SUMMARY
Aims: To investigate the tolerability and efficacy of carbamazepine treatment in patients with partial‐onset seizures and the association with polymorphisms in the sodium channel α‐subunit type 1 (SCN1A), and gamma‐aminobutyric acid (GABA) receptor genes among the Chinese Han population. Methods: 448 patients were genotyped for single nucleotide polymorphisms selected of the SCN1A and GABA‐receptor genes. Monotherapy with carbamazepine (CBZ) was administered to the patients. The effectiveness of CBZ treatment was evaluated with regard to efficacy by the decrease in seizures and tolerability by retention rates. Results: SCN1A rs3812718 A/G with CBZ tolerability (P= 0.038) throughout 24 months of clinical follow‐up and the GABRA1 rs2290732 A/G were significantly associated with CBZ tolerability (P= 0.001). The maintenance dose and serum level of CBZ in AA genotype carriers of rs3812718 A/G were significantly higher than those of GG genotype carriers between 3 and 12 months of follow‐up. The proportion of AA genotype carriers of rs2298771 A/G with seizure free was significantly higher than that of AG+GG genotype carriers from 3 months to 15 months of follow‐up (P < 0.05). Conclusion: rs3812718 A/G and rs2290732 A/G polymorphisms affected the tolerability of CBZ. rs2298771 A/G was associated with efficacy of CBZ treatment.
Keywords: Carbamazepine, Efficacy, Epilepsy, GABA receptor, Polymorphism, SCN1A, Tolerability
Introduction
Epilepsy is one of the most common chronic neurological conditions and its pathogenesis is unclear. The epileptic symptom is usually one of unprovoked seizures [1]. In general, an imbalance between excitatory and inhibitory neurotransmission has been regarded to be the main cause of seizures [2]. The development of epilepsy is associated with genetic factors. Only approximately 2% of cases of idiopathic epilepsy have been associated with monogenetic inheritance; most have been correlated with multiple genes [3, 4]. Epilepsy affects approximately 50 million people worldwide [5, 6]. In China, about seven to nine million individuals suffer from various types of epileptic seizures [7].
At present, treatment with antiepileptic drugs (AEDs) is the main approach to the control of epileptic seizures. Such therapy is used in an attempt to restore the balance between excitatory and inhibitory transmission. Drugs, such as carbamazepine (CBZ), phenytoin, and lamotrigine inhibit hyperexcitable neuron transmission, whereas others, such as valproate, gabapentin, and vigabatri, induce hypoexcitability. CBZ targets the sodium channel α‐subunit type 1 (SCN1A), and is the most important first‐line AED for medication of patients with partial seizures in China. In addition, studies have reported that CBZ may also potentiate gamma‐aminobutyric acid (GABA) receptors [8, 9].
In the treatment of epilepsy, continuation of therapy (“drug tolerability”) and seizure remission are the two main characteristics used to assess the effectiveness of AEDs [10, 11]. Usually, the tolerability of AEDs is measured by the retention rates (the proportions of patients who have continued to take the drug over a defined period) and seizure remission by a decrease in the rates of seizures. Investigations have shown that seizure remission could be maintained in 70% of epileptic patients, but that the remaining 30% developed drug resistance in spite of treatment with various AEDs [5, 12, 13, 14]. Meanwhile, drug resistance is one of the main factors in nonretention of AEDs.
In previous unpublished work with 448 initial participants, we found that almost 40% of patients with new‐onset partial seizures could not continue taking CBZ monotherapy because of lack of efficacy, adverse effects, and other factors, and we also found that seizure remission was maintained after 1 year of treatment (data not published). We still detected heterogeneity in the effects of CBZ treatment that was related to polymorphisms in the SCN1A gene. The maintenance doses and serum levels, as well as the retention rates of CBZ in patients undergoing treatment were associated with the polymorphism rs3812718 A/G in SCN1A. In addition, a decrease in seizure rates was associated with the rs2298771 A/G polymorphism (data not published).
In the study reported herein, we investigated trends in the efficacy and tolerability of long‐term treatment with CBZ using follow‐up assessments of the participants at every 3‐month intervals. We also investigated the association between genetic polymorphisms of SCN1A and of GABA receptors, which comprise α1, β2, and γ2 subunits (encoded by the GABRA1, GABRB2, and GABRG2 genes, respectively), and the efficacy and tolerability of CBZ treatment in Chinese patients of Han ethnicity with a new onset of partial seizures during 2 years of clinical follow‐up.
Methods and Materials
Ethical Approval
The clinical trial of this study was registered in Chinese Clinical Trial Registry and the clinical trial registration number was ChiCTR‐TCH‐10000813. Before the beginning of the study, informed consent was obtained from each participant. The study protocol was approved by the Ethics Committee of Xiangya School of Medicine, Central South University.
Participants
In total, 448 patients who were more than 12 years old, suffered from idiopathic epilepsy, and had reported a new onset of partial seizures were recruited for the study. The study samples were composed of 229 males and 219 females, who were administered CBZ monotherapy for the first time. All the patients were recruited from leading hospitals in Changsha City of China. The treatment period lasted from April 2008 to April 2011. The following were the inclusion criteria for the study: 1) patients had received a confirmed diagnosis of partial seizures; and 2) patients had never received AED treatment. The following patients were excluded: 1) patients who were pregnant or lactating; 2) patients with mental disorders, such as schizophrenia; 3) patients who suffered nonepileptic seizures; and 4) patients with any other medical conditions that might influence their liver and renal function. Informed consent was obtained, and the study protocol was approved by the Ethics Committee of Xiangya School of Medicine, Central South University before the study commenced.
Design
Definition and Determination of the Primary Characteristics of CBZ Tolerability and Efficacy
The tolerability and efficacy of CBZ were defined and evaluated as in previous investigations [10, 11]. In the current study, we focused on three primary outcomes: the serum levels of CBZ, tolerability to CBZ, and decrease in the frequency of seizures. The serum levels of CBZ were determined using high performance liquid chromatography (HPLC). The retention rates for CBZ were used to assess tolerability; they were calculated as the proportion of patients who had continued to take CBZ as medication for seizures over the preceding 3 months [15]. The cumulative survival proportion was used to estimate the tolerability of CBZ as long‐term therapy. To estimate the efficacy of CBZ, four semiquantitative levels of decrease in seizure frequency: seizure free (SF), the efficacy of 75%–SF, the efficacy of 50–75%, and the efficacy of <50%, were introduced in this study. We defined the efficacy of seizure free as a “good” response to medication, and the combination of 75%‐SF, 50–75% and <50% efficacy as a “bad” response to medication. Our previous unpublished study revealed that the G allele of rs2298771 A/G was a risk factor for a bad response to medication under the dominant model (data not published). In the present study, we compared the efficacy of CBZ therapy in carriers of the AA genotype with that in AG+GG carriers in terms of a decrease in seizure rates.
Treatment Protocol
All recruited participants were treated with CBZ monotherapy. At the first and subsequent clinical visits, which occurred every 3 months during 2 years of treatment, clinical data were collected using questionnaires, reviews of medical records, including medical records, family members, and data from primary treating physicians. In the first visit, the data collected included sex, age, age at the first onset of epilepsy, risk factors for epilepsy, family history of epilepsy, medical and neurological history, classification of epilepsy and epileptic syndrome, number of seizures in the past 3 months, and electroencephalographic (EEG, tested by scalp electrodes), and imaging data obtained. For adolescents, the initial dose of CBZ was 200 mg/d with a weekly increase of 100 mg until an appropriate value was reached. For adults and elderly patients, the initial dose was 200 mg/d with a daily increase of 100 mg until an appropriate value was reached. At every visit, the doses of CBZ taken, the serum levels of CBZ, and compliance were also recorded. In participants who experienced a recurrence of seizure, the doses of CBZ were increased. In the case of resistance to CBZ or a serious adverse reaction, CBZ was substituted with another AED. Reports of adverse reactions to the drug, including disorders of liver function, serum lipid levels, and changes in blood cells count, and the cause of discontinuation of medication were also recorded.
Selections of Candidate Genes and Single Nucleotide Polymorphisms (SNPs) and Genotyping of SNPs
We selected four candidate genes that are targeted by CBZ: SCN1A, GABRA1, GABRB2, and GABRG2. The SCN1A gene encodes an excitatory voltage‐gated channel and the GABRA1, GABRB2, and GABRG2 genes encode the α1, β2, and γ2 subunits of the receptor for the inhibitory neurotransmitter GABA, respectively.
We selected SNPs according to a comprehensive consideration of the following criteria: 1) the minor allele frequency (MAF) was more than 10% in the Hapmap CHB (Chinese Han in Beijing) population; 2) tag SNPs was selected by SNPbrowser (version 4.0.1) in the Hapmap database (release 20, January 24, 2006), the selected SNPs had the parameters: pair‐wise r2 (r2≥ 0.8) and haplotype R2 (R2≥ 0.8); and 3) the SNPs had validated functions in the promoter, exons, splice sites of introns or 3’ untranslated region (3’‐UTR). Coefficients of linkage disequilibrium (D′) and pairwise r values were used to assess the degree of linkage disequilibrium (LD) between pairs of loci using SNPbrowser [16]. All the SNPs selected were confirmed in the HapMap (http://www.hapmapp.org) and National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/SNP/) databases. The basic characteristics of the SNPs and the candidate genes are listed in Table 1. DNA was extracted from the peripheral blood of the participants using phenol–chloroform. All DNA samples were shipped to GenScript(Nanjing) Co., Ltd. and all SNPs were genotyped by Sequencing according to the manufacturer's instructions.
Table 1.
Demographics and clinical characteristics of the recruited patients (n = 448)
| Characteristics | Patients with epilepsy |
|---|---|
| Gender (M/F) | 229/219 |
| Adolescents (n, %) | 141 (31.5) |
| Adults and elderly patients (n, %) | 307 (68.5) |
| Age (years) (SD) | 30.4 (16.7) |
| Adolescents | 15.4 (5.2) |
| Adults and elderly patients | 35.1 (19.5) |
| Height (m) (SD) | 153 (8.7) |
| Adolescents | 146 (12.4) |
| Adults and elderly patients | 164 (7.9) |
| Weight (kg) (SD) | 49.3 (9.5) |
| Adolescents | 44.7 (10.8) |
| Adults and elderly patients | 53.9 (9.8) |
| BMI (kg/m2) (SD) | 20.4 (3.0) |
| Adolescents | 18.7 (3.6) |
| Adults and elderly patients | 21.0 (3.2) |
| Seizure type (%)* | – |
| Simple partial | 145 (32.4) |
| Complex partial | 196 (43.8) |
| Secondary generalization | 153 (34.2) |
| EEG (%) | – |
| Normal | 185 (41.3) |
| Epileptiform | 263 (58.7) |
BMI, Body mass index.
EEG, Electroencephalography.
*Some patients might have suffered from more than one type of seizure.
Statistical Analysis
All analyses were performed using the Statistical Package for Social Sciences, version 18.0 (SPSS Inc, Chicago, Illinois). The Pearson χ2 test was used to assess whether the genotypic frequencies among the participants conformed to Hardy–Weinberg equilibrium (HWE), and also to evaluate differences in the proportions of participants who showed particular levels of seizures decrease among the carriers of the various genotypes within the cohort. Analysis of covariance (ANCOVA) was used to assess differences in the maintenance doses and serum levels of CBZ in carriers of various genotypes for all the SNPs, adjusted by age, sex, and body mass index (BMI; defined as the individual's body weight divided by the square of his or her height). Kaplan–Meier survival analysis (using time to event data) was used to assess the CBZ tolerability of the carriers of different genotypes of the SCN1A and GABA receptor genes. Logistic regression analysis was used to adjust the effect among SNPs and other potential factors. The dependent strength between SNPs was evaluated by the haploview software using the r and D′ statistics. The test of significance was two‐tailed, and alpha was set at 0.05.
Results
Characteristics of the Participants and SNPs Selected
Table 1 shows basic information at baseline for the 448 patients recruited. The 448 patients comprised 229 males and 219 females, of whom 141 were adolescents and 307 adults. The average age of all participants was 30.4 years; their mean height was 153 cm, weight 49.3 kg, and BMI 20.4 kg/m2. Of the 448 participants, 145 (32.3%) suffered simple partial seizures, 196 (43.8%) complex partial seizures, 153 (34.2%) secondarily generalized seizures. Some patients might have suffered from more than one type of seizure. On electroencephalogram (EEG) examination, 185 participants (41.3%) showed normal traces and 263 (58.7%) epileptiform abnormalities.
Table 2 lists the basic characteristics of the SNPs selected for the SCN1A and GABA receptor genes. All SNPs conformed to HWE. The MAF of the all SNPs ranged from 12.0% to 46.2%.
Table 2.
Characteristics of the SNPs selected for the SCN1A gene and GABA receptor genes in the recruited participants (n = 448)
| SNP | Gene | Locus | Location (bp) | Allele | Function | MAF (%) | HWE P | Reference |
|---|---|---|---|---|---|---|---|---|
| rs2298771 | SCN1A | exon | 166601034 | A/G | missense | 13.0 | 0.408 | [25] |
| rs3812718 | SCN1A | intron | 166617790 | C/T | spice site | 46.2 | 0.853 | [32] |
| rs4667869 | SCN1A | intron | 166631605 | C/G | NR | 34.1 | 0.399 | NR |
| rs11692675 | SCN1A | intron | 166634674 | C/T | NR | 18.9 | 0.517 | NR |
| rs1020853 | SCN1A | intron | 166588028 | G/T | NR | 44.4 | 0.979 | NR |
| rs10497275 | SCN1A | 3′UTR | 166554976 | A/G | NR | 33.1 | 0.989 | NR |
| rs7577411 | SCN1A | 3′UTR | 166554040 | A/G | NR | 12.0 | 0.200 | NR |
| rs1813502 | SCN1A | 3′UTR | 166554262 | A/G | NR | 21.1 | 0.161 | NR |
| rs12658835 | GABRA1 | 5’‐UTR | 161275302 | A/G | promoter | 29.3 | 0.986 | [33] |
| rs2290732 | GABRA1 | 3’‐UTR | 161324898 | A/G | NR | 38.8 | 0.055 | [34] |
| rs35166395 | GABRA1 | exon | 161281245 | C/T | synonymous | 42.4 | 0.602 | [33] |
| rs592403 | GABRB2 | 3’‐UTR | 160719984 | G/T | NR | 46.1 | 0.079 | [29] |
| rs211037 | GABRG2 | exon | 161528280 | T/C | synonymous | 43.8 | 1.00 | [25] |
| rs424740 | GABRG2 | 3’‐UTR | 161581035 | A/T | NR | 36.3 | 0.643 | [35] |
NR, not reported.
Allele, major/minor allele.
MAF, minor allele frequency.
HWE P, P value for Hardy–Weinberg equilibrium analysis.
Location (bp) from http://hapmap.ncbi.nlm.nih.gov.
The rs3812718 A/G and rs2290732 A/G SNPs were positively correlated with retention rates over 24 months of clinical follow‐up
Significant differences existed in the retention rates of CBZ with respect to the rs3812718 A/G polymorphism of the SCN1A gene over the 24 months of clinical follow‐up by Kaplan–Meier survival analysis (P= 0.038, Figure 1A). For 24 months of CBZ treatment, the overall retention rate was 50.7%. For the GG genotype, the retention rate was 57.3%, for the AG genotype 52.3%, and for the AA genotype 43.8%. Significant differences also existed in the retention rates of CBZ over the 24 months of clinical follow‐up with respect to the rs2290732 A/G polymorphism of the GABRA1 gene by Kaplan–Meier analysis (P= 0.001, Figure 1B). For 24 months of CBZ treatment, the overall retention rate was 50.7%. For the AA genotype it was 62.9%, for the AG genotype 52.5%, and for the AA genotype only 28.2%.
Figure 1.
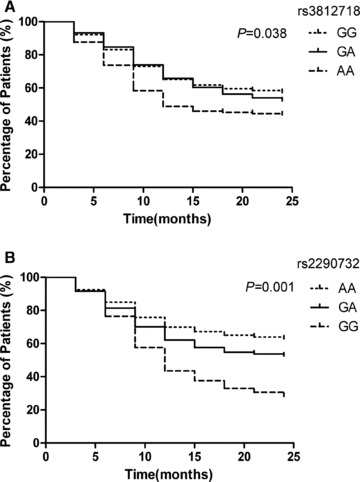
Retention rates for CBZ treatment of carriers of the various genotypes. (A) Retention rates of the carriers of the various rs3812718 A/G genotypes during 24 months of follow‐up. (B) Retention rates of the carriers of the various rs2290732 A/G genotypes during 24 months of follow‐up. *P<0.05
To assess whether rs3812718 and rs2290732 were independent from each other, binary logistic regression analysis was conducted with the tolerability of CBZ as a dependent variable. The results showed that, from the 3‐month to the 6‐month visit, neither rs3812718 nor rs2290732 was associated with the tolerability of CBZ (data not shown). From the 9‐month to the 15‐month visit, rs3812718 and rs2290732 were associated independently with the tolerability of CBZ. The association between rs3812718 and tolerability disappeared from the 18‐month to the 24‐month visit, but this was not the case for rs2290732. These findings indicated that rs3812718 and rs2290732 were associated independently with the tolerability of CBZ (Table 3).
Table 3.
Association between rs3812718 G/A and rs2290732 A/G with the tolerability of CBZ
| 9‐month visit | 12‐month visit | 15‐month visit | 18‐month visit | 21‐month visit | 24‐month visit | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR(95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | |
| rs3812718 | 0.520(0.275–0.981) | 0.041 | 0.424(0.210–0.859) | 0.017 | 0.534(0.322–0.887) | 0.006 | – | – | – | – | – | – |
| rs2290732 | 1.55(1.07–2.98) | 0.023 | 2.03(1.23–3.36) | 0.010 | 2.17(1.24–3.55) | 0.001 | 2.31(1.34–3.87) | 0.001 | 2.21(1.31–3.01) | 0.001 | 2.39(1.27–3.99) | 0.001 |
OR, odds ratio; CI, confidence interval.
OR (95% CI) was used to indicate the risk allele using a stepwise multivariate analysis by binary logistic regression; P‐value adjusted for age, gender, BMI, seizure type, central adverse effect, allergic reaction, and other SNPs.
We did not find significant differences in the retention rates for the other SNPs.
The rs3812718 A/G SNP was positively correlated with the maintenance doses and serum levels of CBZ over 12 months of clinical follow‐up
Figure 2 reveals the trends in the maintenance doses and serum levels of CBZ in the epileptic patients over the 24 months of clinical follow‐up. There were relatively sharp increases in the maintenance doses and serum levels of CBZ for carriers of the AA, AG, or GG genotype from 3 to 15 months of treatment. However, the increases were less dramatic from 15 to 24 months of treatment. ANCOVA showed that the maintenance doses and serum levels of CBZ in carriers of the AA genotype of rs3812718 A/G were significantly higher than those of carriers of the GG genotype from 3 to 12 months of follow‐up, when adjusted for potential confounding factors, such as age, gender, and BMI (Figure 2, P < 0.05). No significant difference existed between carriers of the AG and GG genotypes within 24 months. Furthermore, the significance difference between AA carriers and GG carriers disappeared from 15 to 24 months of follow‐up.
Figure 2.
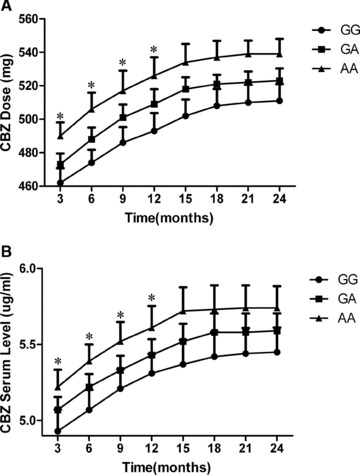
Trends in the maintenance dose and serum levels of CBZ. (A) Trends in the maintenance dose for CBZ (mean ± SE) among rs3812718 A/G genotypes. (B) Trends in the serum levels of CBZ (mean ± SE) among rs3812718 A/G genotypes. P values were analyzed using analysis of covariance (ANCOVA) during each visit to the clinic, after adjustments for age, sex, and BMI as potential compounding covariates. AA genotype carriers vs GG genotype ones. *P<0.05
We did not find significant differences in the maintenance doses and serum levels of CBZ in relation to the other SNPs.
The rs2298771 A/G SNP was positively correlated with decrease in seizure rates within 15 months of clinical follow‐up
Our data revealed that, from 3 months to 24 months of follow‐up, the proportion of patients with seizure free decreased persistently for carriers of either the AA or AG+GG genotypes of the rs2298771 A/G, and the proportion that showed 75%‐SF, 50–75%, and <50% efficacy increased gradually (data not shown). This finding indicated that the efficacy of CBZ decreased during an extended treatment period. The Pearson χ2 analysis showed the significance of the differences in the levels of efficacy between the carriers of the AA and AG+GG genotypes. The proportion of patients with a “good” response to medication among carriers of the AG+GG genotype was significantly lower than that among carriers of the AA genotype from 3 months to 15 months of follow‐up (Figure 3, P < 0.05). However, no significance differences were found at the other time points.
Figure 3.
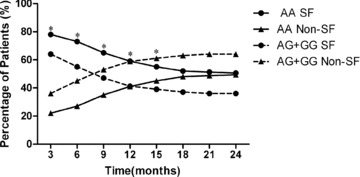
Trends in CBZ efficacy in carriers of various genotypes. The Pearson X3 was used to analyze the differences between carriers of the AA genotypes and carriers of the AG+GG genotypes with respect to the level of efficacy. The proportion of patients with seizure free (SF) among AA genotype carriers was significantly higher than that among AA+GG genotype carriers. Non‐SF indicated the combination of 75 %‐SF effecacy + 50–75% efficacy + < 50 % efficacy. *P<0.05
Meanwhile, we found no significant differences in the levels of efficacy for the other SNPs.
Discussion
We investigated the trends in the retention rates, maintenance doses, and serum levels of CBZ among patients undergoing CBZ treatment during 24 months of clinical follow‐up. We also evaluated the efficacy of CBZ for the treatment of epilepsy with respect to polymorphisms in the SCN1A and GABA receptor genes. We included both adolescents and adults in our study cohort because adolescents’ physiological characteristics are similar to those of adults. With regard to the demographics and clinical characteristics of the participants, our findings differed in some aspects from those of Hu et al. [17], including the proportion of the distribution of the types of seizure. These differences might originate from differences in the age distribution of the two different cohorts, and in the researchers’ choice for subjects, as well as other environmental factors.
In the treatment of epilepsy, seizure recurrence and resistance to AEDs are two formidable challenges. CBZ can induce upregulation of SCN1A and drug transporters [7, 18, 19, 20], which might reduce the response of voltage‐gated channels and even cause the development of tolerance or resistance to the drug. In our previous unpublished work, logistic recession analysis identified many factors that might affect CBZ retention rates for CBZ treatment, including allergic skin reactions, poor efficacy, central adverse effects, and genetic factors. This is consistent with the findings of Hu et al. [17]. Many previous studies have reported retention rates among patients undergoing AED treatment for epilepsy [17, 21, 22, 23, 24]. Using Kaplan–Meier survival curves, we revealed a relationship between the retention rates during long‐term CBZ treatment and different genotypes of the SCN1A and GABA receptor genes.
Among the genes that encoded different subunits of the GABA receptor, the results of our study indicated that only the rs2290732 A/G SNP of the GABRA1 gene was correlated positively with the CBZ retention rates over 2 years of clinical follow‐up. A recent study [25] has shown that GABRA1 IVS11+15A (rs2279020) is significantly correlated with drug resistance. The fact that resistance to CBZ leads to poor efficacy is one of the main contributors to a decrease in retention rates. Target mutations could lead to varying sensitivity to CBZ treatment in different patients [26, 27]. Interestingly, our LD analysis found a high‐LD value between rs2279020 and rs2290732 in the Caucasian population (D′= 1, r2= 1, HapMap Data Rel 28 Phase, August 10, on NCBI B36 assembly, dbSNP b126).
In a previous study, which involved administration of CBZ to patients with epilepsy for 12 months, we found that the maintenance doses and serum levels of CBZ in carriers of the AA genotype of rs3812718 A/G were significantly higher than those in carriers of the GG genotype, which is consistent with the findings of Tate and colleagues [28]. However, up to now, this finding has been controversial. In the previous study, we elucidated the inconsistencies in other studies [23, 29, 30, 31]. The differences among studies may be associated with many factors, including genetic differences, sample size, the age distribution of the cohort, environmental factors, and physicians’ bias. In the current study, the differences appeared from 15 to 24 months of follow‐up (Figure 2). This may have originated from changes in the size of the sample. The sample sizes for the visits from 15 to 24 months were smaller than the ones from 3 to 12 months because some patients had ceased to take CBZ. Intriguingly, when Figure 1A was compared with Figure 2, we found that the maintenance doses and serum levels of CBZ in the carriers of different genotypes of rs3812718 A/G showed an inverse association with CBZ tolerability for the respective genotype carriers. This indicated that larger doses of CBZ could provoke more severe adverse reactions, which would affect the tolerability of CBZ.
Polymorphism at rs2298771 A/G could affect the structure and function of SCN1A, which is targeted by CBZ. This might have caused the variation in the efficacy of epilepsy treatment among carriers of different genotypes. However, the effect requires further elucidation by electrophysiological and pharmacological investigation during the course of long‐term therapy. Moreover, further study is clinically needed to confirm the results.
In summary, in the study reported herein, we revealed that variations in the maintenance dose and serum levels of CBZ in relation to the rs3812718 A/G polymorphism of the SCN1A gene could be partially responsible for the differences in CBZ tolerability that were associated with different genotypes. The rs2290732 A/G polymorphism of the GABRA1 gene might also affect CBZ tolerability. Polymorphism at rs2298771 A/G could cause variations in drug efficacy because of alterations in the structure and function of the SCN1A receptor. These data might aid clinical management by allowing personalized CBZ treatment to be applied to patients with epilepsy.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
We thank leading hospitals of Changsha City of China for help and collaboration, as well as our patients and volunteers for their support for the investigation. We also thank the National Natural Science Foundation of China (81173129), Program for Changjiang Scholars and Innovative Research Team in University (IRT0946), The Science and Technology Plan Key Grant of Hunan Province of China (2009TP4068–2), and The Fundamental Research Funds for the Central Universities (201023100001).
This work was supported by the National Natural Science Foundation of China (81173129), Program for Changjiang Scholars and Innovative Research Team in University (IRT0946), The Science and Technology Plan Key Grant of Hunan Province of China (2009TP4068–2), and The Fundamental Research Funds for the Central Universities (201023100001).
References
- 1. Browne TR, Holmes GL. Epilepsy. N Engl J Med 2001; 344(15): 1145–1151. [DOI] [PubMed] [Google Scholar]
- 2. Terauchi A, Johnson‐Venkatesh EM, Toth AB, Javed D, Sutton MA, Umemori H. Distinct FGFs promote differentiation of excitatory and inhibitory synapses. Nature 2010; 465(7299): 783–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biachi A, Viaggi S, Chiossi E, LICE Episcreen Group . Family study of epilepsy in first degree relatives: Data from the Italian Episcreen Study. Seizure 2003; 12(4): 203–210. [DOI] [PubMed] [Google Scholar]
- 4. Winawer MR, Rabinowitz D, Pedley TA, Hauser WA, Ottman R. Genetic influences on myoclonic and absence seizures. Neurology 2003; 61(11): 1576–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duncan JS, Sander JW, Sisodiya SM, Walkerr MC. Adult epilepsy. Lancet 2006; 367(9516): 1087–1100. [DOI] [PubMed] [Google Scholar]
- 6. Cavalleri GL, Weale ME, Shianna KV, et al Multicentre search for genetic susceptibility loci in sporadic epilepsy syndrome and seizure types: A case‐control study. Lancet Neurol 2007; 6(11): 970–980. [DOI] [PubMed] [Google Scholar]
- 7. Wang WZ, Wu JZ, Wang DS, et al The prevalence and treatment gap in epilepsy in China: An ILAE/IBE/WHO study. Neurology 2003; 60(9): 1544–1545. [DOI] [PubMed] [Google Scholar]
- 8. Granger P, Biton B, Faure C, et al Modulation of the gamma‐aminobutyric acid type: A receptor by the antiepileptic drugs carbamazepine and phenytoin. Mol Pharmacol 1995;47(6): 1189–1196. [PubMed] [Google Scholar]
- 9. Martin MS, Dutt K, Papale LA, et al Altered function of the SCN1A voltage‐gated sodium channel leads to gamma‐aminobutyric acid‐ergic (GABAergic) interneuron abnormalities. J Biol Chem 2011; 285(13): 9823–9834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pauletto G, Bergonzi P, Triveneto Epilepsy Study Group . Oxcarbazepine reduces seizure frequency in a high proportion of patients with both newly diagnosed and refractory partial seizures in clinical practice. Seizure 2006; 15(3): 150–155. [DOI] [PubMed] [Google Scholar]
- 11. Muzykewicz DA, Lyczkowski DA, Memon N, Conant KD, Pfeifer HH, Thiele EA. Efficacy, safety, and tolerability of the low‐glycemic index treatment in pediatric epilepsy. Epilepsia 2009; 50(5): 1118–1126. [DOI] [PubMed] [Google Scholar]
- 12. Zhang C, Wong V, Ng PW, et al Failure to detect association between polymorphisms of the sodium channel gene SCN1A and febrile seizures in Chinese patients with epilepsy. Epilepsia 2011; 51(9): 1878–1881. [DOI] [PubMed] [Google Scholar]
- 13. Elger CE. Pharmacoresistance: Modern concept and basic data derived from human brain tissue. Epilepsia 2003; 44 Suppl 5: 9–15. [DOI] [PubMed] [Google Scholar]
- 14. Sillanpaa M, Schmidt D. Seizure clustering during drug treatment affects seizure outcome and mortality of childhood‐onset epilepsy. Brain 2008; 131(Pt 4): 938–944. [DOI] [PubMed] [Google Scholar]
- 15. Arif H, Buchsbaum R, Pierro J, et al Comparative effectiveness of 10 antiepileptic drugs in older adults with epilepsy. Arch Neurol 2011; 67(4): 408–415. [DOI] [PubMed] [Google Scholar]
- 16. De La Vega FM, Isaac HI, Scafe CR. A tool for selecting SNPs for association studies based on observed linkage disequilibrium patterns. Pac Symp Biocomput 2006; 11: 487–498. [PubMed] [Google Scholar]
- 17. Hu Y, Huang Y, Quan F, Hu Y, Lu Y, Wang XF. Comparison of the retention rates between carbamazepine and valproate as an initial monotherapy in Chinese patients with partial seizures: A 10 year follow‐up, observational study. Seizure 2011; 20(3): 208–213. [DOI] [PubMed] [Google Scholar]
- 18. Yoshimura R, Yanagihara N, Terao T, et al Carbamazepine‐induced up‐regulation of voltage‐dependent Na+ channels in bovine adrenal medullary cells in culture. J Pharmacol Exp Ther 1998; 287(2): 441–447. [PubMed] [Google Scholar]
- 19. Lazarowski A, Czomyj L, Lubienieki F, Girardi E, Vazquez S, D’Giano C. ABC transporters during epilepsy and mechanisms underlying multidrug resistance in refractory epilepsy. Epilepsia 2007; 48 Suppl 5: 140–149. [DOI] [PubMed] [Google Scholar]
- 20. Loscher W. Drug transporters in the epileptic brain. Epilepsia 2007; 48 Suppl 1: 8–13. [DOI] [PubMed] [Google Scholar]
- 21. Mills JK, Lewis TG, Mughal K, Ali I, Ugur A, Whitehouse WP. Retention rate of Clobazam, Topiramate, and Lamotrigine in children with intractable epilepsies at 1 year. Seizure 2011; 20(5): 402–405. [DOI] [PubMed] [Google Scholar]
- 22. Simister RJ, Sander JW, Koepp MJ. Long‐term retention rates of new antiepileptic drugs in adults with chronic epilepsy and learning disability. Epilepsy Behav 2007; 10(2): 336–339. [DOI] [PubMed] [Google Scholar]
- 23. Tate SK, Singh R, Hung CC, et al A common polymorphism in the SCN1A gene associates with phenytoin serum levels at maintenance dose. Pharmacogenet Genomics 2006; 16(10):721–726. [DOI] [PubMed] [Google Scholar]
- 24. Brandt C, May TW, Pohlmann‐Eden B, et al Retention rate of pregabalin in drug‐resistant epilepsy: 1 year follow‐up, single‐centre observation in 105 consecutive, adult patients. Seizure 2009; 18(9): 634–638. [DOI] [PubMed] [Google Scholar]
- 25. Lakhan R, Kumari R, Misra UK, Kalita J, Pradhan S, Mittal B. Differential role of sodium channels SCN1A and SCN2A gene polymorphisms with epilepsy and multiple drug resistance in the north Indian population. Br J Clin Pharmacol 2009; 68(2): 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Avanzini G. Is tolerance to antiepileptic drugs clinically relevant? Epilepsia 2006; 47(8): 1285–1287. [DOI] [PubMed] [Google Scholar]
- 27. Remy S, Gabriel S, Urban BW, et al A novel mechanism underlying drug resistance in chronic epilepsy. Ann Neurol 2003; 53(4): 469–479. [DOI] [PubMed] [Google Scholar]
- 28. Tate SK, Depondt C, Sisodiya SM, et al Genetic predictors of the maximum doses patients receive during clinical use of the antiepileptic drugs carbamazepine and phenytoin. Proc Natl Acad Sci U S A 2005; 102(15): 5507–5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abe T, Seo T, Ishitsu T, Nakagawa T, Hori M, Nakagawa K. Association between SCN1A polymorphism and carbamazepine‐resistant epilepsy. Br J Clin Pharmacol 2008; 66(2): 304–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Manna I, Gambardella A, Bianchi A, et al A functional polymorphism in the SCN1A gene does not influence antiepileptic drug responsiveness in Italian patients with focal epilepsy. Epilepsia 2011; 52(5): e40–e44. [DOI] [PubMed] [Google Scholar]
- 31. Zimprich F, Stogmann E, Bonelli S, et al A functional polymorphism in the SCN1A gene is not associated with carbamazepine dosages in Austrian patients with epilepsy. Epilepsia 2008; 49(6): 1108–1109. [DOI] [PubMed] [Google Scholar]
- 32. Schlachter K, Gruber‐Sedlmayr U, Stogmann E, et al A splice site variant in the sodium channel gene SCN1A confers risk of febrile seizures. Neurology 2009; 72(11): 974–978. [DOI] [PubMed] [Google Scholar]
- 33. Kim MK, Moore JH, Kim JK, et al Evidence for epistatic interactions in antiepileptic drug resistance. J Hum Genet 2011; 56(1): 71–76. [DOI] [PubMed] [Google Scholar]
- 34. Pun FW, Zhao C, Lo WS, et al Imprinting in the schizophrenia candidate gene GABRB2 encoding GABA(A) receptor beta(2) subunit. Mol Psychiatry 2011; 16(5):557–568. [DOI] [PubMed] [Google Scholar]
- 35. Pham X, Sun C, Chen X, et al Association study between GABA receptor genes and anxiety spectrum disorders. Depress Anxiety 2009; 26(11): 998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]


