Summary
This article reviews the recreational use of ketamine (“Special K”; KET) and explores the recent diffusion of its new derivative methoxetamine (“Special M”; MXE). The literature search on the nonclinical/recreational use of KET and MXE was carried out in a range of medical databases. Considering the limitations of peer‐reviewed information, data were integrated with a qualitative assessment of a range of websites, drug fora, and other online resources including e‐newsgroups, chat rooms, mailing lists, e‐newsletters, and bulletin boards. The recreational use of KET has started since its discovery in 1962. This was due to its rapid onset, short duration of action, and peculiar psychotropic effects (“K‐hole”). The latter effect ranges from confusion to dissociation and depersonalization (near‐death experience). However, KET abuse is often associated with physical and psychological side effects, of which the worst is urological/bladder toxicity. Recently, MXE has emerged as a legal and “bladder‐friendly” KET alternative. MXE presents with the same dissociative effect of KET, but with slower onset and longer duration of action. However, MXE seems to be associated with worse side effects than KET, ranging from mood disturbances/suicidal attempts to acute cerebellar toxicity. After 50 years of its discovery, KET has led to the emergence of MXE. However, this latter derivative does not appear to be a safer alternative to KET itself.
Keywords: Ketamine, Methoxetamine, Near‐death experience, Phencyclidine, Psychoactive
Introduction
Ketamine (KET) is a phencyclidine (PCP) derivative that blocks noncompetitively the glutamate N‐methyl‐d‐aspartate (NMDA) receptor; consequently, it inhibits the excitability of pain neurons to induce its dissociative anesthetic activity 1, 2, 3. It binds as well but with a lower affinity to σ and μ opioid receptors 1. KET also inhibits nitric oxide synthase, hence further contributing to analgesia 1. Furthermore, it acts as a noradrenergic and serotonergic uptake inhibitor, both neurotransmitters being involved in descending antinociceptive pathways 4, 5. Because KET binds to both σ(1) and σ(2) receptors with μM affinities, this may suggest that σ receptor‐mediated neuronal remodeling may contribute to the antidepressant effects of KET 6. Regarding MXE, its pharmacology and toxicology have yet to be elucidated. Although no formal studies have demonstrated the mechanism of action of MXE, it is likely to share the mechanism of action of KET through NMDA receptor antagonism and the inhibition of dopamine reuptake 7.
Dissociative activity of KET involves the sensory loss and analgesia as well as amnesia, which are not accompanied by actual loss of consciousness 8. This unique experience could expand to the sense of a near‐death experience (NDE) or even body splitting. For instance, Barbara Collier, an anesthetist, commented: “Ketamine allows some patients to reason that … the strange, unexpected intensity and unfamiliar dimension of their experience means they must have died” 9.
Ketamine causes mild stimulation of the cardiovascular (CVD) system without suppression of the respiration and gag reflex; thus, it has a good safety record 10. It is used in the UK in both emergency departments (EDs) and chronic pain clinics for mild anesthesia in surgeries 11. Furthermore, KET has been used as a therapeutic tool in a range of remaining conditions, including assisted psychotherapy for people with heroin dependence 12, alcoholism 13, 14, resistant depression 15. Furthermore, it has been reported that low‐dose KET can also re‐create a number of physiological abnormalities characteristic of schizophrenia 16, 17. However, administration of the drug in high doses for recreational purposes can cause CVD and respiratory toxicity. This makes its unregulated use outside the controlled environments a concern 18. KET is an arylcyclohexylamine derivative with a molecular weight of 237.73 g/mol. Its chemical name is 2‐(2‐chlorophenyl)‐2‐(methylamino)cyclohexanone (Figure 1). KET has three modifications from the PCP main structure 19 (Figure 1). The first modification involves the replacement of the piperidine ring by a methylamine, which gives the same potency as PCP but increased tendency to induce nausea. The second modification involves the two chloro to the phenyl ring, which decreases the potency but increases the analgesic effect activity. The third substitution involves the addition of carbonyl group to the cyclohexyl ring, which increases the elimination and decreases the duration of action of the anesthetic activity. KET has one chiral center at the C‐2 carbon and thus has two enantiomers (R and S enantiomers). The S enantiomer has the more potent analgesic properties, whereas the postsynaptic properties and agitated behavior are more associated with R enantiomer 2, 20.
Figure 1.
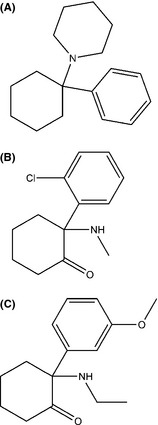
Chemical structures of (A) PCP, (B) KET, and (C) MXE.
Regarding pharmacokinetics, KET is extensively metabolized by N‐demethylation producing norketamine, a noncompetitive NMDA receptor antagonist that might also exhibit enantioselective pharmacological activity, for example, (S)‐norketamine has an 8‐fold higher affinity than (R)‐norketamine 21. The pharmacokinetics of KET in analgesic doses after intravenous (IV), intramuscular (IM), and oral administration was investigated in healthy volunteers 22. Plasma KET concentration–time curves were fitted by a two‐compartment open model with a terminal half‐life of 186 min. Absorption after IM injection was rapid and the bioavailability was 93%. However, only 17% of an oral dose was absorbed because of extensive first‐pass metabolism. This high rate of first‐pass metabolism may well explain why KET is typically not ingested. Similarly, MXE is generally taken by nasal insufflation (snorting), sublingual application, and IV and IM injection, with rectal use having been reported as well 7.
The objective of this paper is to comment on both the recreational use of KET along with its side effects and toxicity and one of its new derivatives, known as methoxetamine (MXE). The latter seems to be particularly popular compared with others such as N‐ethylnorketamine, methoxyketamine, 3‐MeO‐PCP, or remaining derivatives, including tenocyclidine (TCP) and tiletamine (for a review of the gray literature) 23, 24.
Materials and Methods
The literature search on the nonclinical/recreational use of KET and MXE was carried out in six databases: Ingenta, PubMed, Sciencedirect, Scopus, Web of Knowledge, and Wiley (Table 1). Considering the limitations of peer‐reviewed data in relation to latest trends of abuse and new psychoactive substances 11, such as MXE, the results were integrated with a qualitative assessment of a range of Web sites, drug fora, and other online resources including E‐newsgroups, chat rooms, mailing lists, e‐newsletters, and bulletin boards. The keywords used in this study included KET, ketamine, “Special K,” 2‐(2‐chlorophenyl)‐2‐(methylamino)cyclohexanone, phencyclidine, PCP, MXE, “Special M,” 2‐(3‐methoxyphenyl)‐2‐amino)cyclohexanone, methoxetamine, MXE Powder, METH‐O, “Special M,” psychedelics, near‐death experience, NDE, recreational. The search was performed over a period of 10 months (January 2011–October 2011) in eight languages: English, Flemish, German, Hungarian, Polish, Italian, Norwegian, and Spanish. The inclusion criteria were any studies showing the chemistry, pharmacology, psychedelic and recreational use of KET and/or MXE. Nonrelevant studies were excluded. In this respect, the initial search retrieved 246 studies, of which 95 were excluded. The authors ended up with 151 studies being monitored on a regular basis and included 108 Web sites, 41 peer‐reviewed data, one newsletter, and one monograph (see Figure 2). Data collected were kept confidential in a password‐protected online database of the ReDNet (www.rednetproject.eu). Any personal data (that could be identifiable) collected from online fora were kept anonymous. The study was cleared for ethical approval by the School of Pharmacy Ethics Committee, Hatfield, Hertfordshire, UK (December 15, 2010, PHAEC/10‐42).
Table 1.
Comparison between KET and MXE chemistry and effects
| Criteria | KET | MXE |
|---|---|---|
| Chemical name | 2‐(2‐chlorophenyl)‐2‐(methylamino)cyclohexanone | 2‐(3‐methoxyphenyl)‐2‐(amino)cyclohexanone |
| Chemical class | PCP derivative | PCP derivative |
| Molecular weight | 237.73 g/mol | 283.79 g/mol |
| Pharmacological class | Dissociative anesthetic | Dissociative anesthetic |
| Receptors | NMDA, σ, and μ | NMDA, σ, and μ |
| Routes of administration | IV, IM, intranasal, and oral | Intranasal, oral, sublingual, rectal, IM, and very rarely IV |
| Dosage | 10–250 mg | 10–100 mg |
| Onset of action | 30 second–30 min | 30–90 min |
| Duration of action | 3 h 0 min 0 second | 5–7 h |
| Desired effects | Depersonalization and out‐of‐the‐body experiences, including near‐death experiences; stimulation | Euphoria, empathy, coziness, pleasant sensory experience, dissociation, derealization, vivid hallucinations, introspection, antidepressant, dissociation from body (“M‐hole”) |
| Risk of re‐dose | No | Yes |
| Tolerance | Yes | Yes |
| Dependence | Yes | Yes |
| Known adverse effects | Confusion, vivid dreams, hallucination, flashbacks, referential thinking, panic attack, agitation, cardiovascular issues, depression, dissociation, apnea | Confusion, dizziness, time distortion, aphasia, synesthesia, cardiovascular issues, acute cerebellar toxicity, and psychomotor agitation |
| Bladder toxicity | Yes | Not confirmed |
| Cerebellar toxicity | Not reported | Anecdotally reported |
Figure 2.
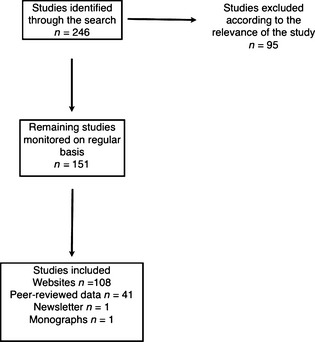
Flow chart illustrating identification of the studies included over the time frame January 2011–October 2011.
Results
Nonclinical Use of KET
Nonclinical use of KET has increased exponentially since its first discovery as a safer anesthetic alternative to PCP 1, 20. It was discovered in 1962 by Calvin Stevens, a consultant for Parke‐Davis/Warner 1, 20. In 1965, its first use as a recreational drug was recorded 20. However, the recreational use became well known from the mid‐1990s, when it was more popular than cocaine. This was partly because cocaine purity dropped and it was sold as a cheaper alternative 25. KET, also known in these contexts as “Special K” or simply “K,” is widely used as a recreational drug in clubs, raves, and squat parties for self‐experimental purposes, and it has caused problems as such in the EU and internationally. It might be difficult to understand why an anesthetic could become a popular substance of misuse. A few reasons can be identified 7, 26. These include its (1) short time‐to‐effect (30 second IV, 5–30 min intranasally, and 20 min orally) and duration of action, which can last up to three hours, (2) low cost, (3) peculiar psychotropic effects. The latter, known among users as the “K‐hole” 27, range from confusional states, vivid dreams, hallucinations, flashbacks, referential thinking, dissociation, and depersonalization to psychotic experiences. It is known that at subanesthetic doses, KET intake has been anecdotally described to be associated with effects somewhat similar to those reported during a near‐death experience (NDE) 8, 28, 29, 30. NDEs usually occur in various situations including cardiac arrest 31; hypovolemic/septic/anaphylactic shock; intracerebral hemorrhage; cerebral infarction; near‐drowning or asphyxia; apnea; electrostimulation of the temporal lobe 32; and prolonged isolation/sensory deprivation 33. Common features of the NDE include (1) the ineffable nature of the experience, (2) a sense of joy (cocaine‐like rush), peace, and love, (3) the detachment from own physical body (out‐of‐the‐body experiences) 34, (4) traveling along a region of darkness toward a light at the end, (5) visualization of past experiences, sometimes organized into a life‐review 28, 35, (6) visions and communications with deceased relatives and friends or “beings of light”, (7) a decision to return to life, and (8) altered perception of time, ataxia, among others 35.
Users reporting the near‐death experiences felt out of the body or lost their senses and sometimes were feeling as out of the planet. One user, Mr. P, reported the above effects after he injected 100 mg of KET IM while he was listening to a piece of music. He said: “I gradually lost my senses. The music was very distorted. I tested myself by asking basic questions about mathematics, the names of those I love, etc., then suddenly I wasn't interested in this anymore. So I tried to concentrate on ‘who I am’ and I lost the interest again. Visions become blurred. It wasn't meaningful who I was any more, because I existed anyway. Then I tried the experience of death. I was going down a tunnel. I saw the planet Earth. I could feel the relationship between the human soul, Earth and the planets. I thought I was a doll, you know the matryoshka? I was the matryoshka of the entire system. I understood that earth is inside something else. I felt its gravity. All this is embraced within a system. I was nothing, but I knew that my place was on Earth” 30.
Another KET user reported: “Two years ago I was with my friends in Valencia. We went to the beach that day and we had some KET. We sat on the sand. The effects started very soon. I felt dizzy and I had to lie down. I closed my eyes. The first thing that I remember is that I felt somehow I was going very fast and that I left my body. It was not frightening. Subsequently, I saw a tunnel and a tiny little light which grew bigger and bigger. I was approaching this light when I heard a voice telling me to go back. So I asked ‘Why? I don't want to go back.’ I had no reply. A being of light appeared. He wanted to show me something. A big screen also appeared. I saw earth and the planets. I have heard them breathing. I touched the stars and talked to the Sun (God). I cannot remember what he said but it was amazing. I kept thinking that it was wonderful and amazing. And then, suddenly, I was lying back on the beach!”
Ketamine effects and NDEs might bear some level of resemblance at a neurobiological level as well. In fact, both KET and NDEs involve events at glutamate N‐methyl‐d‐aspartate (NMDA) receptors 8, 29, 36. However, it is still unclear whether reported psychoactive effects of KET may appropriately fit into typically described features of an NDE.
Adverse Reactions Associated with KET Misuse
Miss L., a 23‐year‐old who tried KET only once in her life at a disco club, observed: “I felt a bit paranoid, I was going to die. The first effects started very soon. I felt very confused and normal reality just disappeared. I was dizzy and unable to walk. I started bumping against walls. I wanted to go out from the room where I was, but it was very cold. I had no‐one close to help me.” 30.
The risk of physical harm from accidents, such as blackouts and bad falls, is also very high 18. There are also reports of people with chronic opiate problems using KET for its anesthetic and analgesic effects 37, 38.
Ketamine may lead to dependence and tolerance can develop quickly; hence, a larger quantity is required to achieve the same effects 18. This can lead users to take it in intense “binges.” An immediate risk of taking KET in recreational settings is accidents, such as bad falls. The disconnection from the body can be dangerous in almost any situation other than lying down in a safe environment. Other adverse effects can include panic attacks and depression, and when taken in large doses, it can exaggerate pre‐existing mental health problems 19, 30, 39. Stimulant‐like weight loss and loss of appetite have also been reported after periods of heavy use. The risks of KET use are increased if it is used with depressant drugs, such as alcohol. It can suppress breathing and heart function in rare cases, although more commonly it stimulates these functions. It is more likely to suppress breathing (i.e., give rise to a period of apnea) if taken as a fast IV 18. When used with stimulant drugs such as ecstasy (MDMA) or amphetamines, it can also cause high blood pressure 3. A number of reports suggest that KET can be used as a “date rape drug” as high doses can cause amnesia for events that happened while under the influence of the drug 27. Three days after the consumption of KET, impairments of working, episodic and semantic memory have been reported 40, 41. One research study has shown that semantic memory impairments associated with recreational KET use are reversible after people stop or substantially reduce its use. However, impairment to episodic and possibly attentional functioning is longer lasting 41, 42, 43. A problem with these studies is that the authors rarely, if ever, provide urine or hair test results to prove that their subjects are not misusing with other drugs at the time of testing. Cannabis and alcohol are particularly likely culprits as many KET users smoke cannabis and drink alcohol daily 27. Some users also experience mild forms of schizophrenic‐like symptoms and perceptual distortions associated with the use of KET for a short period after they have stopped taking the drug 26. Initially, following its anesthetic use, clinicians reported the occurrence of confusional states, vivid dreams, and hallucinations as well as flashbacks 44. The risk of death has been commented in a few reports 18. According to a report by the European Monitoring Centre for Drugs and Drug Addiction 37, some 12 persons have died as a result of KET use (seven in the United States and five in Europe) in the previous 10 years. Only three of these deaths were associated with the ingestion of KET on its own 37. Conversely, Schifano et al. 18 focused on KET misuse mortality figures (UK; 1993–2006), extracted from various sources, and identified 23 victims (typically men, in the 25–44 age group) who self‐administered themselves with a miscellany of psychoactive compounds (including KET) and alcohol. KET was detected in four cases on its own, and they suggested that high safe profile of KET should be questioned.
The bladder toxicity issues associated with KET cannot be disregarded. KET is linked to severe bladder problems including incontinence, painful bladder, bladder shrinkage, and damage to kidney and ureter obstruction, which may lead to bladder removal 45, 46, 47. However, the mechanisms of how KET causes bladder toxicity are still somewhat unclear.
MXE
The recent emergence of new synthetic drugs 7, 48 has also involved the “KET/PCP‐like drugs” market. Since 2010, MXE has been advertised and sold online as a legal alternative to KET 7, 49. Indeed, MXE can be acquired legally without a veterinary license, which is the minimum requirement for the purchase of KET in various countries, including the United States. In the UK, it became the first drug to be banned by the Government under a temporary class drug order in April 2012. Chemical name of MXE is 2‐(ethylamino)‐2‐(3‐methoxyphenyl)cyclohexan‐1‐one (Figure 1). Its molecular weight is 283.79 g/mol. It is available as a white or off‐white hygroscopic powder. It differs from PCP by two modifications 7, 50. The first involves the removal of the piperidine ring and replacement by an ethyl amino group, which gives more potency than PCP but increases the tendency to induce nausea. The second modification involves the 3‐methoxy substitution on the phenyl ring, which increases the μ‐opioid receptor affinity, while at the same time removing its mood‐altering effects.
Methoxetamine is available online as “MXE powder” and “Special M” in the form of white powder. It is labeled as “Not For Human Consumption” to circumvent the regulations regarding recreational drugs 7. Primary route of administration of MXE is intranasal, oral, sublingual, rectal, and IM 51, 52. In addition, very rare cases of IV administration have been reported and included an unconfirmed fatality following an IV injection of both 80–100 mg MXE and 400 mg of 5,6‐methylenedioxy‐2‐aminoindane (MDAI) 51, 52.
Desired effects of MXE and dosages are influenced mainly by the modalities of intake. The dosages can range from 20 to 100 mg for oral administration and 10–50 mg for IM administration. Some users suggest the increase in the dosage gradually without exceeding 50 mg on the first occasion when administered orally 53. The perceived effect could be delayed of some 30–90 min after insufflation 54. This might be dangerous as it often causes the user to ingest another dose of the substance 51, thinking that the first dose was inadequate. Duration of action of MXE has been described as being in the range of 5–7 h 53. However, when taken IM, the effect of MXE is faster than orally (within 5 min) 51 and its duration of action is shorter (about one hour).
MXE Desired Effects and Adverse Reactions
As reported by users, Effects of MXE are similar to those of KET, but with longer delay in onset (90 min) and longer duration (5–7 h) when administered orally 51, 53. MXE ingestion may be associated with NDE whose common features are sensory deprivation, derealization, and dissociation from the physical body 19, 47, 55.
Its reported desired effects include euphoria, empathy, “coziness,” pleasant intensification of sensory experiences especially while listening to the music, mild to strong sense of dissociation from the physical body, distortion of the sense of reality, vivid hallucinations, introspection, and brief antidepressant effects 51, 53, 56, 57. Users' reports described MXE experience as “music sounds great,” “trapped inside a glass chopping board,” “not for social situation,” “feeling like another inanimate object,” “…just seems so absurdly surreal and it makes no sense, but I'm quite happy just to stare at the TV screen, feeling all snugly and warm.” Somebody described MXE as a “big Christmas cardigan,” whose intake was providing both “spinning sensations” and “naturalistic hallucinations in waves,” overall referring to the “M‐hole,” as opposed to the KET “K‐hole” 51. This described the subjective state of dissociation from the body, which may mimic the out‐of‐the‐body experiences or NDE 18, 19. Most users' reports concluded that MXE is different from KET mainly because of “longer come up,” which might lead to a high risk of re‐dose, and its longer‐lasting effects. In summary, MXE seems to work as a short‐acting mood enhancer with powerful (visual) hallucinogenic and dissociative properties. However, it ingestion might be associated with several side effects such as dizziness, confusion, time distortion, aphasia, synesthesia, and psychomotor agitation 53, 57.
Methoxetamine withdrawal symptoms may include low mood and/or depressive thoughts 53, decreased levels of cognitive impairment, insomnia 53, and potential suicidal attempts 51.
Methoxetamine is allegedly used in combination with a variety of other drugs to enhance or prolong its effects and duration of action. These include LSD, 2CC (4‐chloro‐2,5‐dimethoxyphenethylamine), alpha‐MT (alpha‐methyltryptamine), MDAI 53. However, Web fora users do not recommend its consumption with alcohol, tetrahydrocannabinol (THC), selective serotonin reuptake inhibitors (SSRIs), or monoamine oxidase inhibitors (MAOIs).
Some side effects of KET such as agitation and CVD issues (e.g., increased heart rate and blood pressure) may be associated with MXE ingestion. Others have included painful bladder, ureter obstruction, papillary necrosis, and hepatic dysfunction 27, 47, 58. Regarding psychopathological disturbances of MXE, it may seem appropriate to conclude that they are similar to those of KET 59.
Although MXE has been named as the “bladder‐friendly” alternative to “Special K,” work is still needed to confirm that MXE is bladder friendly 47, 50. Users, admitted to accident and emergency department after having ingested MXE, have experienced both KET‐like dissociative/catatonic and sympathomimetic effects such as agitation, tachycardia, hypertension, hallucinations, confusion, stupor, mydriasis, and nystagmus 47, 50. MXE was detected in all the patients' serum. Other patients had acute cerebellar toxicity after nasal insufflations of MXE 60. The toxicity needed several days to recover and was characterized by severe ataxia, slurred speech, nystagmus, incoordination, and reduced consciousness.
User reports on forums confirm these effects. For instance, a chronic user, after 18 months of taking MXE, reported that the drug's effects were dose dependent in most cases 61. He specified his favorite route as sublingual compared to oral as the latter gives slower effect. In low doses (20–35 mg), MXE seemed more of a social drug as “It gave no hangover, lowered inhibitions enough that I could dance and not care if it was bad, and allowed me to feel inebriated enough that I didn't feel like I was missing out on drinking.” However, at higher doses (>40 mg), the dissociative effects started appearing as the user reported: “I found MXE very confusing, numbed the body and yet was still quite suitable for a rave or part setting where socialization would not be required…I occasionally investigated high doses on my own, but did not find them particularly to my liking. I prefer to be functional as I never have much spare time, so the ‘M‐hole’ was not great for me. I only investigated it once. Any time I took doses above 60 mg I found that I would awaken the next morning feeling ‘fuzz’…It is not necessarily an unpleasant feeling, but I certainly feel impaired and would not be comfortable driving a car while experiencing it. It is very hard sensation to describe, but I feel mentally dulled and my vision feels odd.”
Another user have experienced the dissociative effect after about one hour of taking 80 mg MXE sublingually with 15 mg of 1‐(2,5‐dimethoxy‐4‐ethylphenyl)‐2‐aminoethane (2C‐E) intranasally 57. The user reported: “I became unable to follow the movie I was watching while waiting for the chemicals to take effect…From this point on memory is spotty as my mind had deconstructed the concepts of time, order, and reality. Eyes are closed for the duration of the trip. Visuals were truly breathtaking, impossible to relate to my beloved trip report readers. I had the sensation that my body had descended several feet below the earth. I felt as though my mind had disconnected from the confines of its physical structure, projected astrally and was moving though time space at an incalculable speed…I believe I experienced ego death which was terrifying at first but afterward I felt ecstatic.”
Discussion and Conclusions
After 50 years of its discovery, KET, or “Special K,” has led to the emergence of methoxetamine, or “Special M,” and possibly other derivatives such as 3‐MeO‐PCP, PCE, 3‐MeO‐PCE, tiletamine, and 1‐(1‐(2‐thienyl)‐cyclohexyl)morpholine (TCM). Most of these new substances share a number of characteristics that may constitute a public health challenge: (1) they are not approved for human consumption, (2) their intake is possibly associated with a number of unknown side effects/adverse reactions, (3) very few related pharmacological/toxicological data are available in the peer‐reviewed, scientific, literature, with the limited knowledge being mostly restricted to preclinical studies, (4) they are rapidly appearing in always more sophisticated forms and remain unregulated for a long period of time, (5) they are most often synthesized in underground laboratories simply modifying the molecular structure of remaining controlled drugs, hence raising further concerns in terms of the presence of contaminating agents, (6) they are largely available online and thus “just a click” away from our homes and potentially available to everyone 7. In addition, the current legal status of most of its derivatives may arguably facilitate the increasing levels of popularity of the drug and might affect as well the users’ perception of risks associated with its consumption. In fact, the idea that legality can equate with safety still remains well grounded among some recreational users 18, 19. This work has presented an overview of the first 50 years of KET's history and provided an original refection on its role in the future. A possible limitation of the present study could be given by the fact that only publicly available Web sites, fora, and similar sources were monitored. Conversely, to improve the coverage of the study, not only the web pages but also more private ways of communication (including newsgroups, chat rooms, mailing lists, e‐newsletters, and bulletin boards) were here considered.
More studies need to be carried out on the issues here described, especially focusing on the clinical pharmacological and acute/chronic toxicity characteristics of the whole range of the PCP‐like drugs.
Conflict of Interest
The authors declare no conflict of interest.
Funding
This publication is a part of the ReDNet Research project, which has received funding from the European Commission in the framework of the Public Health Programme (2006 348; 2009 12 16).
References
- 1. Rowland LM. Sub‐anesthetic ketamine: how it alters physiology and behavior in humans. Aviation Space Environ Med 2005;76:C52–C58. [PubMed] [Google Scholar]
- 2. Goldberg ME, Torjman MC, Schwartzman RL, Mager DE, Wainer IW. Enantioselective pharmacokinetics of (R)‐ and (S)‐ketamine after a 5‐day infusion in patients with complex regional pain syndrome. Chirality 2011;23:138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Curran HV, Morgan C. Cognitive, dissociative and psychotogenic effects of ketamine in recreational users on the night of drug use and 3 days later. Addiction 2000;95:575–590. [DOI] [PubMed] [Google Scholar]
- 4. Quibell R, Prommer EE, Mihalyo M, Twycross R, Wilcock A. Ketamine*. J Pain Symptom Manage 2011;41(3):640–649. [DOI] [PubMed] [Google Scholar]
- 5. Meller ST. Ketamine: relief from chronic pain through actions at the NMDA receptor? Pain 1996;68:435–436. [DOI] [PubMed] [Google Scholar]
- 6. Robson MJ, Elliott M, Seminerio MJ, Matsumoto RR. Evaluation of sigma (σ) receptors in the antidepressant‐like effects of ketamine in vitro and in vivo. Eur Neuropsychopharmacol 2012;22:308–317. [DOI] [PubMed] [Google Scholar]
- 7. Corazza O, Schifano F, et al. The phenomenon of new drugs on the Internet: a study on the diffusion of the ketamine derivative methoxetamine (‘MXE’). Hum Psychopharmacol Clin Exp 2011;27:145–149. [DOI] [PubMed] [Google Scholar]
- 8. Bonta IL. Schizophrenia, dissociative anaesthesia and near‐death experience; three events meeting at the NMDA receptor. Med Hypotheses 2004;62:23–28. [DOI] [PubMed] [Google Scholar]
- 9. Collier BB. Ketamine and the conscious mind. Anaesthesia 1972;27:120–134. [DOI] [PubMed] [Google Scholar]
- 10. Sehdev RS, Symmons DAD, Kindl K. Ketamine for rapid sequence induction in patients with head injury in the emergency department. Emerg Med Australas 2006;18:37–44. [DOI] [PubMed] [Google Scholar]
- 11. Bell R, Dahl J, Moore R, Kalso E. Perioperative ketamine for acute postoperative pain. Emerg Med Australas 2006;18:37–44. [DOI] [PubMed] [Google Scholar]
- 12. Krupitsky EM, Burakov AM, Dunaevsky IV, Romanova TN, Slavina TY, Grinenko AY. Single versus repeated sessions of ketamine‐assisted psychotherapy for people with heroin dependence. J Psychoactive Drugs 2007;39:13–19. [DOI] [PubMed] [Google Scholar]
- 13. Krupitsky EM, Grinenko AY. Ketamine psychedelic therapy (KPT): a review of the results of ten years of research. J Psychoactive Drugs 1997;29:165–183. [DOI] [PubMed] [Google Scholar]
- 14. Krystal JH, Petrakis IL, Krupitsky E, Schutz C, Trevisan L. D”Souza DC. NMDA receptor antagonism and the ethanol intoxication signal: from alcoholism risk to pharmacotherapy. Ann N Y Acad Sci 2003;1003:176–184. [DOI] [PubMed] [Google Scholar]
- 15. Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science 2012;338:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coyle JT, Basu A, Benneyworth M, Balu D, Konopaske G. Glutamatergic synaptic dysregulation in schizophrenia: therapeutic implications. Handb Exp Pharmacol 2012;213:267–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Javitt DC, Zukin SR, Heresco‐Levy U, Umbricht D. Has an angel shown the way? Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Schizophr Bull 2012;38:958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schifano F, Corkery J, Oyefeso A, Tonia T, Ghodse AH. Trapped in the “K‐hole”: overview of deaths associated with ketamine misuse in the UK (1993–2006). J Clin Psychopharmacol 2008;28:114–116. [DOI] [PubMed] [Google Scholar]
- 19. Corazza O, Schifano F. Ketamine use: a prospective study on the emergence of near‐death states among a group of 50 ketamine recreational users. Subst Use Misuse 2010;45:916–924. [DOI] [PubMed] [Google Scholar]
- 20. Stevenson C. Ketamine: A review, 2003. Update in Anesthesia: http://update.anaesthesiologists.org/wp-content/uploads/2009/08/Ketamine-A-Review.pdf (retrieved on October 2nd, 2012).
- 21. Goldberg ME, Torjman MC, Schwartzman RJ, Mager DE, Wainer IW. Pharmacodynamic Profiles of Ketamine (R)‐and (S)‐ with 5‐Day Inpatient Infusion for the Treatment of Complex Regional Pain Syndrome. Pain Physician 2010;13:379–387. [PMC free article] [PubMed] [Google Scholar]
- 22. Clements JA, Nimmo WS, Grant S. Bioavailability, pharmacokinetics, and analgesic activity of ketamine in humans. J Pharm Sci 1982;71:539–542. [DOI] [PubMed] [Google Scholar]
- 23. DrugsForum . Available from: http://www.Drugs-Forum.com/forum/showwiki.php?title=N-ethyl-nor-ketamine (accessed on November 28th, 2012).
- 24. Beagle JQ. Synthesis and Effects of PCP Analogs; A review by John Q. Beagle. Available from: http://www.erowid.org/archive/rhodium/chemistry/pcp/ (accessed on November 28th, 2012).
- 25. The Independent . Ketamine tops cocaine as new drug of choice, 2009. http://www.independent.co.uk/news/uk/home-news/ketamine-tops-cocaine-as-new-drug-of-choice-1366714.html (retrieved on August 29th, 2012).
- 26. Morgan CJA, Monaghan L, Curran V. Beyond the K‐hole: a 3‐year longitudinal investigation of the cognitive and subjective effects of ketamine in recreational users who have substantially reduced their use of the drug. Addiction 2004;99:1450–1461. [DOI] [PubMed] [Google Scholar]
- 27. Jansen KLR. Ketamine: Dreams and Realities. Sarasota, FL: MAPS, 2011. [Google Scholar]
- 28. Greyson B, Stevenson I. The phenomenology of near‐death experiences. Am J Psychiatry 1980;137:1193–1196. [DOI] [PubMed] [Google Scholar]
- 29. Jansen KLR. Near‐Death Experiences and the NMDA receptor. BMJ 1989;298:1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Corazza O. Near‐Death Experiences: Exploring the Mind‐Body connection. London/New York: Routledge, 2008. [Google Scholar]
- 31. Van Lommel P, Van Wees R, Meyers V, Elfferich I. Near‐death experience in survivors of cardiac arrest: a prospective study in the Netherlands. Lancet 2001;358:2039–2045. [DOI] [PubMed] [Google Scholar]
- 32. Persinger M. Religious and mystical experiences as artefacts of temporal lobe function: a general hypothesis. Percept Mot Skill 1983;57:1255–1262. [DOI] [PubMed] [Google Scholar]
- 33. Comer NL, Madow L, Dixon JL. Observation of sensory deprivation in a life‐threatening situation. Am J Psychiatry 1967;124:164–169. [DOI] [PubMed] [Google Scholar]
- 34. Blanke O, Ortigue S, Landis T, Seeck M. Stimulating illusory own body perceptions. Nature 2002;419:269–270. [DOI] [PubMed] [Google Scholar]
- 35. Moody RA. Life after Life. Atlanta, GA: Mockingbird Books, 1975. [Google Scholar]
- 36. Fenwick P. Is the near‐death experience only N‐methyl‐d‐aspartate blocking? J Near Death Stud 1997;16:43–53. [Google Scholar]
- 37. EMCDDA : Report on the risk assessment of ketamine in the framework of the joint action on new synthetic drugs, European Monitoring Centre for Drugs and Drug Addiction, 2002.
- 38. Dalgarno P, Shewan D. Illicit use of ketamine in Scotland. J Psychoactive Drugs 1996;28:191–199. [DOI] [PubMed] [Google Scholar]
- 39. Wood D, Cottrell A, Baker SC, et al. Recreational ketamine: from pleasure to pain. BJUI 2011;197:1881–1884. [DOI] [PubMed] [Google Scholar]
- 40. Morgan CJA, Mofeez A, Brandner B, Bromley L, Curran V. Ketamine impairs response inhibition and is positively reinforcing in healthy volunteers: a dose‐response study. Psychopharmacology 2004;172:298–308. [DOI] [PubMed] [Google Scholar]
- 41. Morgan CJA, Mofeez A, Brandner B, Lesley B, Curran V. Acute effects of ketamine on memory systems and psychotic symptoms in healthy volunteers. Neuropsychopharmacology 2004;29:208–218. [DOI] [PubMed] [Google Scholar]
- 42. Krystal J, Karper L, Seibyl J, et al. Subanaesthetic effects of the non‐competitive NMDA antagonist, ketamine, in humans”. Arch Gen Psychiatry 1994;51:199–214. [DOI] [PubMed] [Google Scholar]
- 43. Malhotra A, Pinals D, Weingartner H, et al. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology 1996;14:301–308. [DOI] [PubMed] [Google Scholar]
- 44. Siegal R. Phencyclidine and ketamine intoxication: a study of four populations of recreational users In: Peterson RC, Stillman RC, editors. Phencyclidine Abuse: An Appraisal (Natl. Inst. Drug Abuse Res. Monogr. 21). Rockville, MD: National Institute of Drug Abuse, 1978;119–147. [PubMed] [Google Scholar]
- 45. Bhattacharya S. New Scientist, Chronic ketamine use kills bladder cells, 2011. http://www.newscientist.com/article/mg21028174.100-chronic-ketamine-use-kills-bladder-cells.html (retrieved August 29th, 2012).
- 46. Colebunders B, Van Erps P. Cystitis due to the use of ketamine as a recreational drug: a case report. JMCR 2008;2:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wood DM, Davies S, Puchnarewicz M, Johnston A, Dargan PI. Acute toxicity associated with the recreational use of the ketamine derivative methoxetamine. Eur J Clin Pharmacol 2012;68:853–856. [DOI] [PubMed] [Google Scholar]
- 48. Assi S, Fergus S, Stair JL, Corazza O, Schifano F. Emergence and identification of new designer products from the internet. Eur Pharm Rev 2011;16:68–72. [Google Scholar]
- 49. ACMD , 2012. http://www.homeoffice.gov.uk/publications/agencies-public-bodies/acmd1/statement-methoxetamine?view=Binary (accessed on August 29th, 2012).
- 50. Wood DM, Dargan PI. Novel psychoactive substances: how to understand the acute toxicity associated with the use of these substances. Ther Drug Monit 2012;34:363–367. [DOI] [PubMed] [Google Scholar]
- 51. drugs‐forum , 2011. Retrieved on September, 2012, from http://www.drugs-forum.com/index.php.
- 52.LoGiCal Analytical Monograph Methoxetamine, 2012. http://www.logical-standards.com/uploads/pdfs/english/Methoxetamine_Final.pdf (accessed on August 29th, 2012).
- 53. Bluelight , 2010. Retrieved on September 2012, from http://www.bluelight.ru/vb.
- 54. Drugs‐Forum , 2010. Retrieved on September, 2012, from: http://www.drugs-forum.com/index.php.
- 55. Coull J, Morgan H, Cambridge V, et al. Ketamine perturbs perception of the flow time in healthy volunteers. Psychopharmacology (Berl) 2011;218:543–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Purechemicals , 2010. Retrieved on September 2012, from: http://www.purechemicals.co.uk.
- 57. Bluelight , 2011. Retrieved on September 2012, from http://www.bluelight.ru/vb.
- 58. Dillon P, Copeland J, Jansen K. Patterns of use and harms associated with non‐medical ketamine use. Drug Alcohol Depend 2003;69:23–28. [DOI] [PubMed] [Google Scholar]
- 59. Fletcher P, Honey GD. Schizophrenia, ketamine and cannabis: evidence of overlapping memory deficits. Trends Cogn Sci 2006;10:167–174. [DOI] [PubMed] [Google Scholar]
- 60. Shields JF, Dargan PI, Wood DM, Puchnarewicz M, Davies S, Waring WS. Methoxetamine associated reversible cerebellar toxicity: three cases with analytical confirmation. Clin Toxicol (Phila) 2012;50:438–440. [DOI] [PubMed] [Google Scholar]
- 61. Bluelight , 2012. Retrieved on September 2012, from http://www.bluelight.ru/vb.


