Vascular endothelial cells serve as a barrier between tissue and blood stream, which plays an important role in maintaining vascular homeostasis. Oxidative stress has been implicated as a major cause of endothelial injuries in a variety of clinical abnormalities including artherosclerosis, ischemia reperfusion injury, and diabetes [1]. Donepezil, a reversible noncompetitive acetylcholinesterase inhibitor, used to treat Alzheimer's disease, has been reported that it could protect neuronal cells against cell injury [2]. In this study, the protective effect of donepezil against hydrogen peroxide (H2O2)‐induced cell injury in vitro was determined.
Human umbilical vein endothelial cell (HUVEC) line obtained from Department of Pharmacology, College of Pharmacy, Second Military Medical University, was cultured in RPMI‐1640 medium (Gibco, NY, USA), containing 10% fetal bovine serum (Gibco, NY, USA), 100 U/mL penicillin (Invitrogen, NY, USA), and 100 μg/mL streptomycin (Invitrogen, NY, USA). Cells were grown in a 37°C, 5% CO2 incubator with media replenishment every 3 days. Before experimental intervention, confluent cultured cells were preincubated for 12 h in starved medium. The starved cells were then divided into four experimental groups: (1) control; (2) cells incubated with H2O2 (100 μM) (Sinopharm Chemical Reagent Co. Ltd, China) 1.5 h alone; (3) cells pretreated with donepezil (Sigma, MO, USA) for 1 h before coincubated with H2O2; (4) cells incubated with donepezil alone. To evaluate HUVEC cell viability, MTT assay was conducted. After being treated with different medium conditions, the hypoxia‐inducible factor alpha (HIF‐1α) and manganese superoxide dismutase (MnSOD) expression in HUVECs were analyzed by Western blot. Levels of basic fibroblast growth factor (bFGF) and stromal‐derived factor‐1 (SDF‐1) in cellular supernatant of each group were determined by ELISA kits (R&D Systems, MN, USA). All data are presented as mean ± SD. Comparisons of the groups were evaluated by using the t‐test with one‐way analysis of variance (ANOVA). Statistical significance was set at P < 0.05.
During ischemia‐hypoxia, oxidative stress takes place, potentially leading to damage all the principal cellular molecules. It is very important to figure out how the endothelial cell is protected from excessive oxidative stress‐induced cell injury. Donepezil (100 μM) decreased cell viability, but incubation with donepezil at concentrations of 0.1, 1, and 10 μM has no obvious effects on cell viability compared with control (Figure 1A). Our results showed that pretreatment with donepezil at concentrations of 1 and 10 μM, compared to H2O2 group, significantly inhibits the H2O2‐induced reduction in cell viability (Figure 1B). It is demonstrated that lower concentrations of donepezil could protect H2O2‐induced HUVECs injury and may also have a similar protective effect on ischemic tissues.
Figure 1.
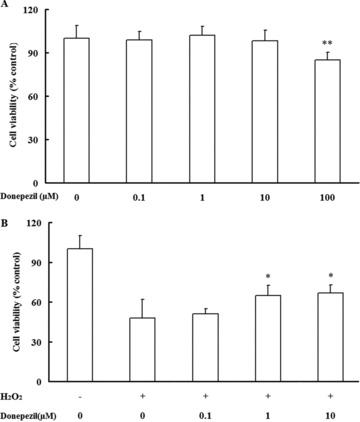
Cell survival as evidenced by MTT in donepezil‐treated groups at four different concentrations (A). Data are expressed as mean ± SD, **P < 0.01 versus control group. Effects of donepezil on viability of H2O2‐stimulated HUVECs by MTT assay (B). All data are expressed as mean ± SD of all 3 experiments, the asterisks represent significant differences between the donepezil‐pretreated group and the H2O2 group (*P < 0.05).
After exposure of HUVECs to donepezil (1 μM) for 2 h could upregulate expression of HIF‐1α in cells significantly (Figure 2A), and HIF‐1α is very essential for endothelium secretion of bFGF and SDF‐1α[3, 4]. We also got the same consequences in a recent study. Donepezil can dramatically increase the secretion of SDF‐1 at concentrations of 1 and 10 μM in cellular supernatant compared with the control (Figure 2C). In the meantime, we also found that donepezil (10 μM) increased bFGF concentrations in cellular supernatant (Figure 2D). It has been confirmed that these cytokines could attenuate the damage or apoptosis of the cells under oxidative stress related cell injury [5, 6, 7]. MnSOD is a very strong antioxidant factor for protection against oxidative damage [8]; we found that donepezil significantly potentiates the expression of MnSOD compared with the control group (Figure 2B).
Figure 2.
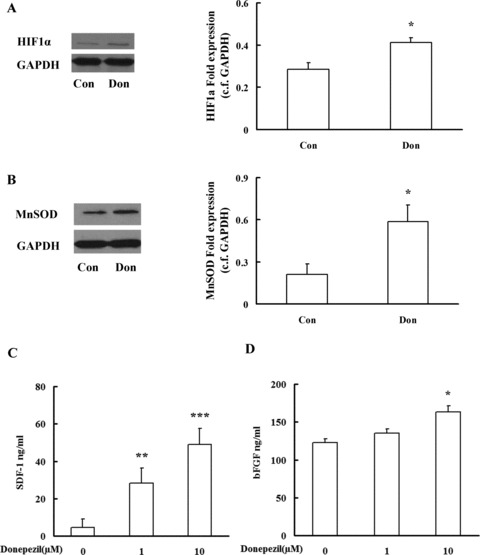
Western blot analysis of HIF1α (A) and MnSOD (B) expressions in HUVECs. SDF‐1 (C) and bFGF (D) were determined by ELISA. Data are expressed as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001 versus control group. Con: control, Don: donepezil.
Few studies have reported that acetylcholinesterase inhibitors could protect endothelial cells in vitro. Endothelial cells can synthesize some of acetylcholine [9], but compared to donepezil, other acetylcholinesterase inhibitors such as neostigmine had no effect on LPS‐induced cell injury [10]. The protective effects of the acetylcholinesterase inhibitors may be related to cell types and the chemical structure. Further experimental evidence is needed to determine if the protection mechanisms of donepezil against hydrogen peroxide‐induced cell injury are all related to acetylcholine.
As stated above, we conclude that donepezil could protect HUVECs against H2O2‐induced cell injury. Due to its efficacy, donepezil might be a potential therapy for oxidative stress in cardiovascular and cerebrovascular diseases.
Acknowledgments
The authors are grateful for grant support from the National Basic Research Program of China (973 Program, 2009CB521901).
References
- 1. Higashi Y, Noma K, Yoshizumi M, Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ J 2009;73:411–418. [DOI] [PubMed] [Google Scholar]
- 2. Zhou J, Fu Y, Tang XC. Huperzine A and donepezil protect rat pheochromocytoma cells against oxygen–glucose deprivation. Neurosci Lett 2001;306:53–56. [DOI] [PubMed] [Google Scholar]
- 3. Calvani M, Rapisarda A, Uranchimeg B, Shoemaker RH, Melillo G. Hypoxic induction of an HIF‐1α‐dependent bFGF autocrine loop drives angiogenesis in human endothelial cells. Blood 2006;107:2705–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF‐1 induction of SDF‐1. Nat Med 2004;10:858–864. [DOI] [PubMed] [Google Scholar]
- 5. O’Driscoll C, Wallace D, Cotter TG. bFGF promotes photoreceptor cell survival in vitro by PKA‐mediated inactivation of glycogen synthase kinase 3β and CREB‐dependent Bcl‐2 up‐regulation. J Neurochem 2007;103:860–870. [DOI] [PubMed] [Google Scholar]
- 6. Lataillade JJ, Clay D, Bourin P, Herodin F, Dupuy C, Jasmin C, Le Bousse‐Kerdiles MC. Stromal cell‐derived factor 1 regulates primitive hematopoiesis by suppressing apoptosis and by promoting G0/G1 transition in CD34+ cells: Evidence for an autocrine/paracrine mechanism. Blood 2002;99:1117–1129. [DOI] [PubMed] [Google Scholar]
- 7. Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF‐1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 2007;129:111–122. [DOI] [PubMed] [Google Scholar]
- 8. Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature 2000;407:390–395. [DOI] [PubMed] [Google Scholar]
- 9. Kirkpatriek CJ, Bittinger F, Nozadze K, Wessler I. Expression and function of the non‐neuronal cholinergic system in endothelial cells. Life Sci 2003;72:2111–2116. [DOI] [PubMed] [Google Scholar]
- 10. Tyagi E, Agrawal R, Nath C, Shukla R. Cholinergic protection via a7 nicotinic acetylcholine receptors and PI3K‐Akt pathway in LPS‐induced neuroinflammation. Neuroche Int 2010;56:135–142. [DOI] [PubMed] [Google Scholar]


