SUMMARY
Background: Stroke is the second most common cause of death and a major cause of disability worldwide. Risperidone is an atypical antipsychotic drug that may increase the risk of stroke. The present work examined whether risperidone enhances the vulnerability to stroke in hypertensive rats and the potential mechanisms underlying such action. Methods: Experiment 1: Wistar‐Kyoto (WKY) rats, spontaneously hypertensive rats (SHRs) and stroke‐prone SHRs (SHR‐SPs) were treated with risperidone (0.8 and 2.4 mg/kg/d) or vehicle for 30 consecutive days. Tissue damage in response to middle cerebral artery occlusion (MCAO) was measured microscopically. The activity of superoxide dismutase, glutathione peroxidase, the levels of malondialdehyde were also determined. Experiment 2: Survival data were recorded in SHR‐SPs that received daily risperidone perpetually. Experiment 3: Effect of risperidone on interleukin‐6 and tumor necrosis factor‐α was examined in quiescent or LPS‐activated cortical microglias from WKY rats. Experiment 4: Potential damage of risperidone exposure to neurons was examined in primary neuronal culture obtained from WKY rats, SHRs, and SHR‐SPs. Results: Risperidone increased infarct areas upon MCAO in SHR‐SPs and SHRs, but not in WKY rats. Survival time in SHR‐SPs was shortened by risperidone. Apoptosis was augmented by risperidone through enhanced Bax. Risperidone also increased endothelial injury. Conclusions: Risperidone enhances the vulnerability to stroke in hypertensive rats through increasing neuronal apoptosis and endothelial injury.
Keywords: Apoptosis, Hypertension, Risperidone, Stroke
Introduction
Stroke is the second most common cause of death and a major cause of disability worldwide [1, 2, 3, 4]. According to a recent report published by the World Health Organization, about 15 million people per year fall victim to stroke, of whom 5 million die and another 5 million are left permanently disabled [5]. Many stroke survivors require lifelong assistance.
Risperidone is an atypical antipsychotic drug that alleviates both positive and negative symptoms and improves cognitive function in schizophrenic patients [6, 7, 8]. Risperidone produces a series of side effects, including extrapyramidal symptoms, hyperprolactinemia, and weight gain [9]. It could also unmask preexisting type 2 diabetes [9]. Clinical studies in the last decade indicated that risperidone may increase the risk of stroke [10, 11, 12]. Such a concern led to official warnings by the FDA [13, 14, 15, 16] and many debates [17, 18, 19].
In the current study, we compared the effects of risperidone in normotensive Wistar‐Kyoto (WKY) rats, spontaneously hypertensive rats (SHRs) and stroke‐prone spontaneously hypertensive rats (SHR‐SPs). Results showed that risperidone sensitized the brain to middle cerebral artery occlusion (MCAO) injury in hypertensive rats. Risperidone also shortened the survival time of SHR‐SPs. Oxidative stress, inflammation, apoptosis, and endothelial injury were examined as potential contributing mechanisms.
Methods
Animals
WKY rats, SHRs, and SHR‐SPs were provided by the Animal Center of the Second Military Medical University, and housed at 23–25°C, with 12/12 h lighting schedule (8:00–20:00 light, 20:00–8:00 dark) and free access to standard food and drinking water. All animal subjects used in this experiment received humane care in compliance with institutional guidelines for health and care of experimental animals.
Protocol
Experiment 1: Effect of Risperidone on Brain Damage upon MCAO
WKY rats, SHRs, and SHR‐SPs (20–22 weeks of age) received daily risperidone (Hengyuan Co., Huzhou, China; 0.8 or 2.4 mg/kg, through food) or vehicle for 30 consecutive days (n = 10–20 per group). Risperidone was milled into rat chow to avoid stress associated with gastric gavage. Food consumption was monitored daily to ensure accurate dosing. Systolic blood pressure (SBP) was measured at the end of the dosing regimen using tail‐cuff plethysmography [20], before MCAO (methods in supplement) [21, 22, 23]. The infarct area was examined 24 h after MCAO. The activity of superoxide dismutase (SOD), glutathione peroxidase (GSH‐Px), and the levels of malondialdehyde (MDA) in the mitochondrial fraction were determined (methods in supplement) [24, 25]. Cerebral ultramicroscopic structure morphology was observed using an electron microscope (methods in supplement) [26].
Experiment 2: Effect of Risperidone on Life Span
SHR‐SPs (20–22 weeks of age) received daily risperidone (0.8 or 2.4 mg/kg, through food) or vehicle for lifelong treatment (n = 15 per group). Rats were observed twice daily (at 8 AM and 6 PM).
Experiment 3: Effect of Risperidone on Inflammation in Cortical Microglias
Effects of risperidone on proinflammatory cytokines were examined in primary cultures of cortical microglias (methods in supplement) [27] from WKY rats. In brief, cultured microglia (quiescent vs. LPS activated, 1 μg/mL) were exposed to risperidone (0.3, 1, 3, 30 μM) or vehicle (0.4% DMSO) for 1 h. Interleukin‐6 (IL‐6) and tumor necrosis factor‐α (TNF‐α) was examined with ELISA kits (Shanghai Transhold Tech. Dev. Co. Ltd, Shanghai, China) [28].
Experiment 4: Effects of Risperidone on Cell Viability and Apoptosis of Neurons
Primary neuronal culture was established from fetal WKY rats, SHRs, and SHR‐SPs (methods in supplement) [29]. Cultured neurons were treated with risperidone (30, 60, 90 μM) or vehicle for 1 h before oxygen–glucose deprivation (OGD) or exposure to hydrogen peroxide (H2O2; 100 μM; methods in supplement) [30]. The cell viability was examined by MTT assay (methods in supplement) [31]. Apoptosis was examined by flow cytometry (methods in supplement) [32] and TUNEL (methods in supplement) [33]. Expression of Bax, Bcl‐2, and caspase‐8 was examined by Western blot (methods in supplement) [34].
Statistical Analysis
Investigators were blind to the procedures during blood pressure recording, weighing and morphological examination. Data are expressed as mean ± SD, and were analyzed with ANOVA, followed by Dunnett t‐test for post hoc comparison. Kaplan–Meier analysis was used to estimate survival probabilities. Log‐Rank testing was used to evaluate equality of survival curves. P< 0.05 was considered statistically significant.
Results
Risperidone dose dependently increased the infarct area induced by MCAO in SHR‐SPs (from 14.8%± 1.5% in control rats to 19.3 ± 2.7% and 21.3 ± 1.9% in rats receiving 0.8 and 2.4 mg/kg/d risperidone, respectively; P= 0.0263 and P= 0.0001, respectively; Figure 1A). Similar findings were observed in SHRs but not in WKY rats (Figure 1A).
Figure 1.
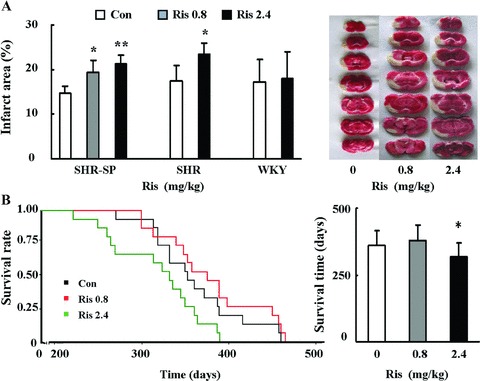
Effects of risperidone on infarct area after MCAO and on life span. (A) Effect of 1‐month treatment of risperidone on brain ischemia after MCAO in SHR‐SPs, SHRs, and WKY rats. Con, control group; Ris 0.8, risperidone 0.8 mg/kg/d; Ris 2.4, risperidone 2.4 mg/kg/d. Dunnett t‐test for SHR‐SPs, unpaired Student's t‐test for SHRs and WKY rats, *P < 0.05, **P < 0.01 for treatment group versus control group. The y‐axis is the percentage of infarct area in cerebral hemisphere. The figures on the right side show the infarct area in each group in SHR‐SPs. n = 10 in each group. (B) Effect of risperidone on the life span of SHR‐SPs. Data are analyzed by Kaplan–Meier survival curves. n = 15 in each group. Log‐Rank test, *P < 0.05 for treatment Ris 2.4 group versus control group.
Risperidone did not influence the body weight and SBP in any rat strain (Figure S1A). Activity of SOD, GSH‐Px, and the level of MDA in ischemic brain tissue were not affected (Figure S2).
Lifelong treatment of risperidone decreased the life span of SHR‐SPs at a dose of 2.4 mg/kg/d (318 ± 53 days vs. 362 ± 53 days in the control rats; P= 0.0472, log‐rank test), but not at a lower dose of 0.8 mg/kg/d (379 ± 58 days vs. 362 ± 53 days in the control rats; P= 0.3368, log‐rank test; Figure 1B). In nearly all subjects, death was caused by stroke as verified by symptoms and/or postmortem morphology observation.
Risperidone did not affect the expression of IL‐6 and TNF‐α in quiescent or LPS‐induced microglias of WKY rats (Figure S2).
Risperidone dose dependently increased the number of apoptic cells induced by OGD or H2O2 exposure and attenuated the cell viability in cultured neurons obtained from SHRs and SHR‐SPs (more significantly in latter), but not in neurons obtained from WKY rats (flow cytometry, Figure 2A; MTT, Figure 2B; TUNEL, Figure 3). Risperidone did not affect cell viability (Figure S3A) and apoptosis (Figure S3B) in cultured cells not exposed to OGD or H2O2.
Figure 2.

Effects of risperidone in neuronal culture. (A) Effect of risperidone on cultured neurons exposed to H2O2: results from flow cytometry (n = 4 in each group). The histogram shows the percentage of apoptotic cells in the treatment groups versus the control group. Flow cytometry analysis of cell apoptosis (Annexin V staining, R2 + R3) and necrosis (PI staining, R3 + R4), R1, alive cells; R2, early apoptotic cells (Annexin V–positive, PI‐negative); R3, late apoptotic or necrotic cells (Annexin V–positive, PI‐positive); R4, dead cells and cell fraction (Annexin V–negative, PI‐positive). (B) Effect of risperidone on neurons exposed to OGD (upper) and H2O2 (lower): results from MTT assay (n = 6 in each group). The values of MTT absorbance and apoptosis rates are expressed as mean ± SD. Dunnett t‐test, *P < 0.05, **P < 0.01 for treatment group versus control group.
Figure 3.
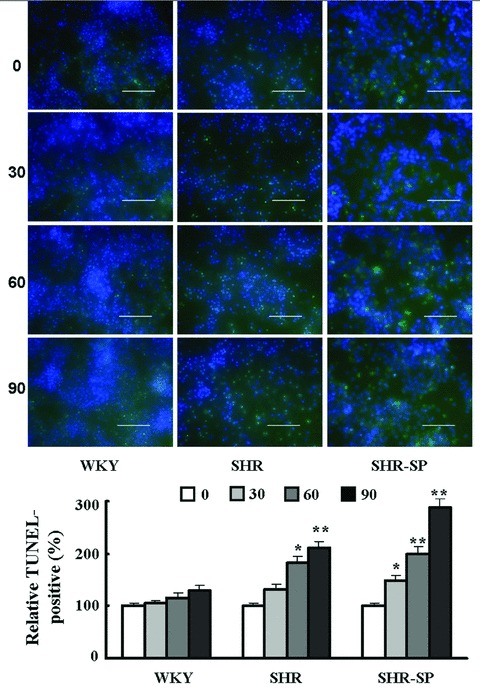
Effects of risperidone on neurons exposed to H2O2: TUNEL labeling. Green: TUNEL‐positive neurons; blue: DAPI‐labeled neurons. The histogram shows the TUNEL‐positive percentage in treatment groups versus the control group. The values of percentages are expressed as mean ± SD. Dunnett t‐test, *P < 0.05, **P < 0.01 for treatment group versus control group (n = 6 in each group). Scale bars = 50 μm.
MCAO resulted in mitochondrial swelling in neurons, microglia, and capillary endothelial damage (Figure 4A). Risperidone increased capillary endothelial vacuoles and edema around the capillaries, aggravated the mitochondrial swelling, ridge loss and membrane rupture, chromatin aggregation, and lysosomes formation. Such actions were more significant in SHR‐SPs than in WKY rats.
Figure 4.

Effects of risperidone on cerebral ultramicroscopic structure in WKY rats and SHR‐SPs under a Hitachi H‐7000 electron microscope. Con, control group; Ris, risperidone 2.4 mg/kg/d group; MCAO, MCAO in control group; Ris+MCAO, MCAO in risperidone 2.4 mg/kg/d group. The injury of capillary endothelium and mitochondrion were shown in panels A and B, respectively. Figures C1, C2, C5, C6: neuronal nuclei; Figures C3, C4, C7, C8: number of lysosomes. Scale bars = 1 μm.
Risperidone increased the expression of Bax dose dependently (from 1.0 ± 0.15 in control group to 1.79 ± 0.21, 2.30 ± 0.30, and 2.89 ± 0.35 in groups receiving 30, 60, and 90 μM risperidone, respectively; P= 0.0395, P= 0.0063, and P= 0.0012, respectively; Figure 5A). The expression of Bcl‐2 and caspase‐8 was not affected (Figures 5B and C).
Figure 5.
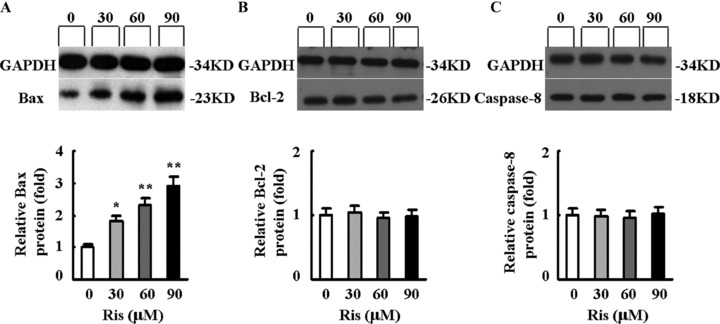
Effects of risperidone on the expression of Bax (A), Bcl‐2 (B), and caspase‐8 (C) in neurons exposed to H2O2 by Western blot analysis. The protein expression in the control group was set at 1. The values of protein expression are expressed as mean ± SD. Dunnett t‐test, *P < 0.05, **P < 0.01 for treatment group versus control group. n = 3 in each group.
Discussion
The main findings of the present work may be summarized as: (1) risperidone increases the infarct area upon MCAO in SHR‐SPs and SHRs, but not in WKY rats; (2) risperidone shortens the life span of SHR‐SPs; (3) risperidone aggravates neuron apoptosis in SHR‐SPs and SHRs, but not in WKY rats.
The risk of stroke associated with risperidone remains controversial up to date [17, 35, 36, 37, 38]. In SHR‐SPs, a widely used stroke model, risperidone increased the infarct area after MCAO, aggravated the damage to cerebral ultramicroscopic structure, and shortened the life span. Similar results were found in SHRs, but not in normotensive WKY rats. These findings were if clinical relevance: risperidone should be avoided in hypertensive patients.
Hypertension is a modifiable factor for stroke [3]. Risperidone is not powerful enough to induce stroke independently, but may aggravate the effects of hypertension on the development of stroke.
Results from the current study clearly indicated that risperidone could aggravate cerebral capillary damage. Risperidone did not influence the expression of proinflammatory cytokines, IL‐6 and TNF‐α and the oxidative stress index, SOD, MDA, GSH‐Px.
Risperidone significantly promoted neuronal apoptosis in primary culture obtained from SHRs and SHR‐SPs but not normotensive WKY rats. There are two major pathways for apoptosis [39, 40]. The extrinsic pathway is activated by the death receptors that results in rapid activation of the initiator caspase‐8. The intrinsic pathway is induced by stress‐inducing stimuli that results in perturbation of mitochondria and the ensuing release of cytochrome c from the intermitochondrial membrane space. The release of cytochrome c is regulated in part by Bcl‐2 family members, with antiapoptotic (Bcl‐2/Bcl‐XL/Mcl1) and proapoptotic (Bax, Bak, and tBid) members inhibiting and promoting the release, respectively. The release of cytochrome c results in activation of the initiator caspase‐9. The activated initiator caspase‐8 and caspase‐9 then activate the effector caspases cascade, resulting in the classical biochemical and morphological changes associated with the apoptotic phenotype [41, 42]. In our experiments, risperidone increased the expression of Bax, but did not affect the expression of Bcl‐2 and caspase‐8. These results suggest that risperidone aggravates neuron apoptosis through the intrinsic pathway.
In conclusion, risperidone could enhance the vulnerability to stroke in hypertensive rats via activating the intrinsic apoptotic pathway in neurons as well as endothelium injury.
Author Contribution
The first two authors contributed equally to this study.
Disclosures
None.
Conflict of Interest
The authors have no conflict of interest.
Supporting information
Figure S1 Effects of risperidone on body weight and SBP in WKY rats, SHRs, and SHR‐SPs. Con, control group; Ris 2.4, risperidone 2.4 mg/kg/d. n = 10 in each group. The values of body weight and SBP are expressed as mean ± SD. Unpaired Student's t‐test, P > 0.05 for treatment group versus control group.
Figure S2 Effects of risperidone on inflammation and oxidative stress. (A) Effect of risperidone on expression of IL‐6 and TNF‐α in quiescent microglias from WKY rats at 0, 0.3, 1, 3, 30 mg/kg. n = 3 in each group. (B) Effect of risperidone on expression of IL‐6 and TNF‐α in LPS‐induced microglias from WKY rats at 0, 0.3, 1, 3, 30 mg/kg. n = 3 in each group. (C) Effect of risperidone on the activity of SOD, GSH‐Px and level of MDA in WKY rats and SHR‐SPs after MCAO. n = 3 in each group. Con, control group; Ris 2.4, risperidone 2.4 mg/kg/d. The values of IL‐6, TNF‐α, MDA, SOD, and GSH‐Px are expressed as mean ± SD. Dunnett t‐test for parts A and B, unpaired Student's t‐test for part C, P > 0.05 for treatment group versus control group.
Figure S3 Effect of risperidone in cerebral cortex neurons not exposed to OGD or H2O2. (A) Effect of risperidone (0, 30, 60, 90 μM) on neurotoxic potential in normal cerebral cortex neurons from SHR‐SPs and WKY rats was examined by flow cytometry (n = 4 in each group). The histogram shows the percentage of apoptotic rate in treatment groups versus the control. Flow cytometry analysis of cell apoptosis (Annexin V staining, R2 + R3) and necrosis (PI staining, R3 + R4), R1, alive cells; R2, early apoptotic cells (Annexin V–positive, PI‐negative); R3, late apoptotic or necrotic cells (Annexin V–positive, PI‐positive); R4, dead cells and cell fraction (Annexin V–negative, PI‐positive). (B) Effect of risperidone (0, 30, 60, 90 μM) on neurotoxic potential in normal cerebral cortex neurons from SHR‐SPs and WKY rats was examined by MTT (n = 6 in each group). The values of MTT and apoptosis rates are expressed as mean ± SD. Dunnett t‐test, P > 0.05 for treatment group versus control group.
Supporting info item
Supporting info item
Supporting info item
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (30730106) and National Basic Research Program of China (973 Program, 2009CB521901).
References
- 1. Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet 2008;371:1612–1623. [DOI] [PubMed] [Google Scholar]
- 2. Cushman M, Cantrell RA, McClure LA, et al Estimated 10‐year stroke risk by region and race in the United States: Geographic and racial differences in stroke risk. Ann Neurol 2008;64:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yu JG, Zhou RR, Cai GJ. From hypertension to stroke: mechanisms and potential prevention strategies. CNS Neurosci Ther 2011;17:577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang P, Tian WW, Song J, Guan YF, Miao CY. Deficiency of NG2+ cells contributes to the susceptibility of stroke‐prone spontaneously hypertensive rats. CNS Neurosci Ther 2011;17:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mackay J, Mensah G. The atlas of heart disease and stroke. Geneva , Switzerland : World Health Organization, 2004. [Google Scholar]
- 6. Pajonk FG. Risperidone in acute and long‐term therapy of schizophrenia–a clinical profile. Prog Neuropsychopharmacol Biol Psychiatry 2004;28:15–23. [DOI] [PubMed] [Google Scholar]
- 7. Janssen PA, Niemegeers CJ, Awouters F, Schellekens KH, Megens AA, Meert TF. Pharmacology of risperidone (R 64 766), a new antipsychotic with serotonin‐S2 and dopamine‐D2 antagonistic properties. J Pharmacol Exp Ther 1988;244:685–693. [PubMed] [Google Scholar]
- 8. Schotte A, Janssen PF, Gommeren W, et al Risperidone compared with new and reference antipsychotic drugs: In vitro and in vivo receptor binding. Psychopharmacology (Berl) 1996;124:57–73. [DOI] [PubMed] [Google Scholar]
- 9. Wooltorton E. Risperidone (Risperdal): Increased rate of cerebrovascular events in dementia trials. Can Med Assoc J 2002;167:1269–1270. [PMC free article] [PubMed] [Google Scholar]
- 10. Douglas IJ, Smeeth L. Exposure to antipsychotics and risk of stroke: Self controlled case series study. BMJ 2008;337:616–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gill SS, Rochon PA, Herrmann N, et al Atypical antipsychotic drugs and risk of ischemic stroke: Population based retrospective cohort study. BMJ 2005;330:445–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herrmann N, Mamdani M, Lanctot KL. Atypical antipsychotics and risk of cerebrovascular accidents. Am J Psychiatry 2004;161:1113–1115. [DOI] [PubMed] [Google Scholar]
- 13. Dorsey ER, Rabbani A, Gallagher SA, Conti RM, Alexander GC. Impact of FDA black box advisory on antipsychotic medication use. Arch Intern Med 2010;170:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Committee on Safety of Medicines . Atypical antipsychotic drugs and stroke: Message from Professor Gordon Duff, chairman, (CEM/CMO/2004/1). London : Committee on Safety of Medicines, 2006. [Google Scholar]
- 15. Mowat D, Fowlie D, MacEwan T. CSM warning on atypical psychotics and stroke may be detrimental for dementia. BMJ 2004;328:1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. European Pharmacovigilance Working Party (PhVWP) . Public assessment report on antipsychotics and cerebrovascular accident. London : Medicines and Healthcare Products Regulatory Agency, 2006. [Google Scholar]
- 17. Curtis D. Effect of antipsychotics on stroke risk remains unproved. BMJ 2008;337:647. [DOI] [PubMed] [Google Scholar]
- 18. Rosack J. Antipsychotics do not appear to increase stroke risk. Psychiatric News 2004;39:22–23. [Google Scholar]
- 19. Pratt NL, Roughead EE, Ramsay E, Salter A, Ryan P. Risk of hospitalization for stroke associated with antipsychotic use in the elderly: A self‐controlled case series. Drugs Aging 2010;27:885–893. [DOI] [PubMed] [Google Scholar]
- 20. Song SW, Liu AJ, Bai C et al. Blood pressure reduction combining baroreflex restoration for stroke prevention in hypertension in rats. Front Pharmacol 2010;1:6. doi: 10.3389/fphar.2010.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wiart M, Davoust N, Pialat JB, et al MRI monitoring of neuroinflammation in mouse focal ischemia. Stroke 2007;38:131–137. [DOI] [PubMed] [Google Scholar]
- 22. Zhu W, Fan Y, Hao Q, et al Postischemic IGF‐1 gene transfer promotes neurovascular regeneration after experimental stroke. J Cereb Blood Flow Metab 2009;29:1528–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Swanson RA, Morton MT, Tsao‐Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab 1990;10:290–293. [DOI] [PubMed] [Google Scholar]
- 24. Xia Z, Luo T, Liu HM, Wang F, Xia ZY, Irwin MG, Vanhoutte PM. L‐Arginine enhances nitrative stress and exacerbates TNF‐Alpha toxicity to human endothelial cells in culture: Prevention by propofol. J Cardiovasc Pharmacol 2010;55:358–367. [DOI] [PubMed] [Google Scholar]
- 25. Zhang XH, Lei H, Liu AJ, Zou YX, Shen FM, Su DF. Increased oxidative stress is responsible for severer cerebral infarction in stroke‐prone spontaneously hypertensive rats. CNS Neursci Ther 2011;17:590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li DJ, Evans R, Yang ZW, et al Dysfunction of cholinergic anti‐inflammatory pathway in hypertensive rats. Hypertension 2011;57:298–307. [DOI] [PubMed] [Google Scholar]
- 27. Zhang W, Shin EJ, Wang T, et al 3‐Hydroxymorphinan, a metabolite of dextromethorphan, protects nigrostriatal pathway against MPTP‐elicited damage both in vivo and in vitro. FASEB 2006;20:2496–2511. [DOI] [PubMed] [Google Scholar]
- 28. Unal‐Cevik I, Kılınc M, Can A, Gursoy‐Ozdemir Y, Dalkara T. Apoptotic and necrotic death mechanisms are concomitantly activated in the same cell after cerebral ischemia. Stroke 2004;35:2189–2194. [DOI] [PubMed] [Google Scholar]
- 29. Ukai W, Ozawa H, Tateno M, Hashimoto E, Saito T. Neurotoxic potential of haloperidol in comparison with risperidone: Implication of Akt‐mediated signal changes by haloperidol. J Neural Transm 2004;111:667–681. [DOI] [PubMed] [Google Scholar]
- 30. Wang P, Xu TY, Guan YF, et al Nampt protects against ischemic stroke through SIRT1‐dependent AMPK pathway. Ann Neurol 2011;69:360–374. [DOI] [PubMed] [Google Scholar]
- 31. Ma H, Guo R, Yu L, Zhang Y, Ren J. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: Role of autophagy paradox and toxic aldehyde. Eur Heart J 2011;32:1025–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lev EI, Leshem‐Lev D, Mager A, et al Circulating endothelial progenitor cell levels and function in patients who experienced late coronary stent thrombosis. Eur Heart J 2010;31:2625–2632. [DOI] [PubMed] [Google Scholar]
- 33. Vogler M, Walczak H, Stadel D, et al Small molecule XIAP inhibitors enhance TRAIL‐induced apoptosis and antitumor activity in preclinical models of pancreatic carcinoma. Cancer Res 2009;69:2425–2434. [DOI] [PubMed] [Google Scholar]
- 34. Lekawanvijit S, Adrahtas A, Kelly DJ, Kompa AR, Wang BH, Krum H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur Heart J 2010;31:1771–1779. [DOI] [PubMed] [Google Scholar]
- 35. Whitaker HJ, Hocine MN, Farrington CP. The methodology of self‐controlled case series studies. Stat Methods Med Res 2009;18:7–26. [DOI] [PubMed] [Google Scholar]
- 36. Liperoti R, Mor V, Lapane KL, Pedone C, Gambassi G, Bernabei R. The use of atypical antipsychotics in nursing homes. J Clin Psychiatry 2003;64:1106–1112. [DOI] [PubMed] [Google Scholar]
- 37. Smith DA, Beier MT. Association between risperidone treatment and cerebrovascular adverse events: Examining the evidence and postulating hypotheses for an underlying mechanism. J Am Med Dir Assoc 2004;5:129–132. [PubMed] [Google Scholar]
- 38. Daneshtalab N, Smeda JS. Alterations in the modulation of cerebrovascular tone and blood flow by nitric oxide synthases in SHR‐SP with stroke. Cardiovasc Res 2010;86:160–168. [DOI] [PubMed] [Google Scholar]
- 39. Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke 2008;40:e331–e339. [DOI] [PubMed] [Google Scholar]
- 40. Wang X. The anti‐apoptotic activity of melatonin in neurodegenerative diseases. CNS Neurosci Ther 2009;15:345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Adams JM. Ways of dying: Multiple pathways to apoptosis. Genes Dev 2003;17:2481–2495. [DOI] [PubMed] [Google Scholar]
- 42. Danlal NN, Korsmeyer SJ. Cell death: Critical control points. Cell 2004;116:205–219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Effects of risperidone on body weight and SBP in WKY rats, SHRs, and SHR‐SPs. Con, control group; Ris 2.4, risperidone 2.4 mg/kg/d. n = 10 in each group. The values of body weight and SBP are expressed as mean ± SD. Unpaired Student's t‐test, P > 0.05 for treatment group versus control group.
Figure S2 Effects of risperidone on inflammation and oxidative stress. (A) Effect of risperidone on expression of IL‐6 and TNF‐α in quiescent microglias from WKY rats at 0, 0.3, 1, 3, 30 mg/kg. n = 3 in each group. (B) Effect of risperidone on expression of IL‐6 and TNF‐α in LPS‐induced microglias from WKY rats at 0, 0.3, 1, 3, 30 mg/kg. n = 3 in each group. (C) Effect of risperidone on the activity of SOD, GSH‐Px and level of MDA in WKY rats and SHR‐SPs after MCAO. n = 3 in each group. Con, control group; Ris 2.4, risperidone 2.4 mg/kg/d. The values of IL‐6, TNF‐α, MDA, SOD, and GSH‐Px are expressed as mean ± SD. Dunnett t‐test for parts A and B, unpaired Student's t‐test for part C, P > 0.05 for treatment group versus control group.
Figure S3 Effect of risperidone in cerebral cortex neurons not exposed to OGD or H2O2. (A) Effect of risperidone (0, 30, 60, 90 μM) on neurotoxic potential in normal cerebral cortex neurons from SHR‐SPs and WKY rats was examined by flow cytometry (n = 4 in each group). The histogram shows the percentage of apoptotic rate in treatment groups versus the control. Flow cytometry analysis of cell apoptosis (Annexin V staining, R2 + R3) and necrosis (PI staining, R3 + R4), R1, alive cells; R2, early apoptotic cells (Annexin V–positive, PI‐negative); R3, late apoptotic or necrotic cells (Annexin V–positive, PI‐positive); R4, dead cells and cell fraction (Annexin V–negative, PI‐positive). (B) Effect of risperidone (0, 30, 60, 90 μM) on neurotoxic potential in normal cerebral cortex neurons from SHR‐SPs and WKY rats was examined by MTT (n = 6 in each group). The values of MTT and apoptosis rates are expressed as mean ± SD. Dunnett t‐test, P > 0.05 for treatment group versus control group.
Supporting info item
Supporting info item
Supporting info item


