state, bound to its inhibitory protein IkB‐α. In response to a wide range of stimuli including oxidative stress, infection, hypoxia, extracellular signals, and inflammation, IkB‐α is phosphorylated by the enzyme IkB‐α kinase. Once liberated from its inhibitory protein, NF‐kB translocates to the nucleus, where it modulates the transcription of a number of pro‐inflammatory genes. Our analysis demonstrated that bioactive R S‐GRA pretreatment inhibits the IkB‐α degradation as well as the NF‐kB activation. A direct consequence of the inhibitory effect of bioactive R S‐GRA on NF‐kB activation is the reduction in pro‐inflammatory mediators production under its control, such as IL‐1β. This cytokine is produced by activated macrophages as a proprotein, which is proteolytically processed to its active form by caspase 1 45. IL‐1β plays an important role in host protection against infections, but can also promote tissue damage in chronic inflammatory diseases. Moreover, IL‐1β is involved in a variety of cellular activities, including cell proliferation, differentiation, and apoptosis.
During EAE, IL‐1β was demonstrated fundamental in the pathogenesis of inflammatory progression 45, 46 implicated in EAE, by activation of T lymphocytes and stimulation of the production of other cytokines in turn 47.
We have clearly confirmed an increase in IL‐1β during EAE. On the contrary, attenuated expression of IL‐1β was observed in mice that received bioactive R S‐GRA as previously demonstrated. A downregulation of IL‐1β in animals treated with bioactive R S‐GRA showed that one of the possible action ways of this treatment is the control of the cytokine production.
Therefore, we have shown that bioactive R S‐GRA administration is able to produce substantial reduction in inflammatory events associated with EAE.
Furthermore, once translocated in the nucleus, NF‐kB activates the transcription of genes involved in the progression of the inflammatory pathway, such as JNK.
JNK is a subfamily of the mitogen‐activated protein kinase (MAPK) superfamily. JNK was originally identified by its ability to specifically phosphorylate the transcription factor c‐Jun on its N‐terminal transactivation domain at two serine residues, Ser63 and Ser73. Kinases of the JNK group of MAPKs are primarily activated by pro‐inflammatory cytokines, such as IL‐1β and TNF‐α, and stress stimuli such as UV radiation, pH changes, hypoxia, and genotoxic and oxidative stress 48. Activation of JNK in response to these stimuli has been linked to several biological responses, including proliferation, differentiation, and apoptosis 48, 49, 50. In normal cells, the activation of JNK is an event that determines the activation of pro‐apoptotic proteins of the Bcl‐2 family, which cause an induction in the release of cytochrome c from mitochondria.
This study has demonstrated that our pharmacological pretreatment with bioactive R S‐GRA prevents the EAE‐induced JNK expression, being that JNK levels substantially decreased in EAE‐injected mice. It is conceivable a mechanism mediated by the upstream inhibition of NF‐kB p65 translocation and probably the inhibition of JNK phosphorylation.
The results obtained from analysis of all these protein parameters led us to investigate the effect of pretreatment with bioactive R S‐GRA on the apoptosis degree after EAE induction.
Apoptosis is a natural form of cell death, which can be induced by an “intrinsic” mitochondria‐mediated pathway 51. This pathway causes activation of caspases (in particular caspase 3), which, by cleavage of cellular substrates, leads to programmed cell death. Caspase 3 is a key regulator of apoptosis, essential for some of the characteristic changes in cell morphology and in some biochemical events associated with the execution and completion of this process 52. As cleaved caspase 3 is considered as marker of apoptosis, we evaluated the cleaved caspase 3 expression by Western blot analysis. We found that EAE causes significant increase in cleaved caspase 3 when compared with sham animals, whereas bioactive R S‐GRA pretreatment decreased the level of cleaved caspase 3.
Another important pro‐apoptotic factor, playing a pivotal role in developmental cell death and in CNS injury, is Bax. For this reason, we have detected pro‐apoptotic transcriptional changes and upregulation of Bax in EAE. In particular, we have shown that pretreatment with bioactive R S‐GRA reduced Bax expression, leading to a reduction in the pro‐apoptotic pathway activation. This finding suggests that the neuroprotective effect of bioactive R S‐GRA is associated with apoptosis regulation.
Finally, Canistro et al. 53 concluded their study reporting that the regular administration of extract containing dietary constituents (120 or 240 mg/kg), including glucosinolates, to healthy humans for chemopreventive purposes should be used with caution. Conversely, in our study, we evaluated the neuroprotective effect (10 mg/kg) of pure glucosinolate R S‐GRA, bioactivated with myrosinase, in an animal model of MS, and we found that the mechanisms involved were both the modulation of the inflammatory pathways and the reduction in the activation of cell death by apoptosis.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
We thank all the authors who have contributed to the manuscript. Also, the authors would like to thank the secretary office of IRCCS Centro Neurolesi “Bonino‐Pulejo”, Messina, for their excellent technical assistance, together with Dott. Ferrantelli V. and his staff of the Institute of Experimental Zooprophylaxy of Sicily “A. Mirri”, Palermo (Italy), for the experimental assistance during this study. We gratefully acknowledge also the financial support from the Italian Ministry of Agriculture, Food and Forestry Policies (Research grant, G.R.D.N.).
The first two authors contributed equally to this work.
References
- 1. Calabresi PA. Diagnosis and management of multiple sclerosis. Am Fam Physician 2004;70:1935–1944. [PubMed] [Google Scholar]
- 2. Lassmann H, Bruck W, Lucchinetti CF. The immunopathology of multiple sclerosis: An overview. Brain Pathol 2007;17:210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med 2000;343:938–952. [DOI] [PubMed] [Google Scholar]
- 4. Compston A, Coles A. Multiple sclerosis. Lancet 2008;372:1502–1517. [DOI] [PubMed] [Google Scholar]
- 5. Duquette P, Pleines J, Girard M, Charest L, Senecal‐Quevillon M, Masse C. The increased susceptibility of women to multiple sclerosis. Can J Neurol Sci 1992;19:466–471. [PubMed] [Google Scholar]
- 6. Lassmann H. Multiple sclerosis pathology: Evolution of pathogenetic concepts. Brain Pathol 2005;15:217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 2005;128:2705–2712. [DOI] [PubMed] [Google Scholar]
- 8. Brownell B, Hughes JT. The distribution of plaques in the cerebrum in multiple sclerosis. J Neurol Neurosurg Psychiatry 1962;25:315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bo L, Vedeler CA, Nyland H, Trapp BD, Mork SJ. Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler 2003;9:323–331. [DOI] [PubMed] [Google Scholar]
- 10. Keegan BM, Noseworthy JH. Multiple sclerosis. Annu Rev Med 2002;53:285–302. [DOI] [PubMed] [Google Scholar]
- 11. Lucchinetti CF, Parisi J, Bruck W. The pathology of multiple sclerosis. Neurol Clin 2005;23:77–105. [DOI] [PubMed] [Google Scholar]
- 12. Fugger L, Friese MA, Bell JI. From genes to function: The next challenge to understanding multiple sclerosis. Nat Rev Immunol 2009;9:408–417. [DOI] [PubMed] [Google Scholar]
- 13. Ebers GC. Environmental factors and multiple sclerosis. Lancet Neurol 2008;7:268–277. [DOI] [PubMed] [Google Scholar]
- 14. Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain 2006;129:1953–1971. [DOI] [PubMed] [Google Scholar]
- 15. Hemmer B, Archelos JJ, Hartung HP. New concepts in the immunopathogenesis of multiple sclerosis. Nat Rev Neurosci 2002;3:291–301. [DOI] [PubMed] [Google Scholar]
- 16. Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol 2010;162:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Waldner H, Whitters MJ, Sobel RA, Collins M, Kuchroo VK. Fulminant spontaneous autoimmunity of the central nervous system in mice transgenic for the myelin proteolipid protein‐specific T cell receptor. Proc Natl Acad Sci U S A 2000;97:3412–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bettelli E, Pagany M, Weiner HL, Linington C, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein‐specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med 2003;197:1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Litzenburger T, Fassler R, Bauer J, et al. B lymphocytes producing demyelinating autoantibodies: Development and function in gene‐targeted transgenic mice. J Exp Med 1998;188:169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Verhoeven DT, Verhagen H, Goldbohm RA, van den Brandt PA, van Poppel G. A review of mechanisms underlying anticarcinogenicity by brassica vegetables. Chem Biol Interact 1997;103:79–129. [DOI] [PubMed] [Google Scholar]
- 21. Conaway CC, Getahun SM, Liebes LL, et al. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr Cancer 2000;38:168–178. [DOI] [PubMed] [Google Scholar]
- 22. Song L, Thornalley PJ. Effect of storage, processing and cooking on glucosinolate content of Brassica vegetables. Food Chem Toxicol 2007;45:216–224. [DOI] [PubMed] [Google Scholar]
- 23. Abdull Razis AF, Bagatta M, De Nicola GR, Iori R, Ioannides C. Up‐regulation of cytochrome P450 and phase II enzyme systems in rat precision‐cut rat lung slices by the intact glucosinolates, glucoraphanin and glucoerucin. Lung cancer 2011;71:298–305. [DOI] [PubMed] [Google Scholar]
- 24. Abdull Razis AF, Iori R, Ioannides C. The natural chemopreventive phytochemical R‐sulforaphane is a far more potent inducer of the carcinogen‐detoxifying enzyme systems in rat liver and lung than the S‐isomer. Int J Cancer 2011;128:2775–2782. [DOI] [PubMed] [Google Scholar]
- 25. Fimognari C, Nusse M, Cesari R, Iori R, Cantelli‐Forti G, Hrelia P. Growth inhibition, cell‐cycle arrest and apoptosis in human T‐cell leukemia by the isothiocyanate sulforaphane. Carcinogenesis 2002;23:581–586. [DOI] [PubMed] [Google Scholar]
- 26. Leoni O, Iori R, Palmieri S. Hydrolysis of glucosinolates using nylon‐immobilized myrosinase to produce pure bioactive molecules. Biotechnol Bioeng 2000;68:660–664. [DOI] [PubMed] [Google Scholar]
- 27. Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1‐Nrf2‐ARE pathway. Annu Rev Pharmacol Toxicol 2007;47:89–116. [DOI] [PubMed] [Google Scholar]
- 28. Hayes JD, Kelleher MO, Eggleston IM. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur J Nutr 2008;47:73–88. [DOI] [PubMed] [Google Scholar]
- 29. Chittenden T, Harrington EA, O'Connor R, et al. Induction of apoptosis by the Bcl‐2 homologue Bak. Nature 1995;374:733–736. [DOI] [PubMed] [Google Scholar]
- 30. White FA, Keller‐Peck CR, Knudson CM, Korsmeyer SJ, Snider WD. Widespread elimination of naturally occurring neuronal death in Bax‐deficient mice. J Neurosci 1998;18:1428–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abdull Razis AF, Bagatta M, De Nicola GR, Iori R, Ioannides C. Intact glucosinolates modulate hepatic cytochrome P450 and phase II conjugation activities and may contribute directly to the chemopreventive activity of cruciferous vegetables. Toxicology 2010;277:74–85. [DOI] [PubMed] [Google Scholar]
- 32. Wagner AE, Ernst I, Iori R, Desel C, Rimbach G. Sulforaphane but not ascorbigen, indole‐3‐carbinole and ascorbic acid activates the transcription factor Nrf2 and induces phase‐2 and antioxidant enzymes in human keratinocytes in culture. Exp Dermatol 2010;19:137–144. [DOI] [PubMed] [Google Scholar]
- 33. EEC Regulation 1864/90, Enclosure VIII. Off J Eur Communities 1990; L170: 27–34. [Google Scholar]
- 34. Pessina A, Thomas RM, Palmieri S, Luisi PL. An improved method for the purification of myrosinase and its physicochemical characterization. Arch Biochem Biophys 1990;280:383–389. [DOI] [PubMed] [Google Scholar]
- 35. Rodrigues DH, Vilela MC, Barcelos LS, Pinho V, Teixeira MM, Teixeira AL. Absence of PI3Kgamma leads to increased leukocyte apoptosis and diminished severity of experimental autoimmune encephalomyelitis. J Neuroimmunol 2010;222:90–94. [DOI] [PubMed] [Google Scholar]
- 36. Minagar A, Alexander JS. Blood‐brain barrier disruption in multiple sclerosis. Mult Scler 2003;9:540–549. [DOI] [PubMed] [Google Scholar]
- 37. Bruck W. The pathology of multiple sclerosis is the result of focal inflammatory demyelination with axonal damage. J Neurol 2005;252:3–9. [DOI] [PubMed] [Google Scholar]
- 38. Ahn YH, Hwang Y, Liu H, et al. Electrophilic tuning of the chemoprotective natural product sulforaphane. Proc Natl Acad Sci U S A 2010;107:9590–9595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Conaway CC, Wang CX, Pittman B, et al. Phenethyl isothiocyanate and sulforaphane and their N‐acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res 2005;65:8548–8557. [DOI] [PubMed] [Google Scholar]
- 40. Cornblatt BS, Ye L, Dinkova‐Kostova AT, et al. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis 2007;28:1485–1490. [DOI] [PubMed] [Google Scholar]
- 41. Dinkova‐Kostova AT, Jenkins SN, Fahey JW, et al. Protection against UV‐light‐induced skin carcinogenesis in SKH‐1 high‐risk mice by sulforaphane‐containing broccoli sprout extracts. Cancer Lett 2006;240:243–252. [DOI] [PubMed] [Google Scholar]
- 42. Talalay P, Fahey JW, Healy ZR, et al. Sulforaphane mobilizes cellular defenses that protect skin against damage by UV radiation. Proc Natl Acad Sci U S A 2007;104:17500–17505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Markiewicz I, Lukomska B. The role of astrocytes in the physiology and pathology of the central nervous system. Acta Neurobiol Exp 2006;66:343–358. [DOI] [PubMed] [Google Scholar]
- 44. Aloisi F, Ria F, Adorini L. Regulation of T‐cell responses by CNS antigen‐presenting cells: Different roles for microglia and astrocytes. Immunol Today 2000;21:141–147. [DOI] [PubMed] [Google Scholar]
- 45. Neuhaus O, Hartung HP. In search of a disease marker: The cytokine profile of primary progressive multiple sclerosis. Mult Scler 2001;7:143–144. [DOI] [PubMed] [Google Scholar]
- 46. Link H. The cytokine storm in multiple sclerosis. Mult Scler 1998;4:12–15. [DOI] [PubMed] [Google Scholar]
- 47. McGeachy MJ, Anderton SM. Cytokines in the induction and resolution of experimental autoimmune encephalomyelitis. Cytokine 2005;32:81–84. [DOI] [PubMed] [Google Scholar]
- 48. Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell 2000;103:239–252. [DOI] [PubMed] [Google Scholar]
- 49. Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature 2001;410:37–40. [DOI] [PubMed] [Google Scholar]
- 50. Kennedy NJ, Davis RJ. Role of JNK in tumor development. Cell Cycle 2003;2:199–201. [PubMed] [Google Scholar]
- 51. Green DR, Reed JC. Mitochondria and apoptosis. Science 1998;281:1309–1312. [DOI] [PubMed] [Google Scholar]
- 52. Porter AG, Janicke RU. Emerging roles of caspase‐3 in apoptosis. Cell Death Differ 1999;6:99–104. [DOI] [PubMed] [Google Scholar]
- 53. Canistro D, Barillari J, Melega S, et al. Black cabbage seed extract affects rat Cyp‐mediated biotransformation: Organ and sex related differences. Food Chem Toxicol 2012;50:2612–2621. [DOI] [PubMed] [Google Scholar]


