Stroke is the second most common cause of death and a leading cause of adult disability worldwide 1, 2. Endothelial dysfunction has been observed in stroke patients and has been related to stroke physiopathology, clinical severity, and outcome 3. Endothelial progenitor cells (EPCs) are bone marrow‐derived cells that are mobilized to the peripheral circulation when vascular repair and neovascularization are required. EPCs play a critical role in maintaining endothelial function and might affect the progression of vascular disease 4.
Assays that measure EPC number and function may provide important information for the risk stratification of stroke. It has been demonstrated that the tube formation assay is a robust, rapid, reproducible, and comprehensive method 5. However, bone marrow (BM)‐EPCs' ability to form tubular‐like network remains controversial. The overwhelming majority of the works assessed the tube formation function of BM‐EPCs by coplating with mature endothelial cells, such as human umbilical vein endothelial cells (HUVECs) 6, 7. In this study, we established a BM‐EPCs tube formation method, which is able to generate tubular‐like network on Matrigel without coplating with mature endothelial cells.
Mouse bone marrow‐derived EPCs were isolated and cultured according to described technique 8. Cultured BM‐EPCs were characterized as cells coexpressing Sca‐1 and Flk‐1 (BD Pharmingen, San Diego, CA, USA) under flow cytometry examination. To confirm the BM‐EPCs phenotypes, cells were stained for the uptake of Dil‐acLDL (Molecular Probes Inc., Eugene, OR, USA) and FITC‐labeled Ulex europaeus agglutinin (lectin; Sigma‐Aldrich, St. Louis, MO, USA). The in vitro angiogenic activity of BM‐EPCs was determined by Matrigel tube formation assay. Briefly, BM‐EPCs were replated at the density of 30,000, 60,000, 120,000, and 180,000 cells per cm2 (10,000, 20,000, 40,000 and 60,000 cells per well) in 96‐well plates precoated with 50 μl/well growth factor‐reduced Matrigel (BD Biosciences, Bedford, MA, USA). Tube formation ability of BM‐EPCs was assessed by tubes number. Tube was defined as a structure exhibiting a length 4 times its width 9. Values are presented as mean ± SD. Multiple comparisons involving more than three groups were performed using one‐way ANOVA. Post hoc comparisons were performed with the Neuman–Keuls test. A value of P < 0.05 was considered statistically significant.
Mouse BM‐EPCs grew to confluence and displayed a typical spindle‐like morphology (Figure 1A). Flow cytometric analysis showed about 17.3% of cell population was positive for Flk‐1 and Sca‐1 (Figure 1B). Characterization of BM‐EPCs was further confirmed as Dil‐acLDL and lectin double‐positive adherent cells under a fluorescence microscope (Figure 1C).
Figure 1.
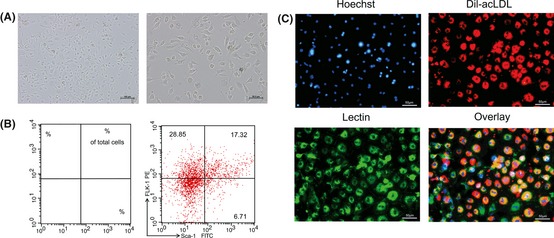
Characterization of mouse bone marrow‐endothelial progenitor cells (BM‐EPCs). (A) Representative photographs of BM‐EPCs after 7 days culture. Scale bars: Left, 100 μm; Right, 50 μm. (B) Cell phenotypes were assessed by flow cytometric analysis with stem (Sca‐1) and endothelial (Flk‐1) cell markers. (C) BM‐EPCs were further confirmed as Dil‐acLDL (red) and lectin (green) double‐positive cells under a fluorescence microscope. Nuclei were counterstained with Hoechst (blue). Scale bar: 50 μm.
Examples of BM‐EPCs tube formation on top of the gelled Matrigel are presented in Figure 2. Successful development of the dispersed BM‐EPCs to tubular‐like network mainly depends on both the culture time and the cell density on the Matrigel. Plated BM‐EPCs initially attached on the Matrigel, then migrated toward each other over the next 2–4 h, and gradually formed tubular‐like network, which matured by 6–9 h. After that, the network was prone to detach from the Matrigel and break apart (Figure 2A). Cell density is another critical determinant. Inadequate cell density yielded incomplete networks, while too many cells resulted in large areas of monolayers. The optimal cell density was found to be about 120,000 cells/cm2 (40,000 cells per well on a 96‐well plates, Figure 2B).
Figure 2.
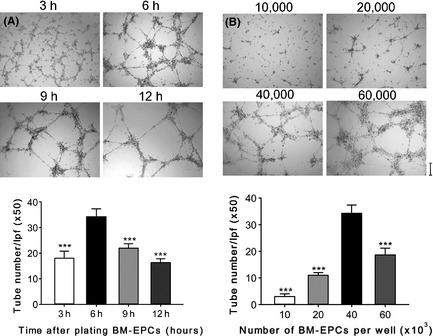
Dynamics of the behavior of bone marrow‐endothelial progenitor cells (BM‐EPCs) after plating on the Matrigel. (A) Mouse BM‐EPCs tube formation on Matrigel with time. (B) Mouse BM‐EPCs tube formation on Matrigel with cell density. Data are expressed as the mean tubes number/LPF (×50) ± SD of three independent experiments; ***P < 0.001 vs. 6 h group (A) or vs. 40,000 cells per well group (B). Scale bar: 200 μm.
In summary, it was found that the preferred density on Matrigel is 120,000 cells per cm2, and the optimal observing time for tube formation is 6 h after seeding. Our results, that mouse BM‐EPCs without coplating with mature endothelial cells are able to generate tubular‐like network on Matrigel, suggest that BM‐EPCs may structurally contribute to growing vessels and provide an insight into better understanding the nature of BM‐EPCs.
Conflict of Interest
The authors declare no conflict of interests.
Acknowledgments
This study was supported by the grants from National Natural Science Foundation of China (81070118, 81170115) and the Natural Science Foundation of Zhejiang (2012C33108).
References
- 1. Cushman M, Cantrell RA, McClure LA, et al. Estimated 10‐year stroke risk by region and race in the United States: Geographic and racial differences in stroke risk. Ann Neurol 2008;64:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yu JG, Zhou RR, Cai GJ. From hypertension to stroke: Mechanisms and potential prevention strategies. CNS Neurosci Ther 2011;17:577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roquer J, Segura T, Serena J, Castillo J. Endothelial dysfunction, vascular disease and stroke: The ARTICO study. Cerebrovasc Dis 2009;27(Suppl 1):25–37. [DOI] [PubMed] [Google Scholar]
- 4. Urbich C, Dimmeler S. Endothelial progenitor cells: Characterization and role in vascular biology. Circ Res 2004;95:343–353. [DOI] [PubMed] [Google Scholar]
- 5. Staton CA, Reed MW, Brown NJ. A critical analysis of current in vitro and in vivo angiogenesis assays. Int J Exp Pathol 2009;90:195–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Groleau J, Dussault S, Haddad P, et al. Essential role of copper‐zinc superoxide dismutase for ischemia‐induced neovascularization via modulation of bone marrow‐derived endothelial progenitor cells. Arterioscler Thromb Vasc Biol 2010;30:2173–2181. [DOI] [PubMed] [Google Scholar]
- 7. Jung SY, Choi JH, Kwon SM, Masuda H, Asahara T, Lee YM. Decursin inhibits vasculogenesis in early tumor progression by suppression of endothelial progenitor cell differentiation and function. J Cell Biochem 2012;113:1478–1487. [DOI] [PubMed] [Google Scholar]
- 8. Xie HH, Zhou S, Chen DD, Channon KM, Su DF, Chen AF. GTP cyclohydrolase I/BH4 pathway protects EPCs via suppressing oxidative stress and thrombospondin‐1 in salt‐sensitive hypertension. Hypertension 2010;56:1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tepper OM, Galiano RD, Capla JM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 2002;106:2781–2786. [DOI] [PubMed] [Google Scholar]


