Summary
Background
Parkinson's disease (PD) is a neurodegenerative disorder characterized by progressive death of dopaminergic neurons in the substantia nigra pars compacta (SNpc).
Aims
To study if tenuigenin (TEN), the main active component of Polygala tenuifolia, can protect dopaminergic neurons from inflammation‐mediated damage in vivo.
Methods
We observed the effects of TEN on lipopolysaccharide (LPS) induced PD model by behavioral analysis, high‐performance liquid chromatography, immunohistochemistry and enzyme‐linked immunoadsorbent assay, etc.
Results
We showed that a single intranigral dose of LPSs (10 μg) induced microglial activation, reduced the survival ratio of tyrosine hydroxylase‐immunoreactive (TH‐ir) neurons in the SNpc and reduced dopamine (DA) content in the striatum. Treatment with 300 mg/kg TEN once per day over 14 weeks improved the survival rate of TH‐ir neurons in the SNpc to 75%, on the non‐injected side. Treatment with 200 or 300 mg/kg TEN once per day over 14 weeks significantly improved DA levels in the striatum to 73% and 81% on the non‐injected side, respectively. The excessive production of cytokines, such as tumor necrosis factor (TNF)‐α and interleukin (IL)‐1β, was abolished by TEN administration.
Conclusion
Our results suggest that TEN may play a role in protecting dopaminergic neurons against inflammatory challenge.
Keywords: Neuroinflammation, Parkinson's disease, Proinflammatory cytokines, Tenuigenin
Introduction
Parkinson's disease (PD) is a neurodegenerative disorder characterized by progressive death of dopaminergic neurons in the substantia nigra pars compacta (SNpc). Although the mechanism of neuronal degeneration remains to be elucidated, postmortem analysis and animal experiments have provided evidence to suggest that inflammation plays an important role in the occurrence and development of PD 1, 2, 3. Postmortem studies have shown that there is a large number of reactive microglia in the SNpc of PD patient, particularly in areas of maximal neurodegeneration, specifically the ventral and lateral portions of the SNpc 4. Under normal physiological conditions, microglia are involved in immune surveillance and host defense against infectious agents. However, microglia readily become activated in response to injury or immunological challenges. Activated microglia are believed to contribute to neurodegeneration through the release of inflammatory cytokines 5. The most obvious immune change in the brain of PD patients is a significant increase in inflammatory cytokines, such as IL‐1β, IL‐2, IL‐6, and tumor necrosis factors (TNFs) 6, 7. In contrast to the transient damage exerted on the serotonergic system, inflammatory reactions induced by intranigral injections of lipopolysaccharides (LPSs) had irreversible neurodegenerative effect on the cell body and terminal of dopaminergic neurons 8. There appears to be a delay in the time taken for significant LPS‐induced damage to occur in dopaminergic neurons. This is possibly because LPS acts via microglial activation with a subsequent release of “toxic” cytokines 9.
Given the role of microglia in mediating neurodegeneration, much effort has been made to develop novel PD treatments by targeting microglia and associated inflammatory factors 10. A previous study from our laboratory explored the use of novel drugs for the treatment of PD 11, 12. Polygala tenuifolia, also known as Yuan Zhi, is a plant in the Polygalaceae family. It is primarily used as an expectorant and is one of the 50 primary herbs used in traditional Chinese medicine. Tenuigenin (TEN), the main active component of P. tenuifolia, has garnered attention. Recent studies have indicated that TEN has anti‐dementia, anti‐aging, anti‐inflammatory, anti‐oxidant and a number of other properties 13, 14, 15, 16. P. tenuifolia root extracts have been reported to exhibit neuroprotective and neuroregenerative effects 14, 17. In a rat model of Alzheimer's disease (AD), P. tenuifolia root extracts ameliorate spatial cognition disorders and protect neuronal cells against in vitro toxins 18, 19, 20, as well as improving the cognitive behavior of AD animal models 16. Cell culture results have shown that treatment with P. tenuifolia decreases secretion of amyloid β‐protein, which is responsible for neuronal pathogenesis and cell death in AD 21, 22. BT‐11, the extract from P. tenuifolia Willdenow roots, has been shown to ameliorate memory impairment induced by scopolamine and stress in rats 15. It has also been shown to protect cultured neuronal cells against toxins. Recent studies indicate that P. tenuifolia root extracts can enhance cognitive function in elderly individuals and provide memory enhancement in healthy adults 13, 23. The extracts of P. tenuifolia have become more widely used in the research for the treatment of neurodegenerative diseases.
Our previous work revealed that TEN provides neuroprotection for dopaminergic neurons against 6‐hydroxydopamine (6‐OHDA)‐induced damage. The neuroprotective mechanisms might involve antioxidative effects, maintenance of mitochondrial function, regulation of caspase‐3 and tyrosine hydroxylase (TH) expression, and their related activities 14. To further examine if TEN can protect dopaminergic neurons against inflammation‐mediated damage in vivo, the effects of TEN treatment on microglial activation, along with the survival and function of dopaminergic neurons were investigated.
Materials and Methods
Tenuigenin
Tenuigenin was generously provided by Dr. Zhan‐Jun Zhang (National Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University). The purity of the powder was 99% determined by reverse phase high‐performance liquid chromatography (HPLC). It is an active, lipophilic ingredient from P. tenuifolia Willd. (C30H45ClO6, 537 kDa; Figure S1).
Animals and Surgery
Fifty adult male Sprague–Dawley rats weighing 280–330 g were supplied by the Beijing Vital River Lab Animal Technology Co., Ltd., (Beijing, China) and housed under standard conditions with a standard 12‐h on/off light cycle, with food and water supplied ad libitum. Rats were allowed to acclimate to their new surroundings for 10 days before experimental manipulation. The rats were anesthetized with 350 mg/kg chloral hydrate and positioned in a stereotaxic apparatus (David Kopf Instruments, CA, USA) to conform to the brain atlas of Paxinos and Watson (1986). LPS from Escherichia coli (serotype O26:B6; Sigma, MO, USA) was dissolved (5 mg/mL) in phosphate‐buffed saline (PBS), with 2.0 μL injected into the right SNpc. The injection needle was lowered through a drill hole 5.3 mm posterior, 2 mm lateral, and 7.8 mm ventral to the bregma. The injection was delivered over 2 min, and after each injection, the needle was left in situ for an additional 5 min to avoid reflux along the injection track. Thereafter, the skull surface was covered with fiber sponge, and the skin was sutured. Sham‐operated animals were subjected to the same surgical procedures, except that 2 μL PBS was injected into the SNpc. All experimental procedures were approved by the Committee on Animal Care and Usage of Capital University of Medical Sciences Center, and every effort was made to minimize animal suffering.
Application of TEN
Rats were randomly divided into 5 groups: the sham‐operated group, the LPS‐injected group followed by vehicle treatment, the LPS‐injected group followed by treatment with 200 mg/kg TEN, the LPS‐injected group followed by treatment with 300 mg/kg TEN, and the group with no LPS injected but treated with 300 mg/kg TEN. There are 10 rats in each group. Two weeks prior to, and 12 weeks after experimental manipulation, animals were intragastrically administered 0.9% saline or the corresponding concentration of TEN (1 mL) once per day at the same time.
Behavioral Analysis and Evaluation of Locomotor Activity
Locomotor activity was assessed in automated activity chambers connected to a digiscan analyzer that transmitted the number of beam breaks (activity data) to a computer (VersaMon Version 2.11; Accuscan Instruments, OH, USA). Each chamber consisted of an individual cage with a grid of infrared beams mounted horizontally every 2.5 cm. The locomotor activity was recorded as total distance traveled (cm), horizontal activity (times), movement time (s), rest time (s), and stereotypy counts during the 30‐min recording period. The lights were off, and the room was sealed from noise.
Tissue Collection and Processing
On the second day after the commencement behavioral analysis, 4 rats from each group were randomly selected for morphological studies. All other rats were exsanguinated from the heart to quantify blood proinflammatory cytokines. Rats were then decapitated, and the bilateral SNpc and striatum were rapidly dissected and stored at −80°C until required. The SNpc was used for the quantification of proinflammatory cytokines, and the striatum for determination of dopamine (DA) content. For the morphological studies, rats were anesthetized with chloral hydrate, then transcardially perfused with 100 mL saline followed by 200 mL of 4% paraformaldehyde in phosphate buffer. Brains were removed and post‐fixed in the same fixative and then cryoprotected with 20% and 30% sucrose for 3–5 days. The brains were frozen on powdered dry ice and then arranged for frontal sectioning according to the rat brain atlas of Paxinos and Watson. Frozen sections (35 μm thickness) were cut with a cryostat (Leica, Germany) at −20°C and used in immunohistochemistry analyses.
Quantification of DA and dihydroxyphenylacetic acid (DOPAC)
The striatum was processed and stored at −80°C as described above. The DA contents, and its metabolite DOPAC, were determined using an HPLC apparatus with an electrochemical detector (Model 5600A CoulArray Detector System ESA, MA, USA). Tissues were homogenized in 200 mM ice‐cold perchloric acid and the homogenate placed in an ice bath for 60 min. The sample was then centrifuged at 15,000 × g for 20 min at 4°C, and the supernatant was transferred to a clean tube and the volume determined. A one‐half volume of a solution containing 20 mM potassium citrate, 300 mM potassium dihydrogen phosphate, and 2 mM ethylenediaminetetraacetic acid (EDTA)·2Na was added and mixed thoroughly to precipitate the perchloric acid. After incubating in an ice bath for 60 min, the mix was centrifuged at 15,000 × g for 20 min at 4°C. The supernatant was filtered through a 0.22‐μm filter and injected into the HPLC system. The mobile phase was 125 mM sodium citrate buffer supplemented with 20% methanol, 0.1 mM EDTA·2Na, 0.5 mM 1‐octanesulfonic acid sodium salt (Acros Organics, NJ, USA) and adjusted to pH 4.3. The flow rate was set at 1.2 mL/min. Striata from 5 or 6 animals of each treatment group were used.
Immunohistochemistry and Quantification of Dopaminergic Neuronal Survival Rate
All sections spanning the SNpc (bregma −4.80 to −6.30 mm) were collected and used for immunohistochemistry. Every sixth section through the compacta region was processed for immunohistochemical detection of the dopaminergic neuronal marker TH. The anti‐TH mouse monoclonal antibody (Sigma) was used at 1:2000 dilutions. Sections were incubated with primary antibodies for 24 h at 4°C. As a negative control, diluted non‐immune goat serum instead of the primary antibodies was used. Sections were incubated with a biotinylated goat anti‐mouse antibody and then with s avidin–biotin–peroxidase complex for 30 min at 37°C. The bound complex was visualized by incubating sections in a solution containing 0.1% 3,3′‐diaminobenzidine (Sigma), 1% H2O2, and 8% ammonium nickel sulfate (Fluka Chemie GmbH, Buchs, Switzerland).
The TH‐ir neurons with distinct nuclei were counted among the 6 sections throughout the entire rostrocaudal extent of the SNpc. All sections were coded and examined blind. The survival rate of dopaminergic neurons in the SNpc was determined by counting the number of TH‐ir neurons on the LPS‐injected side relative to the number of TH‐ir neurons on the non‐injected side.
Quantitation of Proinflammatory Cytokines
We quantified the level of proinflammatory cytokines in each group of rats from their blood and in the SNpc. The blood from the rat hearts was placed in clean tubes containing anticoagulants, then centrifuged at 1500 × g for 15 min at 4°C. The supernatant was collected and used immediately. For the SNpc, it was processed and stored at −80°C as described above. Tissues were homogenized in ice‐cold tissue lysis buffer containing 137 mM NaCl, 20 mM Tris (pH 8.0), 1% (v/v) glycerol, 1% (v/v) Nonidet P‐40 (NP40), 1 mM phenylmethylsulfonyl fluoride, and 0.5 mM sodium vanadate. The homogenate was centrifuged at 1500 × g for 15 min at 4°C. The supernatant was collected and kept at 4°C; if not used immediately, the supernatant was stored at −80°C until required. The levels of TNF‐α and IL‐1β were detected using rat TNF‐α and IL‐1β enzyme‐linked immunoadsorbent assay (ELISA) kits (Shanghai ExCell Biology Inc., Shanghai, China), respectively, according to the manufacturer's instructions. The sensitivity of the ELISA assay was 15 pg/mL for both the TNF‐α and the IL‐1β. Standards were assayed in duplicate. The protein concentration was determined using a detergent‐compatible protein assay with bovine serum albumin as the standard. The SNpc tissue from 5 or 6 animals of each treatment group was used.
Statistical Analysis
Data were analyzed using graphpad prism 4.0 software (www.graphpad.com/welcome.htm). All results were presented as the mean ± SEM. One‐way analysis of variance with Dunnett and Newman‐Keul's post hoc test was used to compare means within groups, and Student's t‐test was applied for unpaired samples. A P‐value < 0.05 was considered statistically significant.
Results
TEN Administration Improves Functional Recovery
The locomotion activity results show that whole activity in the vehicle‐treated control group was significantly lower than in other groups. A summary of activities such as total movement distance, movement time, horizontal activity and stereotypy counts for this group is shown in Figure 1(A–D). However, the rest time for the rats in the vehicle‐treated control group was significantly higher than the others (Figure 1E). Treatment with TEN (200 or 300 mg/kg) for 14 weeks significantly enhanced all measured activities except for rest time, which was reduced. Figure 1F shows the effects of TEN on the 30‐min track‐plot‐pictures of LPS rats.
Figure 1.
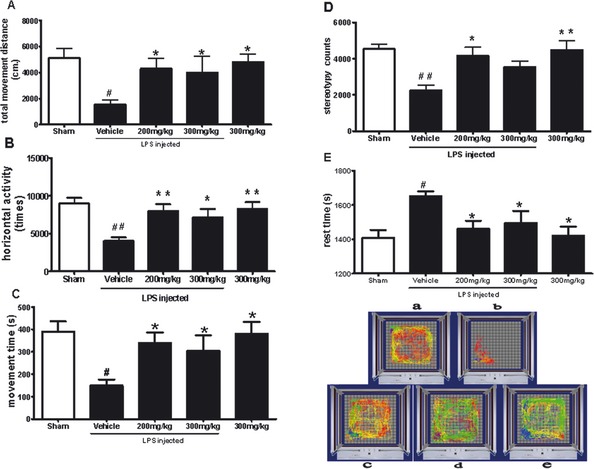
Effects of tenuigenin (TEN) on the locomotor activity of lipopolysaccharide (LPS)‐treated rats. Rats were randomly grouped and then pre‐treated with TEN (200 or 300 mg/kg day) or vehicle 2 weeks before LPS injection and for 12 weeks after LPS injection. On day 92, rats were placed in automated activity chambers, and locomotor activity over 30 min was measured and analyzed by an accuscan 2.11 system (OH, USA) (A) total movement distance; (B) horizontal activity; (C) movement time; (D) stereotypy counts; and (E) rest time are shown as mean ± SEM. n = 9; *P < 0.05, **P < 0.01, ***P < 0.001; * vs. vehicle, # vs. sham. (F) shows the effects of TEN on the track‐plot pictures of rats. (a) sham (without LPS and TEN); (b) vehicle; (c) 200 mg/kg TEN with LPS injection; (d) 300 mg/kg TEN with LPS injection; and (e) 300 mg/kg TEN.
TEN Administration Attenuates Depletion of DA and DOPAC
The adequacy of the LPS injection was confirmed by a decrease of striatal DA content. In the vehicle‐treated control group, the levels of DA and DOPAC on the LPS‐injected side were reduced to 36% and 47%, respectively, when compared with the non‐injected side. Treatment with TEN (200 or 300 mg/kg) for 14 weeks significantly attenuated DA depletion in the striatum, which was induced by LPS intranigral injection (Figure 2). The levels of DA on the LPS‐injected side were increased to 73% and 81% of the non‐injected side in animals treated with 200 and 300 mg/kg TEN, respectively (Figure 2A). The levels of DOPAC on the LPS‐injected side were increased to 79% and 84% of the non‐injected side in groups treated with 200 and 300 mg/kg TEN, respectively (Figure 2B).
Figure 2.
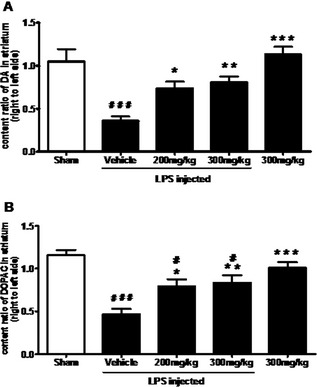
Effect of tenuigenin (TEN) treatment on dopamine (DA) and dihydroxyphenylacetic acid (DOPAC) contents in the striatum. Rats were randomly grouped and then pretreated with TEN (200 and 300 mg/kg day) or vehicle 2 weeks before lipopolysaccharide (LPS) injection and 12 weeks after LPS injection. On day 98, rats were decapitated and striatum were dissected. DA (A) and DOPAC (B) contents in the striatum were detected by high‐performance liquid chromatography, and the ratios of right to left side were calculated. n = 5–6. *,# P < 0.05, **,## P < 0.01, ***,### P < 0.001; * vs. vehicle; # vs. sham.
TEN Treatment Protects Dopaminergic Neurons from LPS‐Induced Injury
In sham‐operated animals, the number of TH‐ir neurons was similar on the ipsilateral and contralateral sides to the injection site (Figure 3A). The survival ratio of TH‐ir neurons was 89% (Figure 3E). Animals that received vehicle treatment after LPS intranigral injection showed a marked loss of TH‐ir neurons and their dendrites (Figure 3B); only 37% of TH‐ir neurons in the SNpc on the LPS‐injected side survived, compared with those on the non‐ injected side (Figure 3E). In contrast, a significantly large proportion of the TH‐ir neurons survived in the animals treated with TEN, and the dendritic processes surrounding the TH‐ir neurons were largely preserved (Figure 3D). At a dose of 300 mg/kg, TEN preserved as many as 75% of TH‐ir cells on the LPS‐injected side, compared with those on the non‐injected side (Figure 3E). At a dose of 200 mg/kg, TEN was less effective, preserving only 59% of TH‐ir neurons in the LPS‐injected vehicle‐treated group (Figure 3C,E).
Figure 3.
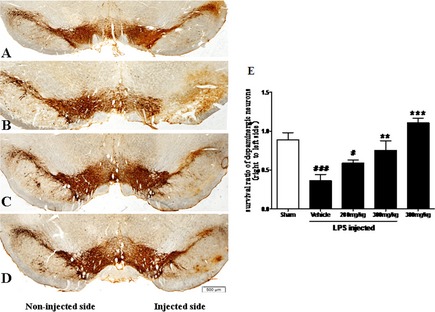
Morphological evidence of the protective effect of tenuigenin (TEN) against lipopolysaccharide (LPS)‐induced damage to dopaminergic neurons in the substantia nigra pars compacta (SNpc). Rats were randomly grouped and pre‐treated with TEN (200 and 300 mg/kg day) or vehicle 2 weeks before LPS injection and 12 weeks after LPS injection. On day 96, rats were transcardially perfused with 4% paraformaldehyde. Frozen sections (35‐μm‐thick sections) were cut and tyrosine hydroxylase (TH) was detected by immunohistochemical staining to show dopaminergic neurons in the SNpc. Photomicrographs were captured using a spot‐2 imaging analysis system (MI, USA). (A) TH staining in sham‐operated rats was similar on the non‐injected (left) and phosphate‐buffed saline‐injected (right) sides. (B) Twelve weeks after the LPS injection, a profound loss of tyrosine hydroxylase‐immunoreactive (TH‐ir) cells is seen on the injected side in animals treated with vehicle after LPS injection. (C and D) In rats treated with 200 mg/kg (C) or 300 mg/kg (D) TEN for 14 weeks, a greater proportion of TH‐ir cells survived on the injected side compared with the injected side in vehicle‐treated rats. The scale bar represents 500 μm. (E) TH‐ir neurons in the SNpc were counted, and the survival ratio of the dopaminergic neurons in the SNpc (the injected side vs. the non‐injected side) was calculated. n = 4. *,# P < 0.05, **,## P < 0.01, ***,### P < 0.001; * vs. vehicle; # vs. sham.
TEN Inhibits Microglial Release of Proinflammatory Cytokines
Focal injection of LPS induced excessive release of proinflammatory cytokines. In the SNpc of sham‐operated animals and on the non‐injected side of LPS‐injected animals, the concentrations of TNF‐α and IL‐1β were significantly lower than in the vehicle‐treated control group. In the LPS‐injected vehicle‐treated controls, the concentrations of TNF‐α and IL‐1β in the SNpc were 3.85 ± 0.66 and 7.66 ± 2.39 pg/mg protein, respectively. Treatment with 200 mg/kg TEN significantly reduced the concentration of TNF‐α and IL‐1β by 38.4% and 50.5%, respectively. Treatment with 300 mg/kg TEN reduced the production of TNF‐α and IL‐1β by 31.4% and 64.4%, respectively (Figure 4A,B).
Figure 4.
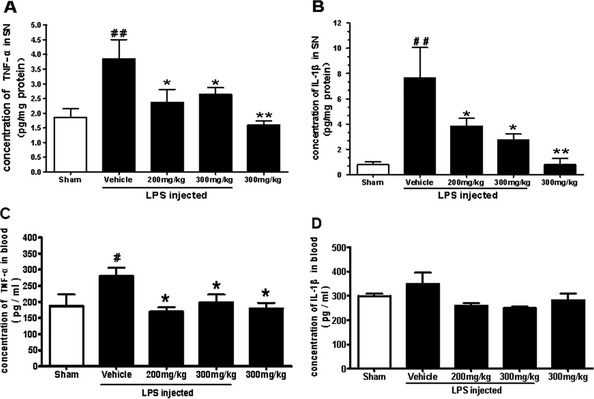
Effects of tenuigenin (TEN) treatment on the concentrations of proinflammatory cytokines in the substantia nigra pars compacta (SNpc) of lipopolysaccharide (LPS)‐injected rats. Rats were randomly grouped and then pretreated with TEN (200 or 300 mg/kg day) or vehicle 2 weeks before LPS injection and 12 weeks after LPS injection. On day 98, rats were decapitated, and the SNpc were dissected. The concentration of tumor necrosis factor (TNF)‐α (A) and interleukin (IL)‐1β (B) was detected using commercial enzyme‐linked immunoadsorbent assay (ELISA) kits. n = 5–6. *,# P < 0.05, **,## P < 0.01, ***,### P < 0.001; * vs. vehicle; # vs. sham. The concentration of TNF‐α (C) and IL‐1β (D) in blood obtained from rat hearts was determined by ELISA. n = 5. *,# P < 0.05, **,## P < 0.01, ***,### P < 0.001; * vs. vehicle; # vs. sham.
In the LPS‐injected vehicle‐treated control group, the concentration of TNF‐α in blood was significantly higher than in the other groups (Figure 4C). The concentration of IL‐1β in blood of the LPS‐injected vehicle‐treated group was slightly higher than in the other groups but not statistically significant (Figure 4D).
Discussion
Lipopolysaccharide, a component of the Gram‐negative bacterial cell wall, is a potent inducer of inflammation. LPS treatment can activate microglia in vitro to release proinflammatory cytokines such as TNF‐α and IL‐1β 24. In the current study, we showed that focal injection of LPS induced microglial activation and release of proinflammatory cytokines in the SNpc (Figure 4A, B). The locomotor activity in the LPS‐injected group followed by vehicle treatment was significantly reduced (Figure 1A–D). Furthermore, there was a significant decrease in the number of TH‐ir cells in the SNpc, and depletion of DA and DOPAC contents in the striatum. These results are in agreement with previous studies showing that intranigral LPS injection induces an inflammatory reaction and damages the nigrostriatal dopaminergic system 8. In our experiments, intranigral LPS injection led to the local degeneration of dopaminergic neurons in the SNpc, thereby representing a model for PD‐like neurodegeneration and motor deficits. This is the model that our laboratory has been using to conduct research into PD 25.
Our results demonstrated that TEN, an active compound of the traditional Chinese medicine P. tenuifolia, significantly improved the behavioral manifestation of dopaminergic degeneration and prevented the loss of dopaminergic neurons. We also found that TEN potently inhibited LPS‐induced activation of microglia and production of deleterious cytokines, clearly demonstrating the neuroprotective effects of TEN.
Tenuigenin passes through the blood–brain barrier easily because of its lipophilic characteristics and small molecular size (MW 537). We studied its potential anti‐inflammatory effects in the central nervous system (CNS). Our previous work demonstrated that treatment with TEN attenuated the release of inflammatory cytokines, such as TNF‐α and IL‐1β, in LPS‐treated primary cultured microglia. Experiments indicated that TEN had potent anti‐inflammatory activity in the CNS.
In the brain, the concentrations of TNF‐α and IL‐1β were both significantly enhanced by LPS treatment. However, in the blood, only the concentrations of TNF‐α was statistically increased compared to other groups. We consider that inflammatory cytokines in the blood mainly come from the brain, since TNF‐α is usually the first inflammatory cytokine released by inflammatory tissues, and represents the early event of inflammation. Our observation suggests that the increase in inflammatory cytokines in blood might result from the impaired blood brain barrier or peripheral inflammation secondary to brain damage.
Neuroprotection by TEN has been demonstrated in models of AD 18, 19, 20. Recently, P. tenuifolia was reported to provide sedative and anti‐dementia neuroprotective efficacy in several in vitro pharmacological studies, as well as anti‐oxidative and anti‐neurotoxic effects in cultured neurons 14, 21, 22. The present study shows that the neuron protective effects of TEN might result from the inhibition of LPS‐induced microglial activation and the release of inflammatory cytokines. Taken together, our in vitro and in vivo experiments indicate that TEN can protect dopaminergic neurons from multiple pathways including anti‐inflammatory mechanism.
The neuroinflammatory reaction is not specific to PD, as it has been conclusively observed in many other neurodegenerative diseases including AD and multiple sclerosis. Neuroinflammatory processes possibly participate in the propagation of the neurodegenerative process in these diseases. Drugs targeting specific neuroinflammatory‐associated deleterious mechanisms may prove effective in slowing or even halting the progress of neurodegeneration.
Conclusion
Our study has demonstrated in vivo that microglial activation induced by the intranigral injection of LPS had a degenerative effect on dopaminergic neurons, and that TEN, a traditional Chinese herb with anti‐inflammatory and immunosuppressive properties, can improve the survival of these injured neurons by inhibiting microglial activation and the release of proinflammatory cytokines. Our recent study also suggests that TEN could protect dopaminergic neurons from lesions induced by LPS treatment and activation of microglia in vitro (L. Lu, unpublished data). Taken together with our previous results, this study further suggests the effectiveness of TEN in protecting dopaminergic neurons against inflammatory challenge.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Figure S1 Chemical structure of tenuigenin.
Acknowledgments
This study was supported by the National Basic Research Program of China (2011CB504100), National Nature Science Foundation of China (30973540, 81030062).
References
- 1. Grunblatt E, Mandel S, Maor G, Youdim MB. Gene expression analysis in N‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine mice model of Parkinson's disease using cDNA microarray: Effect of R‐apomorphine. J Neurochem 2001;78:1–12. [DOI] [PubMed] [Google Scholar]
- 2. Hunot S. Neuroinflammatory processes in Parkinson's disease. Ann Neurol 2003;53(Suppl 3) :S49–S60. [DOI] [PubMed] [Google Scholar]
- 3. Miller RL, James‐Kracke M, Sun GY, Sun AY. Oxidative and inflammatory pathways in Parkinson's disease. Neurochem Res 2009;34:55–65. [DOI] [PubMed] [Google Scholar]
- 4. Hirsch EC, Hunot S, Damier P, Faucheux B. Glial cells and inflammation in Parkinson's disease: A role in neurodegeneration? Ann Neurol 1998;44:S115–S120. [DOI] [PubMed] [Google Scholar]
- 5. Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog Neurobiol 1999;57:563–581. [DOI] [PubMed] [Google Scholar]
- 6. Long‐Smith CM, Sullivan AM, Nolan YM. The influence of microglia on the pathogenesis of Parkinson's disease. Prog Neurobiol 2009;89:277–287. [DOI] [PubMed] [Google Scholar]
- 7. Tansey MG, Goldberg MS. Neuroinflammation in Parkinson's disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis 2010;37:510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Castano A, Herrera AJ, Cano J, Machado A. Lipopolysaccharide intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system. J Neurochem 1998;70:1584–1592. [DOI] [PubMed] [Google Scholar]
- 9. Hunter RL, Cheng B, Choi DY, Liu M, Liu S, Cass WA, Bing G. Intrastriatal lipopolysaccharide injection induces parkinsonism in C57/B6 mice. J Neurosci Res 2009;87:1913–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Castano A, Herrera AJ, Cano J, Machado A. The degenerative effect of a single intranigral injection of LPS on the dopaminergic system is prevented by dexamethasone, and not mimicked by rh‐TNF‐alpha, IL‐1beta and IFN‐gamma. J Neurochem 2002;81:150–157. [DOI] [PubMed] [Google Scholar]
- 11. Li FQ, Cheng XX, Liang XB, et al. Neurotrophic and neuroprotective effects of tripchlorolide, an extract of Chinese herb Tripterygium wilfordii Hook F, on dopaminergic neurons. Exp Neurol 2003;179:28–37. [DOI] [PubMed] [Google Scholar]
- 12. Luo D, Zhang Q, Wang H, et al. Fucoidan protects against dopaminergic neuron death in vivo and in vitro. Eur J Pharmacol 2009;617:33–40. [DOI] [PubMed] [Google Scholar]
- 13. Lee JY, Kim KY, Shin KY, Won BY, Jung HY, Suh YH. Effects of BT‐11 on memory in healthy humans. Neurosci Lett 2009;454:111–114. [DOI] [PubMed] [Google Scholar]
- 14. Liang Z, Shi F, Wang Y, Lu L, Zhang Z, Wang X. Neuroprotective effects of tenuigenin in a SH‐SY5Y cell model with 6‐OHDA‐induced injury. Neurosci Lett 2011;497:104–109. [DOI] [PubMed] [Google Scholar]
- 15. Shin KY, Won BY, Heo C, et al. BT‐11 improves stress‐induced memory impairments through increment of glucose utilization and total neural cell adhesion molecule levels in rat brains. J Neurosci Res 2009;87:260–268. [DOI] [PubMed] [Google Scholar]
- 16. Zhang H, Han T, Zhang L, et al. Effects of tenuifolin extracted from radix polygalae on learning and memory: A behavioral and biochemical study on aged and amnesic mice. Phytomedicine 2008;15:587–594. [DOI] [PubMed] [Google Scholar]
- 17. Park CH, Choi SH, Koo JW, et al. Novel cognitive improving and neuroprotective activities of Polygala tenuifolia Willdenow extract, BT‐11. J Neurosci Res 2002;70:484–492. [DOI] [PubMed] [Google Scholar]
- 18. Egashira N, Yuzurihara M, Hattori N, Sakakibara I, Ishige A. Ninjin‐yoei‐to (Ren‐Shen‐Yang‐Rong‐Tang) and Polygalae radix improves scopolamine‐induced impairment of passive avoidance response in mice. Phytomedicine 2003;10:467–473. [DOI] [PubMed] [Google Scholar]
- 19. Sun XL, Ito H, Masuoka T, Kamei C, Hatano T. Effect of Polygala tenuifolia root extract on scopolamine‐induced impairment of rat spatial cognition in an eight‐arm radial maze task. Biol Pharm Bull 2007;30:1727–1731. [DOI] [PubMed] [Google Scholar]
- 20. Zhang D, Zhang JJ, Liu GT. The novel squamosamide derivative FLZ protects against 6‐hydroxydopamine‐induced apoptosis through inhibition of related signal transduction in SH‐SY5Y cells. Eur J Pharmacol 2007;561:1–6. [DOI] [PubMed] [Google Scholar]
- 21. Jia H, Jiang Y, Ruan Y, et al. Tenuigenin treatment decreases secretion of the Alzheimer's disease amyloid beta‐protein in cultured cells. Neurosci Lett 2004;367:123–128. [DOI] [PubMed] [Google Scholar]
- 22. Lv J, Jia H, Jiang Y, et al. Tenuifolin, an extract derived from tenuigenin, inhibits amyloid‐beta secretion in vitro. Acta Physiol (Oxf) 2009;196:419–425. [DOI] [PubMed] [Google Scholar]
- 23. Shin KY, Lee JY, Won BY, et al. BT‐11 is effective for enhancing cognitive functions in the elderly humans. Neurosci Lett 2009;465:157–159. [DOI] [PubMed] [Google Scholar]
- 24. Le W, Rowe D, Xie W, Ortiz I, He Y, Appel SH. Microglial activation and dopaminergic cell injury: An in vitro model relevant to Parkinson's disease. J Neurosci 2001;21:8447–8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou HF, Liu XY, Niu DB, Li FQ, He QH, Wang XM. Triptolide protects dopaminergic neurons from inflammation‐mediated damage induced by lipopolysaccharide intranigral injection. Neurobiol Dis 2005;18:441–449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Chemical structure of tenuigenin.


