Stroke is the second most common cause of death and ischemic stroke is the largest subtype 1, 2, 3, 4. To investigate the mechanism of cerebral ischemic injury as well as to develop effective therapies to this disease, the use of ischemic stroke animal models is very important. Rodents, especially mice, are widely used for this purpose 5. Clinically, the occlusion of middle cerebral artery is most of the cases in ischemic stroke 6. Therefore, middle cerebral artery occlusion (MCAO) is a representative model for ischemic stroke. It is well known that there are many factors exert important effects on the outcome in the MCAO stroke model, including the age and sex of the animal used 6. Here, we examined the outcomes of MCAO in the different strains of mice.
Four different strains of mice (25 ± 2 g in each group) were used in this study. Male ICR mice (n = 33), KM mice (n = 25), BALB/c mice (n = 31), and C57BL/6 mice (n = 29) were purchased from Sino‐British SIPPR/BK Lab Animals (Shanghai, China). All animals were used in accordance with the guidelines of Second Military Medical University for Animal Care. The animals were anesthetized with chloral hydrate (300 mg/kg) and remained anesthetized during the following operation. Then, the mice were subjected to MCAO as previously described 7 without reperfusion. After 24 h, neurological deficits were examined using a 5‐point scale 7. Mice were then sacrificed. Brain slices were prepared and stained with 1% 2, 3, 5‐triphenyltetrazolium chloride (TTC, Sinopharm Chemical Reagent Co. Ltd. Shanghai, China) 8. The infarction size was measured using an image analyzer (Image J 1.41; NIH, Bethesda, MD, USA). Data are expressed as mean ± SD and analyzed with one‐way analysis of variance (ANOVA) followed by Dunnett's t‐test for pairwise comparison.
During the following 24 h, 6, 6, 25, and 4 mice died in ICR, KM, BALB/c, and C57BL/6, respectively. The mortality is 18%, 24%, 81%, and 14%, respectively (Table 1). Then, all these brains of dead mice were removed, and signs of hemorrhage, edema or infarction were examined. The data showed that no animals died because of anesthetic or surgical accident. Edema or infarction but not hemorrhage existed in the brain, which suggested ischemic injury might be the main death reason. There are also 7 mice in ICR and 18 in C57BL/6 that failed to be induced cerebral infarction. The infarct size is 54.4 ± 14.8%, 38.8 ± 13.9%, 54.4 ± 14.8%, 12.9 ± 1.2% in ICR, KM, BALB/c, and C57BL/6, respectively (shown in Figure 1). The infarct size is the smallest in C 57BL/6 mice compared with the other 3 stains (P < 0.01). The infarct size in KM mice is smaller than ICR or BALB/c mice (P < 0.05). The neurological deficit score is significantly higher in BALB/c mice than all the other 3 strain mice, and the neurological function has no difference in ICR, KM, and C57BL/6 mice (P > 0.05).
Table 1.
The infarction rate and mortality of operation in different strains of mice with MCAO
| ICR | KM | BALB/c | C57BL/6 | |
|---|---|---|---|---|
| Number of mice with MCAO | 33 | 25 | 31 | 29 |
| The death of the animals | 6 | 6 | 25 | 4 |
| Mortality, % | 18 | 24 | 81 | 14 |
| Mice without infarction | 7 | 0 | 0 | 18 |
| Failure rate, % | 21 | 0 | 0 | 62 |
Figure 1.
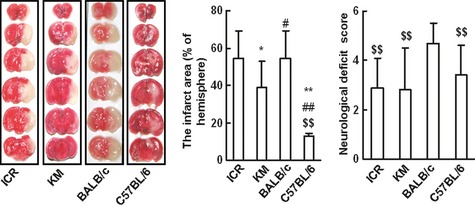
The disparity in the infarct size and neuralgic function in 4 mice strains with MCAO. The left panel: representative coronal brain sections from 4 strains of mice (ICR, KM, BALB/c, and C57BL/6). Sections were stained with 2‐3‐5‐triphenyltetrazolium chloride (TTC). Data are expressed as mean ± SD and analyzed by one‐way analysis of variance (ANOVA) followed by Dunnett's t‐test. *P < 0.05, **P < 0.01 versus ICR; #P < 0.05, ##P < 0.01 versus KM; P < 0.05, P < 0.01 versus BALB/c.
Our results show that individual susceptibility to cerebral ischemic injury and neurological functions induced by MCAO vary greatly among these different strains of mice. BALB/c mice are susceptible and sick to the ischemic injury induced by MCAO, while C57BL/6 mice are insensitive to MCAO. We recommend the ICR and KM strains but not BALB/c or C57BL/6 mice for the application of ischemic cerebral injury study in mice with MCAO.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This study was supported by China Basic Research Program (2009CB521901), National Natural Science Foundation of China (30901809) and China Postdoctoral Science fund special grant (201104279).
The first two authors contributed equally to this work.
References
- 1. Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet 2008;371: 1612–1623. [DOI] [PubMed] [Google Scholar]
- 2. Cushman M, Cantrell RA, McClure LA, Howard G, Prineas RJ, Moy CS, Temple EM, Howard VJ. Estimated 10‐year stroke risk by region and race in the United States: geographic and racial differences in stroke risk. Ann Neurol 2008;64: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yu JG, Zhou RR, Cai GJ. From hypertension to stroke: mechanisms and potential prevention strategies. CNS Neurosci Ther 2011;17: 577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics: 2008 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008;117: e25–e146. [DOI] [PubMed] [Google Scholar]
- 5. Liu F, McCullough LD. Middle cerebral artery occlusion model in rodents: methods and potential pitfalls. J Biomed Biotechnol 2011;2011: 464701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Durukan A, Tatlisumak T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav 2007;87: 179–197. [DOI] [PubMed] [Google Scholar]
- 7. Lin LL, Wang W, Cheng MH, Liu AJ. Protection of different components of Danshen in cerebral infarction in mice. CNS Neurosci Ther 2012;18: 511–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang P, Tian WW, Song J, Guan YF, Miao CY. Deficiency of NG2+ cells contributes to the susceptibility of stroke‐prone spontaneously hypertensive rats. CNS Neurosci Ther 2011;17: 327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]


