Summary
Aim and methods
Changes in cerebrospinal fluid osmotic pressure modulate brain excitability. Transient receptor potential vanilloid 4 (TRPV4), which is sensitive to hypotonic stimulation, is expressed in hippocampus. The present study investigated the effect of hypotonic stimulation on hippocampal synaptic transmission and the role of TRPV4 in hypotonicity‐action using electrophysiological recording and pharmacological technique.
Results
Accompanied with the decrease in paired pulse facilitation, field excitatory postsynaptic potential (fEPSP) was enhanced by hypotonicity and TRPV4 agonist 4α‐PDD in hippocampal slices, which was sensitive to TRPV4 antagonist HC‐067047. Hypotonicity‐induced increase in fEPSP was blocked by α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) receptor antagonist, but not N‐methyl‐d‐aspartate receptor or N‐ or P/Q‐type voltage‐gated calcium channel antagonist. High voltage‐gated calcium current (I Ca) in hippocampal CA3 pyramidal neurons was not affected by hypotonicity. AMPA‐activated current (I AMPA) in hippocampal CA1 pyramidal neurons was increased by hypotonicity and 4α‐PDD, which was attenuated by HC‐067047. Inhibition of protein kinase C or protein kinase A markedly attenuated hypotonicity‐increased I AMPA, whereas antagonism of calcium/calmodulin‐dependent protein kinase II had no such effect.
Conclusion
TRPV4 is involved in hypotonicity‐induced enhancement of hippocampal synaptic transmission, which may be mediated through promoting presynaptic glutamate release and increasing postsynaptic AMPA receptor function.
Keywords: Hypotonicity, TRPV4, Synaptic transmission, High voltage‐gated calcium channels, AMPA receptor
Introduction
Information processed at synapses, which underlies all basic and higher‐order processes essential for normal brain function, is sensitive to changes in the internal environment. For example, a reduction in the extracellular osmolarity has long been proved to enhance spontaneous and evoked excitatory synaptic transmission in neocortical slices and whole‐cell synaptic current in hippocampal slices 1, 2, which is related to restless and increased seizure susceptibility under hypo‐osmolar clinic conditions. On the other hand, hypertonic treatment is effective in arresting these events 3, 4. Transient receptor potential vanilloid 4 (TRPV4), widely expressed in brain including hippocampus, hypothalamus, and cerebellum, etc., is sensitive to hypotonic stimulation 5. As an osmotic sensor, TRPV4 is involved in hyper‐ or hypo‐osmotic regulation in the central nervous system 6, 7. However, our recent study excludes the involvement of TRPV4 in hypertonicity‐decreased hippocampal synaptic transmission 8.
TRPV4 can be activated by various stimuli (including hypotonic stimulation, warm temperature, lipids downstream of arachidonic acid metabolism, and mechanical stimulation) 9, and there is evidence that TRPV4 plays a role in the central nervous system function 10. TRPV4 has been proved to contribute to the resting membrane potential at physiological temperature (~37°C), which plays an important role in regulating neural excitability in hippocampus 10. It is reported that activation of TRPV4 by 4α‐PDD increases the frequency of miniature excitatory postsynaptic currents (mEPSCs) in dorsal root ganglion‐spinal dorsal horn (DRG‐DH) neuronal cocultures and hippocampal neurons cultures 11. TRPV4 is sensitive to hypotonic stimulation, but whether TRPV4 is responsible for the modulation of synaptic transmission in hypotonic condition remains unclear.
Synaptic transmission is processed in the form of a chemical message released from presynaptic terminal and received by specific receptors in postsynaptic membrane, where the message is processed, integrated, and propagated 12, 13. In hippocampus, the influx of calcium through voltage‐gated calcium channels (VGCCs) plays an important role in presynaptic depolarization to vesicle release, and the excitatory synaptic transmission is mediated primarily by two classes of ionotropic glutamate receptors, α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionate, and N‐methyl‐d‐aspartate receptors (AMPAR and NMDAR), respectively 13. This study firstly examined the effect of hypotonic stimulation on synaptic transmission in hippocampal slices and the role of TRPV4 in hypotonicity‐action. Then, we explored the action site of hypotonicity using antagonists of N‐ and P/Q‐type VGCC, NMDAR, and AMPAR. Finally, we examined the effect of hypotonic stimulation on high voltage‐gated calcium channels (I Ca) in hippocampal CA3 pyramidal neurons and on AMPA‐activated current (I AMPA) in hippocampal CA1 pyramidal neurons to elaborate the mechanisms underlying hypotonic stimulation/TRPV4‐action on synaptic transmission. Our results showed that TRPV4‐mediated hypotonicity‐induced enhancement of hippocampal synaptic transmission through promoting presynaptic glutamate release and increasing postsynaptic AMPAR function.
Materials and Methods
Experimental Animals
Male mice (3‐week‐old, ICR, Oirental Bio Service Inc., Nanjing) were used in the study. Care of animals conformed to standards established by the National Institutes of Health. All animal protocols were approved by the Nanjing Medical University Animal Care and Use Committee (ID: 20110632). All efforts were made to minimize animal suffering and to reduce the number of animals used.
Slice Preparation
Mice were decapitated under deep anesthesia with ethyl ether. The brains were rapidly removed, and the coronal brain slices (400 μm) were cut using a vibrating microtome (Microslicer DTK 1500, Dousaka EM Co, Kyoto, Japan) in ice‐cold oxygenated (95% O2/5% CO2) modified artificial cerebrospinal fluid (mACSF) composed of (in mM) NaCl 126, CaCl2 1, KCl 2.5, MgCl2 1, NaHCO3 26, KH2PO4 1.25, and d‐glucose 20. After 1‐h recovery, hippocampal slices were transferred to a recording chamber.
Electrophysiological Recording
All experiments were performed at room temperature (22–24°C). For recording field excitatory postsynaptic potential (fEPSP), slices were perfused continually with the oxygenated bath solution composed of (in mM) NaCl 74, CaCl2 2, KCl 2.5, MgCl2 1, NaHCO3 26, KH2PO4 1.25, D‐glucose 20, and D‐mannitol 80 at osmolarity of 300 mOsm/kg. A glass microelectrode (No. 64‐0817(G85150T‐3), Warner Instruments Inc., Hamden, CT, USA) with the resistance of 4–5 MΩ filled with 2 M NaCl was inserted into the stratum radiatum region of CA1 area. fEPSP was generated by stimulating the Schaffer collateral/commissural pathway using a stimulator (SEN‐3301, Nihon Kohden, Japan). Stimulus pulses of 0.1 ms duration were delivered every 15 seconds. Signals were obtained using an Axoclamp 2B amplifier (Axon Instruments, Foster City, CA, USA), sampled at 20 kHz and filtered at 10 kHz, and the output was digitized with a Digidata 1200 converter (Axon Instruments). The synaptic transmission in hippocampal CA1 area was examined by evaluating the response to stimuli ranging from 0.1 mA to 1.0 mA (I/O curve). The basal synaptic transmission was recorded using the stimulation intensity yielding the half‐maximal fEPSP slope for a given slice.
For whole‐cell patch clamp recording, hippocampal neurons were viewed with an upright microscope equipped with infrared‐sensitive camera (DAGE‐MTI, IR‐1000) and perfused continually with the oxygenated bath solution containing 0.1 μM tetrodotoxin (TTX). I Ca and I AMPA were recorded using an EPC‐10 amplifier (HEKA Elektronik, Lambrecht/Pfalz, Germany). Data were acquired at a sampling rate of 10 kHz and filter (Bessel) of 2.9 kHz. In the experiments, the capacitance and series resistance were compensated. Data obtained from neurons in which uncompensated series resistance resulted in voltage‐clamp errors >5 mV were not taken in further analysis. Hypotonic solution was obtained by adjusting the concentration of D‐mannitol. The osmolarity was measured using the Advanced Micro Osmometer, model 3300 (Advanced instruments Inc, Norwood, Massachusetts).
I Ca was recorded using the glass pipettes with the resistance of 4–5 MΩ filled with (in mM) CsCl 140, MgCl2 2, Na2‐ATP 5, TEA‐Cl 2, HEPES 10, EGTA 10 at pH 7.2. The hippocampal pyramidal neuron was depolarized from –80 mV (50 ms) to –40 mV (200 ms) and then to 0 mV (200 ms). I Ca was activated by the second depolarization. Nifedipine (10 μM) was added in the bath solution to block L‐type VGCC, and therefore, I Ca mainly included N‐ and P/Q‐type calcium current. The voltage‐dependent activation curve (G–V curve) was measured by a series of depolarizing pulses (200 ms) from –60 mV to +40 mV stepping by 10 mV with interval time of 5 second. The voltage‐dependent inactivation curve (inactivation–voltage curve) was measured by double pulses: precondition pulses (3 second) ranging from –80 mV to +20 mV by stepping 10 mV and following +10 mV test pulse (200 ms) with internal time of 5 second. For recording I AMPA, the glass pipette was filled with (in mM) CsCl 140, MgCl2 1, CaCl2 0.2, Tris‐ATP 2, HEPES 10, EGTA 10 at pH 7.2. The hippocampal pyramidal neuron was perfused in the bath solution containing D‐APV (50 μM) and bicuculline (10 μM) and held at –60 mV. AMPA was dissolved in the bath solution and applied for 1.5 second using a rapid drug delivery system. After testing the effect of hypotonicity on I Ca or I AMPA, 10 μM 4α‐PDD was added in mASCF (with 0.1 μM TTX) to test whether the neuron had TRPV4 using a ramp protocol depolarizing from −80 mV to +80 mV over 700 ms.
Chemicals
4α‐phorbol‐12, 13‐didecanoate (4α‐PDD) was obtained from CALBIOCHEM (San Diego, CA, USA) and others, unless stated, all came from Sigma Chemical Company
4α‐PDD, HC‐067047, NBQX, D‐APV, D‐Sphingosine, bisindolylmaleimide II (BIM), phorbol 12‐myristate 13‐acetate (PMA), KN93, KN62, 8‐Bromoadenosine 3′, 5′‐cyclic monophosphate (8‐Br‐cAMP), H‐89, and KT5720 were prepared as stock solutions in DMSO. The final concentration of DMSO in the bath chamber or pipette solution was <0.1%. Concentrations of the above‐listed chemicals were chosen as previously reported (Supplementary 1). H‐89, KT5720, KN93, KN67, D‐Sphingosine were present in the pipette solution, while others were applied in the bath solution.
Data Analysis
Data are expressed as means ± SEM and were analyzed with pClamp (Axon Instruments), PulseFit (HEKA Elektronik) and SigmaPlot (SPSS Inc., Chicago IL, USA) software. All data were obtained from the neurons where 4α‐PDD‐evoked current could be recorded. Paired or unpaired t‐test was used for statistical analysis with the significant level set at P < 0.05 (*) and P < 0.01 (**). G–V curve and inactivation–voltage curve of I Ca were fitted by Boltzmann functions, in which G/Gmax = 1/(1 + exp (V0.5–Vm)/k) or I/Imax = 1/(1 + exp (V0.5–Vm)/k), with V0.5 being membrane potential at which 50% of activation or inactivation was observed and k being the slope of the function. In dose–response curve, I AMPA induced by different dose of AMPA was normalized to I AMPA induced by 300 μM AMPA in isotonic solution (300 mOsm/kg) in the same neuron and the data were fitted by logistic equation in which I = I max/[1 + (EC50/C)n], with n being the Hill coefficient and EC50 being the concentration producing 50% maximal response value. When exploring current–voltage relationship (I−V curve), I AMPA induced at different holding potential was normalized to I AMPA with the holding potential being –60 mV in isotonic solution in the same neuron.
Results
Hypotonic Stimuli Enhance fEPSP in Hippocampal Slices
The basal transmission of Schaffer collaterale‐CA1 synapse was examined by plotting the fractional changes in fEPSP slope against the testing stimuli at 0.1−1.0 mA. It was found that the slope of fEPSP was enhanced when isotonic bath solution (300 mOsm/kg) was changed into hypotonic solution (240 mOsm/kg) (n = 14, paired t‐test, P < 0.01). The enhancement of fEPSP was reversible after hypotonic stimulation was washed out (Figure 1A,B). To elucidate the responsible synaptic sties of hypotonicity‐enhanced synaptic transmission, we measured paired pulse facilitation (PPF), an index of presynaptic facilitation, with interpulse interval (IPI) of 25−100 ms. PPF data were expressed as a ratio (PPR) of the second response slope relative to the first. Figure 1C shows that PPR with IPI of 25 ms in hypotonic solution was smaller than that in isotonic solution (n = 14, paired t‐test, P < 0.05), indicative of an increase in presynaptic glutamate release by hypotonic stimulation. Here, it was noted that the increase in fEPSP was more evident with larger osmotic gradient (Figure 1D), and the following experiments were performed using hypotonic stimulation of 240 mOsm/kg which produced a significant increase in fEPSP.
Figure 1.

Effect of hypotonic stimulation on the synaptic transmission in hippocampal slices. (A) Typical recordings show that fEPSP was reversibly enhanced by hypotonic stimulation (240 mOsm/kg). (B) Slopes of fEPSP are plotted against stimulus intensity ranging from 0.1 mA to 1.0 mA. The plots were fitted by linear regression analysis, and the slope of the regression line for I/O curve was 1.13 and 1.46 in hypotonic and isotonic solution, respectively (n = 14, paired t‐test, P < 0.05). Additionally, the maximal response to 1.0 mA was larger in hypotonic solution (n = 14, paired t‐test, P < 0.05). (C) PPR evoked with IPI at 25 ms was markedly reduced in hypotonic solution (115.52 ± 4.18), when compared with that in isotonic solution (128.26 ± 6.16). *P < 0.05 versus 300 mOsm/kg (D) fEPSP was enhanced by hypotonic stimulation, and the enhancement was prominent at larger osmotic pressure gradient. fEPSP almost did not change in isotonic solution (300 mOsm/kg) when fructose was used to adjust the osmolality.
TRPV4 is Involved in Hypotonicity‐Enhanced fEPSP in Hippocampal Slices
To explore the role of TRPV4 in hypotonicity‐enhanced fEPSP, we firstly tested the effect of TRPV4 agonist 4α‐PDD on fEPSP in hippocampal slices. The slope of fEPSP was significantly enhanced by application of TRPV4 agonist 4α‐PDD (10 μM) (n = 10, paired t‐test, P < 0.01). fEPSP almost recovered to the control level after washout (Figure 2A,B). Figure 2C shows that PPR with IPI of 25 ms was markedly smaller after application of 4α‐PDD (n = 10, paired t‐test, P < 0.05). These results suggest that TRPV4 agonist mimics hypotonicity‐enhanced fEPSP in hippocampal slices.
Figure 2.

Effect of 4α‐PDD on the synaptic transmission in hippocampal slices. (A) Typical recordings show that fEPSP was reversibly enhanced by 4α‐PDD. (B) Slopes of fEPSP are plotted against stimulus intensity ranging from 0.1 mA to 1.0 mA. The plots were fitted by linear regression analysis and the slope of the regression line for I/O curve was 1.07 and 1.23 before and during 4α‐PDD treatment, respectively (n = 10, paired t‐test, P < 0.05). Additionally, the maximal response to 1.0 mA was larger after 4α‐PDD treatment (n = 10, paired t‐test, P < 0.05). (C) PPR evoked with IPI at 25 ms was significantly reduced by 4α‐PDD (control: 130.75 ± 5.16; 4α‐PDD: 115.52 ± 4.18). *P < 0.05 versus 300 mOsm/kg (D) In the presence of HC‐067047, hypotonicity‐enhanced fEPSP was markedly attenuated from 48.33 ± 4.34% to 4.99 ± 3.36%. **P < 0.01 versus 240 mOsm/kg. In the presence of HC‐067047, fEPSP was only enhanced 2.01 ± 0.69% by 4α‐PDD, which was significantly different from the enhancement by 4α‐PDD alone (25.07 ± 3.13%). ## P < 0.01 versus 4α‐PDD (E,F) In the presence of HC‐067046, PPR evoked with IPI at 25 ms was not affected by hypotonic stimulation (E) or 4α‐PDD (F).
We then tested the effect of TRPV4 antagonist on hypotonicity‐ and 4α‐PDD‐action on fEPSP. Application of HC‐067047 slightly reduced fEPSP and increased PPR (with IPI of 25 ms) in isotonic solution (n = 7, paired t‐test, P > 0.05 in each case). As shown in Figure 2D–F, preapplication of HC‐067047 (1 μM) markedly attenuated the increase in fEPSP (n = 10) and decrease in PPR by both hypotonicity (n = 8) and 4α‐PDD (n = 8). Collectively, the above results indicate that TRPV4 mediates hypotonicity‐enhanced EPSP.
Antagonism of AMPA‐Type Glutamate Receptor Attenuates Hypotonicity‐Action
In general, a rise in presynaptic intracellular calcium level triggers neurotransmitter release and N‐ or P/Q‐type VDCCs are crucial for calcium influx related to glutamate release 14, 15, 16. Here, we firstly tested the effect of N‐ or P/Q‐type VDCC antagonist (ω‐conotoxin GVIA (1 μM) and ω‐agatoxin IVa (300 nM)) on fEPSP in normal condition. Application of ω‐conotoxin GVIA or ω‐agatoxin IVa reduced fEPSP by 55.97 ± 4.78% (n = 6) and 35.17 ± 5.04% (n = 7) in isotonic solution, respectively (P < 0.01 in each case). In the presence of ω‐conotoxin GVIA or ω‐agatoxin IVa, fEPSP was increased by 53.73 ± 3.97% (n = 10) and 48.66 ± 2.09% (n = 11), respectively, when isotonic solution was changed into hypotonic solution (Figure 3A,B).
Figure 3.

Effect of VGCC, NMDAR, and AMPAR antagonists on hypotonicity‐enhanced fEPSP. (A,B) The enhancement of fEPSP by hypotonic stimulation was not affected by N‐ or (A) P/Q‐type VGCC antagonist (B). (C,D) Preapplication of AMPAR (C) but not NMDAR antagonist (D) markedly blocked hypotonicity‐enhanced fEPSP. NBQX markedly attenuated hypotonicity‐enhanced fEPSP from 48.33 ± 4.34% to 1.07 ± 0.56%. **P < 0.01 versus 240 mOsm/kg.
As AMPARs in postsynaptic neurons are a major type of receptors which mediate rapid electrophysiological response to glutamate, we then tested the role of AMPAR in hypotonicity‐enhanced fEPSP. Application of AMPAR antagonist NBQX (1 μM) reduced fEPSP by 95.48 ± 3.17% in isotonic solution (n = 6, paired t‐test, P < 0.01), while NMDAR antagonist D‐APV (50 μM) almost did not affect fEPSP (n = 7, paired t‐test, P > 0.05). This result consists with the previous report that AMPARs mediate most fast excitatory synaptic transmission 17. It was noted that in the presence of NBQX, fEPSP was almost not affected by the hypotonic stimulation (n = 8). Additionally, after application of D‐APV, fEPSP was increased 46.89 ± 4.00% by hypotonic stimulation (n = 12) (Figure 3C,D). These results suggest that AMPAR is likely involved in hypotonicity‐enhanced fEPSP.
Hypotonic Stimulation does not Affect I Ca in Hippocampal CA3 Pyramidal Neurons
This study also tested the effect of hypotonicity on presynaptic VGCCs. In pyramidal neurons of hippocampal CA3 area, the amplitude and I−V curve of I Ca were almost not affected when the bath solution was changed from isotonicity into hypotonicity (n = 15, paired t‐test, P > 0.05) (Figure 4A–C). Besides this, neither G–V curve nor inactivation–voltage curve shifted before and during hypotonic treatment (Figure 4D,E).
Figure 4.
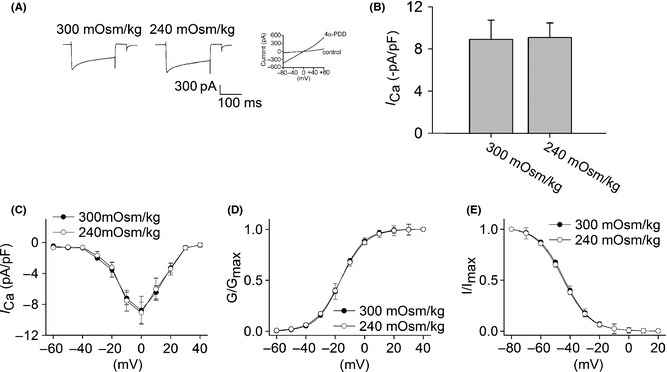
Effect of hypotonic stimulation on I Ca in hippocampal CA3 pyramidal neurons. (A) Typical recordings show that I Ca almost did not change when the bath solution was changed from isotonicity (–511.74 pA) to hypotonicity (–547.98 pA) for 10 min. 4α‐PDD‐induced current was recorded in the same neuron. (B) On the average, I Ca was −8.91 ± 1.28 pA/pF and −9.09 ± 1.37 pA/pF in isotonic and hypotonic solution, respectively. (C) I–V curve was measured by a series of depolarizing pulses (200 ms) from –60 to +40 mV stepping by 10mV with interval time of 5 second. I–V curve did not change before and during hypotonic treatment. (D) G–V curve was assessed using the data transformed from I–V curve shown in (C). G–V curve did not shift by hypotonic treatment, in which V0.5 were –16.40 ± 1.71 mV and –16.38 ± 1.21 mV (n = 7, paired t‐test, P > 0.05), k were 8.10 ± 0.41 and 8.65 ± 1.09, (n = 7, paired t‐test, P > 0.05) for isotonicity and hypotonicity, respectively. (E) In the presence of hypotonicity, inactivation–voltage curve did not shift, with V0.5 being –44.75 ± 2.11 mV and –43.93 ± 1.97 mV (n = 7, paired t‐test, P > 0.05) and k being –9.15 ± 1.39 and –8.99 ± 2.67 for isotonicity and hypotonicity, respectively (n = 7, paired t‐test, P > 0.05).
Hypotonic Stimulation Increases I AMPA in Hippocampal CA1 Pyramidal Neurons
As NBQX selectively attenuated hypotonicity‐enhanced fEPSP, we then tested whether the response of AMPAR in pyramidal neurons of hippocampal CA1 region was affected by hypotonic stimulation. It was found that I AMPA (activated by 15 μM AMPA) was increased from −14.69 ± 1.55 pA/pF to −18.52 ± 1.61 pA/pF by hypotonic stimulation (n = 17, paired t‐test, P < 0.01). After hypotonicity was washed out, I AMPA recovered to −14.74 ± 2.01 pA/pF (Figure 5A). Upon exposure to hypotonic stimulation, the maximal I AMPA was significantly increased, but EC50 (300 mOsm/kg: 16.89 ± 1.31 μM; 240 mOsm/kg: 16.24 ± 1.30 μM) and n values (300 mOsm/kg: 1.73; 240 mOsm/kg: 1.75) of the dose–response curve were not markedly different in isotonic or hypotonic condition (n = 9, paired t‐test, P > 0.05 in each case) (Figure 5C). Hypotonicity‐increased I AMPA at every voltage ranging from −80 mV to + 60 mV, but the reversal potential (300 mOsm/kg: 0.08 ± 0.01 mV; 240 mOsm/kg: 0.07 ± 0.02 mV) and I(+60 mV)/I(−80 mV) ration were not affected (300 mOsm/kg: −0.56 ± 0.07; 240 mOsm/kg: −0.58 ± 0.03) (n = 9, paired t‐test, P > 0.05 in each case) (Figure 5D). Here, we also found that AMPAR antagonist NBQX markedly blocked I AMPA in isotonic and hypotonic solution (Figure 5B).
Figure 5.
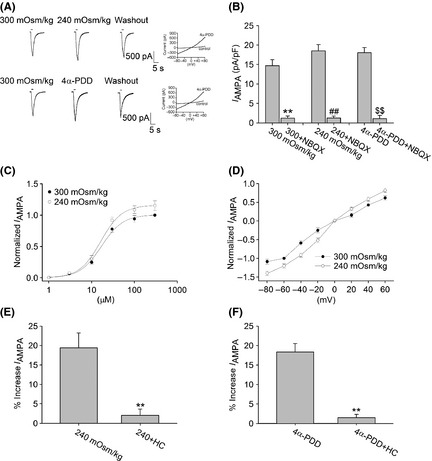
Effect of hypotonic stimulation and TRPV4 agonist 4α‐PDD on I AMPA in hippocampal CA1 pyramidal neurons. (A) Typical recordings show that I AMPA was increased from –1.13 nA to –1.37 nA when the bath solution was changed from isotonicity to hypotonicity. After washout, I AMPA recovered to –1.15 nA (upper). I AMPA was reversibly increased by application of TRPV4 agonist 4α‐PDD, and I AMPA was –1.08 nA, –1.30 nA, and –1.11 nA before, during, and after 4α‐PDD treatment (below). 4α‐PDD‐induced current was recorded in the same neuron. (B) Application of NBQX markedly blocked I AMPA in isotonic and hypotonic solution as well as in the presence of 4α‐PDD. **P < 0.01 versus 300 mOsm/kg, ## P < 0.01 versus 240 mOsm/kg, $$ P < 0.01 versus 4α‐PDD. (C) Dose–response curves for I AMPA in isotonic and hypotonic solution. Each point represents the normalized current from 8 ~ 17 hippocampal neurons. (D) I–V curves were shown in isotonic and hypotonic solution. (E) In the presence of HC‐067047, the increase in I AMPA by hypotonic stimulation was markedly attenuated from 19.40 ± 3.82% to 2.04 ± 1.58%. **P < 0.01 versus 240 mOsm/kg (F) 4α‐PDD‐induced increase in I AMPA was significantly attenuated from 18.36 ± 2.16% to 1.49 ± 0.86%. **P < 0.01 versus 4α‐PDD
Additionally, I AMPA was increased from −14.45 ± 2.01 pA/pF to −18.03 ± 1.08 pA/pF by application of 10 μM 4α‐PDD (n = 9, paired t‐test, P < 0.05) (Figure 5A). Application of 4α‐PDD did not affect EC50 value of the dose–response curve (300 mOsm/kg: 16.74 ± 1.11 μM; 4α‐PDD: 15.96 ± 1.67 μM, n = 9) or I(+60 mV)/I(−80 mV) ration (300 mOsm/kg: 0.07 ± 0.03 mV; 4α‐PDD: 0.07 ± 0.05 mV, n = 9) and reversal potential of I−V curve (300 mOsm/kg: −0.55 ± 0.08; 4α‐PDD: −0.57 ± 0.04, n = 9) (P > 0.05 in each case). The increase in I AMPA after 4α‐PDD treatment was significantly blocked by NBQX (Figure 5B). In the presence of HC‐067047, I AMPA was almost not affected by hypotonic treatment (300 mOsm/kg: −14.62 ± 2.38 pA/pF, 240 mOsm/kg: −14.92 ± 2.48 pA/pF, n = 14, parted t‐test, P > 0.05) or 4α‐PDD (300 mOsm/kg: −13.71 ± 3.01 pA/pF, 4α‐PDD: −13.95 ± 1.76 pA/pF, n = 8, paired t‐test, P > 0.05) (Figure 5E,F). These results suggest that activation of TRPV4 increases the response to AMPAR.
PKC and PKA Intracellular Signaling Pathways are Involved in Hypotonicity‐Increased I AMPA
Phosphorylation of AMPAR is an important mechanism responsible for the modulation of their function 18. We continued to explore whether some intracellular pathways were responsible for the increase in I AMPA by hypotonicity. We evaluated the involvement of calcium/calmodulin‐dependent protein kinase II (CaMKII), protein kinase C (PKC), and protein kinase C (PKA) in the modulation of I AMPA in isotonic solution using the specific agonists and antagonists and found that activation of these intracellular signaling pathways was responsible for the increase in I AMPA (Supplementary 2). As shown in Figure 6A, with CaMKII antagonist KN62 (n = 10) or KN93 (n = 11) in the pipette solution, hypotonicity‐induced increase in I AMPA was not significantly affected (unpaired t‐test, P > 0.05 in each case). Figure 6B shows that in the presence of PKC antagonist D‐Sphingosine (n = 11) or BIM (n = 13), the increase in I AMPA by hypotonic stimulation was markedly reduced (unpaired t‐test, P < 0.01, in each case). Preapplication of PKA antagonist H‐89 or KT5720, I AMPA was increased 6.01 ± 2.58% (n = 10) and 5.73 ± 1.28% (n = 13) by hypotonic stimulation, both of which were significantly different from the increase in I AMPA without antagonism of PKA (unpaired t‐test, P < 0.01 in each case).
Figure 6.

Effect of CaMKII, PKC, and PKA antagonists on hypotonicity‐increased I AMPA. (A) In the presence of KN62 or KN93, I AMPA was increased 17.16 ± 3.19% and 16.01 ± 1.93% by hypotonic stimulation, respectively. (B) I AMPA was increased 6.02 ± 2.78% and 7.16 ± 1.13% by hypotonic stimulation in the presence of D‐sphingosine or BIM, respectively. **P < 0.01 versus 240 mOsm/kg (C) Preapplication of H‐89 or KT5720 markedly attenuated the increase in I AMPA by hypotonic stimulation. **P < 0.01 versus 240 mOsm/kg
Discussion
Brain excitability is sensitive to changes in osmolarity. In clinic, a reduction in extracellular osmolarity (such as overhydration that may happen in hastened rehydration therapy, dialysis disequilibrium syndrome, and the syndrome of inappropriate ADH secretion) can induce seizure activity. Electrophysiological evidence has reported that hypotonic stimulation increases the endogenous burst firing in hippocampal neurons and promotes epileptic‐form activity by strengthening both excitatory synaptic communications in neocortex and field effects among the entire cortical population 3. Moreover, this study showed that hypotonic solutions potentiated fEPSP in hippocampal slices (Figure 1), consistent with condition associated with hyperexcitability. TRPV4, a cellular osmoreceptor sensitive to hypotonic stimulation, is a member of transient receptor potential vanilloid subfamily and widely expressed in the central nervous system 5. This study found that TRPV4 agonist 4α‐PDD enhanced fEPSP in hippocampal slices, and the enhancement of fEPSP by both hypotonic stimulation and 4α‐PDD was markedly blocked by the specific TRPV4 antagonist HC‐067047 (Figure 2), indicating an involvement of TRPV4 in hypotonicity‐enhanced hippocampal synaptic transmission.
Neurons communication is primarily through chemical synapses that transmit information by releasing neurotransmitters to produce brief changes in the electrical potential on the postsynaptic cells. Previous study in cultured DRG‐DH and hippocampal neurons has shown that 4α‐PDD increases the frequency of miniature excitatory postsynaptic currents (mEPSCs) 11. Consistently, this study found that hypotonic stimulation and 4α‐PDD decreased PPF which is generally considered as a presynaptic factor (Figures 1C and 2C). These results point to the presynaptic action site of hypotonicity/TRPV4 activation, which leads to the increase in presynaptic glutamate release and the subsequent enhancement of synaptic transmission. Synaptic transmission is triggered by calcium influx mainly through presynaptic VGCC, specifically N‐ and P/Q‐type VGCC 12. However, preapplication of N‐ or P/Q‐type VGCC antagonist failed to affect hypotonicity‐enhanced fEPSP (Figure 3A,B). Besides this, hypotonicity had no effect on I Ca in hippocampal CA3 pyramidal neurons (Figure 4). These results strongly suggest that hypotonicity‐induced increase in presynaptic neurotransmitter release is independent of the action on presynaptic VGCC. Our previous study has reported that activation of TRPV4 by hypotonicity can increase neuronal excitability in primary sensory neurons 19. Although TRPV4 does not affect functional synapses development in hippocampus, TRPV4 activation causes cation influx in hippocampal neurons, which helps to facilitate the depolarization of cell membrane, and CA3 pyramidal neurons are less excitable in TRPV4 knockout mice 10. Therefore, activation of TRPV4 by hypotonicity per se may increase presynaptic neurotransmitter release. On the other hand, besides VGCC, there are other factors, such as metabotropic glutamate receptors and nicotinic acetylcholine receptors, participating in the modulation of presynaptic glutamate release 20, 21. More experiments in the future are needed to determine whether hypotonicity/TRPV4 has effect on these receptors.
Glutamate‐induced excitatory neurotransmission in hippocampus is mainly mediated through ionotropic and metabotropic receptors. Among ionotropic receptors, AMPAR and NMDAR are frequently colocalized in postsynaptic neurons. Our present study and previous report show that AMPAR is the major type of glutamate receptor involved in the formation of fEPSP 17. Here, it was found that blockage of AMPAR significantly attenuated hypotonicity‐induced enhancement of fEPSP (Figure 3C). Additionally, I AMPA was increased by hypotonicity and 4α‐PDD, which was blocked by TRPV4 antagonist HC‐067047 (Figure 5). Therefore, besides the action on presynapse, the effect of hypotonicity/TRPV4 also involves postsynaptic mechanism, especially an increase in AMPAR function. NMDAR is another important inotropic glutamate receptor which is mainly involved in a slower phase of neurotransmission and plays an important role in induction of long‐term potentiation (LTP) 13. We recently report that TRPV4‐activation increases NMDA‐activated current, therefore, it is possible that hypotonicity may facilitate LTP induction. More experiments are needed to prove this possibility. It is generally reported that acute hypotonic condition leads to epileptiform seizures, whereas hypertonic condition causes depression and coma 3, 22. We previously reported that synaptic transmission was depressed in hypertonic solution, which is likely due to an inhibition of presynaptic calcium influx through VDCC to subsequently decrease neurotransmitter release 8. Although the in vivo data indicate that TRPV4 plays a role in sensing hypo‐ and hyperosmolar stimuli in mice 6, the in vitro experiment performed on rat and mouse hippocampal slices show that TRPV4 is selectively responsible for hypotonicity‐induced enhancement of synaptic transmission 8. Besides this, hippocampal synaptic transmission may be modulated by hypo‐ and hyper‐tonic stimuli through totally different mechanisms. A large amount of in vitro evidence show that TRPV4 is specially activated by reduced osmotic pressure 23. One explanation of the discrepancy between the involvement of TRPV4 in osmotic sensation in vivo and in vitro may be that in mammals, there exists other proteins, which directly interact with TRPV4 to form an osmosensor complex, essential for the response to hyperosmolar stimuli and such an osmosensor complex could still function in the residual sensing of TRPV4 mutant mice 24.
In this study, it was found that upon exposure to hypotonic stimulation, EC50 value of dose–response curve for I AMPA was not changed although the maximal response was markedly increased, which indicates that hypotonicity‐action on I AMPA is not due to increasing ligand binding affinity. Hypotonic stimulation increased I AMPA at every holding potential, leaving the reversal potential or current ratio at +60 mV/−80 mV unchanged, which implies that hypotonicity‐action is voltage‐independent. Phosphorylation of AMPAR is an important mechanism for short‐term modulation of their function 18. Our previous studies on primary sensory neurons have reported that some intracellular signaling pathways may be involved in hypotonicity‐induced modulation on voltage‐gated ion channels. For example, antagonism of PKG pathway attenuated hypotonicity‐induced inhibition of high voltage‐gated calcium channel 25 and inactivation of PKC selectively blocked the increase in tetrodotoxin‐sensitive sodium channel 26 and slow‐inactivating potassium channel by hypotonicity in trigeminal ganglion neurons 27. Here, we found that antagonists of PKC and of PKA markedly attenuated the increase in I AMPA by hypotonic stimulation, whereas antagonists of CaMKII had no such effect, indicating that PKC and PKA signaling pathways are responsible for hypotonicity‐increased I AMPA (Figure 6). However, in cultured hippocampus neurons, the amplitude of mEPSC was not affected by 4α‐PDD 11, suggesting that TRPV4 activation has no effect on postsynaptic glutamate receptors, and this discrepancy may be due to the different cell types (hippocampal culture vs. slice).
The neural circuitry in hippocampus is crucial for higher brain function. This study found that TRPV4 mediated hypotonicity‐enhanced hippocampal synaptic transmission through promoting presynaptic glutamate release and increasing postsynaptic AMPAR response, which amplifies the excitatory neurotransmission. Excessive glutamate‐mediated excitatory neurotransmission may lead to neuronal injury in pathological conditions such as in epilepsia and in cerebral ischemia. On the other hand, hypotonicity can enhance synchronization among CA1 hippocampal neurons through nonsynaptic mechanisms to induce seizure susceptibility. Whether TRPV4 activation is involved in this nonsynaptic mechanisms remains unclear. TRPV4 is expressed in hippocampal neurons and astrocytes 10, 28, and therefore, it may be a promising target in modulating hippocampal neural functions.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Data S1. Materials and Methods.
Table S1. Effect of second messengers systems on I AMPA.
Figure S1. Effect of hypotonic stimulation on miniature excitatory postsynaptic current (mEPSC) in mice hippocampal slices.
Figure S2. Effect of hypotonic stimulation on AMPAR‐mediated EPSC in mice hippocampal slices.
Acknowledgment
This work was supported by National Natural Science Foundation of China (31271206 and 30900577), Basic Medical Advantage Disciplines Project of Nanjing Medical University (JX10131801055), Science and Technology Project of Jiangsu Province (BK2011029) and Graduate Students Scientific Research Innovation Project of Jiangsu Province (CXZZ12_0567).
References
- 1. Huang R, Bossut DF, Somjen GG. Enhancement of whole cell synaptic currents by low osmolarity and by low [NaCl] in rat hippocampal slices. J Neurophysiol 1997;77:2349–2359. [DOI] [PubMed] [Google Scholar]
- 2. Rosen AS, Andrew RD. Osmotic effects upon excitability in rat neocortical slices. Neuroscience 1990;38:579–590. [DOI] [PubMed] [Google Scholar]
- 3. Andrew RD. Seizure and acute osmotic change: clinical and neurophysiological aspects. J Neurol Sci 1991;101:7–18. [DOI] [PubMed] [Google Scholar]
- 4. Azouz R, Alroy G, Yaari Y. Modulation of endogenous firing patterns by osmolarity in rat hippocampal neurones. J Physiol 1997;502:175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kauer JA, Gibson HE. Hot flash: TRPV channels in the brain. Trends Neurosci 2009;32:215–224. [DOI] [PubMed] [Google Scholar]
- 6. Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4‐/‐ mice. Proc Natl Acad Sci USA 2003;100:13698–13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mizuno A, Matsumoto N, Imai M, et al. Impaired osmotic sensation in mice lacking TRPV4. Am J Physiol Cell Physiol 2003;285:C96–C101. [DOI] [PubMed] [Google Scholar]
- 8. Li L, Yin J, Liu C, et al. Hypertonic stimulation inhibits synaptic transmission in hippocampal slices through decreasing pre‐synaptic voltage‐gated calcium current. Neurosci Lett 2012;507:106–111. [DOI] [PubMed] [Google Scholar]
- 9. Plant TD, Strotmann R. TRPV4. Handb Exp Pharmacol 2007;179:189–205. [DOI] [PubMed] [Google Scholar]
- 10. Shibasaki K, Suzuki M, Mizuno A, et al. Effects of body temperature on neural activity in the hippocampus: regulation of resting membrane potentials by transient receptor potential vanilloid 4. J Neurosci 2007;27:1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cao DS, Yu SQ, Premkumar LS. Modulation of transient receptor potential vanilloid 4‐mediated membrane currents and synaptic transmission by protein kinase C. Mol Pain 2009;5:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iremonger KJ, Benediktsson AM, Bains JS. Glutamatergic synaptic transmission in neuroendocrine cells: Basic principles and mechanisms of plasticity. Front Neuroendocrinol 2010;31:296–306. [DOI] [PubMed] [Google Scholar]
- 13. Scannevin RH, Huganir RL. Postsynaptic organization and regulation of excitatory synapses. Nat Rev Neurosci 2000;1:133–141. [DOI] [PubMed] [Google Scholar]
- 14. Horne AL, Kemp JA. The effect of ω‐conotoxin GVIA on synaptic transmission within the nucleus accumbens and hippocampus of the rat in vitro. Br J Pharmacol 1991;103:1733–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bargas J, Ayala GX, Hernández E, et al. Ca2 + ‐channels involved in neostriatal glutamatergic transmission. Brain Res Bull 1998;45:521–524. [DOI] [PubMed] [Google Scholar]
- 16. Honda I, Kamiya H, Yawo H. Re‐evaluation of phorbol ester‐induced potentiation of transmitter release from mossy fiber terminals of the mouse hippocampus. J Physiol 2000;529:763–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sprengel R. Role of AMPA receptors in synaptic plasticity. Cell Tissue Res 2006;326:447–455. [DOI] [PubMed] [Google Scholar]
- 18. Carvalho AL, Duarte CB, Carvalho AP. Regulation of AMPA receptors by phosphorylation. Neurochem Res 2000;25:1245–1255. [DOI] [PubMed] [Google Scholar]
- 19. Chen L, Liu C, Liu L. Osmolality‐induced tuning of action potentials in trigeminal ganglion neurons. Neurosci Lett 2009;452:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem 2000;75:889–907. [DOI] [PubMed] [Google Scholar]
- 21. McKay BE, Placzek AN, Dani JA. Regulation of synaptic transmission and plasticity by neuronal nicotinic acetylcholine receptors. Biochem Pharmacol 2007;74:1120–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ziai WC, Toung TJ, Bhardwaj A. Hypertonic saline: first‐line therapy for cerebral edema? J Neurol Sci 2007;261:157–166. [DOI] [PubMed] [Google Scholar]
- 23. Liedtke W, Choe Y, Martí‐Renom MA, et al. Vanilloid receptor‐related osmotically activated channel (VR‐OAC), a candidate vertebrate osmoreceptor. Cell 2000;103:525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liedtke W, Tobin DM, Bargmann CI, et al. Mammalian TRPV4 (VR‐OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc Natl Acad Sci USA 2003;100:14531–14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen L, Liu C, Liu L. Changes in osmolality modulate voltage‐gated calcium channels in trigeminal ganglion neurons. Brain Res 2008;1208:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li L, Liu C, Chen L, et al. Hypotonicity modulates tetrodotoxin‐sensitive sodium current in trigeminal ganglion neurons. Mol Pain 2011;7:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen L, Liu C, Liu L. The modulation of voltage‐gated potassium channels by anisotonicity in trigeminal ganglion neurons. Neuroscience 2008;154:482–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Butenko O, Dzamba D, Benesova J, et al. The increased activity of TRPV4 channel in the astrocytes of the adult rat hippocampus after cerebral hypoxia/ischemia. PLoS ONE 2012;7:e39959. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Materials and Methods.
Table S1. Effect of second messengers systems on I AMPA.
Figure S1. Effect of hypotonic stimulation on miniature excitatory postsynaptic current (mEPSC) in mice hippocampal slices.
Figure S2. Effect of hypotonic stimulation on AMPAR‐mediated EPSC in mice hippocampal slices.


