Summary
Aims
Administration of antigens into the anterior chamber (AC) of the eye induces a form of antigen‐specific immune tolerance termed anterior chamber‐associated immune deviation (ACAID). This immune tolerance effectively impairs host delayed‐type hypersensitivity (DTH) responses. We hypothesized that ACAID could be generated in BALB/c mice following AC inoculation of the encephalitogenic antigens myelin oligodendrocyte glycoprotein (MOG) and myelin basic protein (MBP).
Methods
We used DTH assays and local adoptive transfer (LAT) assays to test whether MOG/MBP‐induced ACAID following their administration into the AC, whether they elicited this immune tolerance via CD8+ T cells, and whether their AC coadministration (MOG/MBP) induced specific immune tolerance to one or both antigens.
Results
We showed that MOG/MBP‐induced AC‐mediated specific immune tolerance, as evident from impaired DTH responses. This antigen‐driven DTH suppression was solely mediated via splenic CD8+ T cells as confirmed by LAT assays. Finally, a single AC injection with both antigens was sufficient to induce specific immune tolerance to these antigens, as evident from DTH and LAT assays.
Conclusion
ACAID T‐cell regulation could be used as a therapeutic tool in the treatment of complicated autoimmune diseases that involve multiple antigens such as multiple sclerosis.
Keywords: ACAID, Multiple sclerosis, Peripheral tolerance, T regulatory cells
Introduction
The anterior chamber (AC) of the eye is an immunologically privileged site that is protected from inflammation 1, 2, 3, 4. Following injection of soluble antigens into the AC of eye, an antigen‐specific cell‐mediated systemic peripheral tolerance mechanism called anterior chamber‐associated immune deviation (ACAID) is elicited 5, 6, 7. ACAID is characterized by the inhibition of delayed hypersensitivity (DTH) reactions to the AC‐injected antigens 8, 9. This immune tolerance is elicited by at least two distinct cell populations of AC‐induced antigen‐specific regulatory T cells (Tregs); CD4+ CD25+ antigen‐specific Tregs that impair the induction of DTH responses and CD8+ Tregs that suppress the expression of DTH responses 8, 9, 10.
Multiple sclerosis (MS) is a chronic type IV hypersensitivity‐associated autoimmune disease involving the central nervous system (CNS) 11, characterized by the onset of perivascular inflammation, demyelination, and axonal lesions 12. Although the pathogenesis of MS remains elusive, it is believed that the disease results from autoimmune mechanisms that follow the activation of potentially pathogenic self‐reactive T cells that recognize myelin proteins of the CNS in the host. Certain key autoantigens have been linked to onset of MS such as myelin oligodendrocyte glycoprotein (MOG) 13, 14, myelin basic protein (MBP) 15, 16, proteolipid protein (PLP) 15, 17, myelin‐associated oligodendrocyte basic protein (MOBP) 18, 19, and oligodendrocyte‐specific protein (OSP) 20, 21. Antigen‐specific therapy targeting Treg‐mediated specific suppression of autoreactive responses holds promise in the development of treatments for MS. The suppressive roles of CD8+ Tregs could be utilized in the context of autoimmune disease 22. Studies have underlined the potential roles of ACAID CD8+ Tregs in delaying the progression of MOG 23‐ and MBP 24‐induced experimental autoimmune encephalomyelitis (EAE) in animal models.
In this study, we sought to address three main questions regarding encephalitogenic antigens (MOG and MBP). The first is whether they induce ACAID‐mediated immune tolerance following their administration into the AC of the eye in BALB/c mice. The second is whether they elicit this immune tolerance via CD8+ T cells harvested from the MOG‐induced and MBP‐induced mice. The third is whether the coadministration of MOG and MBP into the AC of the eye induces specific immune tolerance to one or both antigens. This approach is likely to open up new avenues and strategies for therapy of heterogeneous autoimmune diseases such as MS.
Materials and Methods
Mice
Six‐to‐eight‐week‐old wild‐type BALB/c mice were procured from Jackson Laboratories (Bar Harbor, ME, USA). All mice were housed at the Eugene Applebaum College of Pharmacy and Health Science Animal Care Facility of Wayne State University and were used in accordance with the guidelines of the Institutional Animal and Care Use Committee (IACUC).
AC Injection
AC inoculation of antigens was carried out using a Hamilton automatic dispensing apparatus (Hamilton, Whittier, CA, USA), as explained previously 5, 6. 50–100 μg of MOG or MBP (or a combination of both antigens) was injected (in 5‐μL Phosphate‐buffered saline [PBS]) (Sigma, St. Louis, MO, USA) into the AC of BALB/c mice. This procedure was performed under anesthetizing conditions (2–3% isoflurane with oxygen supply). Mice receiving PBS alone via intracameral injection were used as controls.
Subcutaneous Immunization
Mice were immunized by s.c. injection on day 7 with 250 μg of MOG or MBP (or a combination of both antigens) (Sigma). Antigens were emulsified 1:1 in complete Freund's adjuvant (CFA; Sigma). Each animal received 200 μL of a MOG or MBP (or a combination of both) in an emulsion with CFA. On day 14 post‐AC injection of antigens either a DTH assay or a LAT assay was carried out as described below.
DTH Assay
On day 14 post‐AC injection of MOG, MBP, or a combination of both antigens (which is 7 days after s.c. immunization with MOG, MBP, or MOG/MBP in CFA), a DTH assay was performed to measure DTH, as described in our previous publications 5, 6, 25. Antigens were injected (500 μg/20 μL) intradermally into the left ear pinna, and PBS alone was injected into the right ear pinna as an internal control. Both ears were measured 24 h later using a Mitutoyo engineer's micrometer (Mitutoyo Corp., Kawasaki, Japan), and the difference in ear swelling was used as a measure of DTH (n = 5 mice per group). The ear swelling measurements were performed before and 24 h after MOG or MBP injection using the following equation: specific ear swelling = (24 h measurement − 0 h measurement) for the left ear − (24 h measurement − 0 h measurement) for the right ear.
Local Adoptive Transfer (LAT) Assay
The LAT assay is a pivotal functional approach that is performed to test for regulatory cells in ACAID 4, 5. On day 14 post‐AC injection of MOG, MBP, or a combination of both antigens (7 days after the s.c. immunization with MOG, MBP, or MOG/MBP in CFA), spleens were diced, and expressed through a 40‐μm Nylon mesh. All isolated spleen cells were made into single‐cell suspensions. Spleen cells containing putative regulatory cells were washed twice and used in a local adoptive transfer (LAT) assay, as explained in our previous publications 5, 6. One million in vivo‐generated putative regulatory cells (in 10 μL) combined with immune spleen cells (one million cells in 10 μL; isolated from s.c. immunized donors) and along with 500 μg/20 μL of MOG or MBP were intradermally injected into the left ear pinna of naïve mice. 20 μL of PBS alone was injected into the right ear pinna as an internal control. The presence of regulatory cells was evaluated by suppression of ear swelling responses induced by immune spleen cells after 24 and 48 h. Two positive control groups of mice were included: The first received ovalbumin (OVA)‐specific spleen cells (isolated from mice that received OVA via AC injection) plus MOG/MBP immunized spleen cells and MOG/MBP antigens. The second received naive spleen cells plus MOG/MBP immunized spleen cells and MOG/MBP antigens. Negative controls included mice that received naïve spleen cells along with MOG/MBP and mice that received MOG/MBP alone. Positive and negative control groups of mice were injected with PBS in the right pinna as an internal control. (n = 5 mice per group). The presence of Tregs was confirmed by the inhibition of ear swelling responses induced by immune spleen cells after 24 h and 48 h.
Immunomagnetic Cell Separation
Whole splenic T cells or CD8+ T cells were isolated from AC‐injected mice (to be used in LAT assays) by incubating their splenic cells with CD90.2 (Thy‐1.2) or CD8 microBeads (10 μL per 107 cells) (Miltenyi Biotec Inc., Auburn, CA, USA) for 15 min at 4–8°C. The cell suspension was passed through a LS MACS column placed in the magnetic field of a MACS separator. Cells were washed 3× with 3 mL of buffer (PBS containing 0.5% Bovine Serum Albumin and 2 mm EDTA), and the magnetically labeled T cells or CD8+ cells were retained in the column followed by elution and resuspension in PBS for injection. The mouse CD90.2 alloantigen is a pan–T‐cell marker that is expressed on peripheral T cells in BALB/c mice.
Statistical Analysis
The Student's t test was used to evaluate the significance of experiments. Data were expressed as mean ± SD for all experimental measurements.
Results
AC Delivery of MOG or MBP Induces ACAID in BALB/c Mice
We hypothesized that AC injection of the encephalitogenic antigens MOG and/or MBP induces specific immune tolerance in Balb/c mice. To test the hypothesis, we conducted DTH assays. Mice were primed in the AC with MOG or MBP on day 0, and then immunized (s.c.) with MOG or MBP emulsified with CFA on day 7. On day 14, the mice ears were challenged with MOG or MBP by intradermal injections. Ear swelling was measured after 24 and 48 h. Results demonstrated that mice primed in the AC with MOG or MBP did develop immune tolerance that was confirmed by significant suppression of MOG‐ or MBP‐specific DTH responses compared with positive control mice that did not receive the AC injection (Figure 1). This proves that ACAID was efficiently induced as a result of MOG or MBP inoculation into the AC of Balb/c mice.
Figure 1.
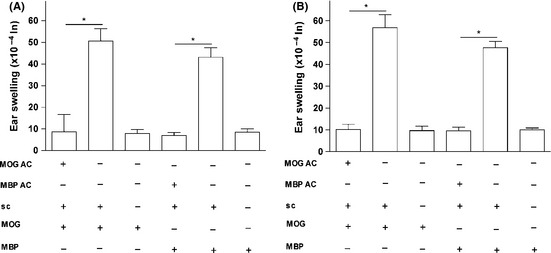
AC‐injected MOG or MBP inhibits DTH responses in Balb/c mice via induced peripheral tolerance. ACAID was induced in Balb/c mice via the AC injection of MOG or MBP (except for the control mice) followed by the s.c. immunization with MOG in CFA or MBP in CFA on day 7. On day 14, the mice were challenged with MOG or MBP (500 μg/20 μL) intradermally in the left ear pinna. 20‐μL PBS was injected into the right ear pinna as internal control. Positive control mice received the s.c. immunization with MOG/MBP in CFA on day 7 and were subsequently challenged intradermally with MOG or MBP on day 14. Negative control mice received only intradermal injections of MOG or MBP on day 14. Ear swelling responses were determined after 24 h (A) and 48 h (B) of the intradermal injection of MOG or MBP. P values < 0.05 were considered significant (*).
Codelivery of MOG and MBP (as a Single Injection) into the AC Triggers ACAID Specific to Both Antigens
To test whether the coinoculation of MOG and MBP into the AC of Balb/c mice induces specific immune tolerance to both antigens, we performed DTH assays in which Balb/c mice were primed in the AC with a cocktail of MOG and MBP on day 0, immunized (s.c.) with the same cocktail in CFA on day 7, and finally ear‐challenged with both antigens on day 14. Results of ear swelling measurements on days 15 and 16 showed that mice coinjected with MOG/MBP in the AC developed specific immune tolerance as evidenced by significant suppression of DTH responses as compared with the positive controls (Figure 2). We also injected MOG alone and MBP alone in the ear and noticed suppression as well. Another group of mice that received the coinjection of MOG/MBP in the AC on day 0 was s.c immunized with ovalbumin (OVA) in CFA on day 7 and then ear‐challenged with OVA on day 14. OVA was unable to suppress DTH responses in these mice (data not shown). This confirms the specificity of the ACAID induction. To summarize, specific peripheral tolerance was induced to both antigens coinjected into the AC of the eye.
Figure 2.
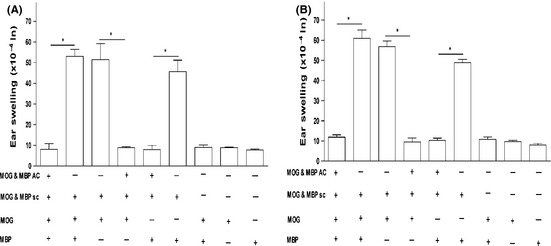
MOG/MBP coinjection via the AC of the eye in Balb/c mice inhibits MOG/MBP‐induced DTH responses. ACAID induction was performed by injecting Balb/c mice once with the MOG/MBP cocktail via the AC (except for the control mice) followed by the s.c. immunization with MOG/MBP in CFA on day 7. On day 14, the mice were challenged with MOG/MBP, MOG alone, or MBP alone (500 μg/20 μL) intradermally in the left ear pinna. 20‐μL PBS was injected into the right ear pinna as internal control. Positive control mice received the s.c. immunization with MOG/MBP in CFA on day 7 and were subsequently challenged intradermally with MOG/MBP, MOG, or MBP on day 14. Negative control mice received only intradermal injections of MOG/MBP, MOG, or MBP on day 14. Ear swelling responses were determined after 24 h (A) and 48 h (B) of the intradermal injection of MOG/MBP, MOG, or MBP. P values < 0.05 were considered significant (*).
LAT Assay‐Based Approaches
Inhibition of DTH responses is a crucial confirmative sign of ACAID induction at the efferent arm of the immune response 8, 9, 10, 26, 27. We used LAT assays to detect the capacity of in vivo‐generated efferent suppressor cells to inhibit the expression of DTH responses. Three LAT assay‐based approaches were performed to address two specific questions: (1) Does the coinjection of MOG and MBP inhibits the expression of DTH responses in a specific manner? (as suggested by our findings in Figure 2) (2) If so, what is the phenotype of the efferent cells associated with induction of this peripheral immune tolerance? To address these questions, we inoculated a cocktail of MOG/MBP into the AC of Balb/c mice (on day 0). Immunization (s.c.) with MOG/MBP in CFA was performed on day 7 followed by harvesting spleen cells on day 14. The harvested spleen cells, that contain putative suppressor cells, were admixed with immune spleen cells (isolated from mice subcutaneously immunized with MOG/MBP), and then injected together with the respective antigens into the ear pinna of naïve mice. The presence of regulatory cells is indicated by a substantial reduction in ear swelling responses that are normally associated with the injection of immune spleen cells.
LAT Assay 1: In Vivo‐Generated Putative Regulatory Spleen Cells of Mice that Received a Coinjection of MOG/MBP in the AC Suppress MOG/MBP‐Induced DTH Responses
We used whole spleen cell populations to address whether spleen cells isolated from mice injected with a cocktail of MOG/MBP in the AC had the ability to exhibit regulatory activities when mixed with immune spleen cells and MOG/MBP, as suggested by our results in Figure 2. Spleen cells of naïve mice and of mice subcutaneously immunized with MOG/MBP in CFA (immune spleen cells) were isolated. Spleen cells with putative suppressor cells were isolated from mice injected with MOG/MBP in the AC followed by s.c. immunization with MOG/MBP in CFA on day 7. These were mixed together with an equal number of immune spleen cells and a cocktail of MOG/MBP antigens. The suspension was injected directly into the left ear pinna of naïve mice to test for regulatory functions of the putative suppressor cells by monitoring the reduction in ear swelling responses after 24 and 48 h. Two positive controls were included (cell combinations that were injected into the ear pinna of naive mice): The first included OVA‐specific regulatory spleen cells isolated from mice that received AC‐injected OVA mixed with immune spleen cells from MOG/MBP subcutaneously immunized mice and the MOG/MBP antigen cocktail. The second positive control included naive spleen cells, immune spleen cells from MOG/MBP subcutaneously immunized mice, and the MOG/MBP antigen cocktail. Both controls were deficient in MOG/MBP‐specific regulatory splenocytes. A negative control group included naïve spleen cells and the MOG/MBP antigen cocktail injected into the ear pinna of naive mice. Another negative control involved the injection of the MOG/MBP antigen cocktail alone.
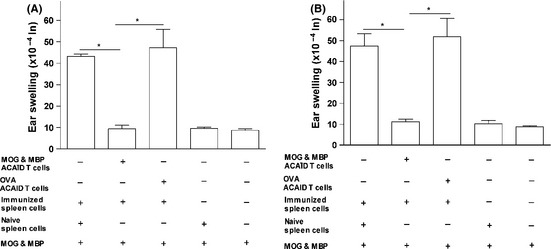
As evident in Figure 3, the presence of putative regulatory splenocytes in mice that received the MOG/MBP AC coinjection inhibited DTH swelling responses as compared with the positive control mice. This indicates the ability of ACAID spleen cells to regulate DTH responses of primed immune effector cells after 24 and 48 h (Figure 3). The statistical significance of ear swelling measurements obtained were as follows: ACAID mice versus positive control (no MOG/MBP‐specific regulatory spleen cells), *P = 0.0027 (24 h); *P = 0.0015 (48 h). ACAID mice versus positive control (OVA‐specific regulatory spleen cells), *P = 0.0002 (24 h); *P = 0.000008 (48 h). Both negative control groups showed negative ear swelling responses as expected (Figure 3). These results show that DTH responses induced by primed immune effector cells were specifically suppressed by the regulatory spleen cell populations of mice injected with MOG/ MBP in the AC, suggesting the existence of Tregs within the T‐cell compartment of the spleen cell population. Therefore, we set out to examine the potential regulatory abilities of T cells within the spleen cell compartment.
Figure 3.

In vivo‐generated putative regulatory spleen cells of mice that received a coinjection of MOG/MBP in the AC inhibit MOG/MBP‐induced DTH responses. On day 14, spleen cells were isolated from Balb/c mice that received a coinjection of MOG/MBP in the AC on day 0 and a s.c. immunization of MOG/MBP on day 7. These putative regulatory spleen cells were mixed with immune spleen cells (isolated from MOG/MBP subcutaneously immunized donors) and the MOG/MBP antigen cocktail. The suspension was injected into the left ear pinna of naïve mice to test for regulatory functions of the putative suppressor cells by monitoring the reduction in ear swelling responses after 24 (A) and 48 h (B). Two positive controls were included: The first included OVA‐specific regulatory spleen cells isolated from mice that received AC‐injected OVA mixed with immune spleen cells from MOG/MBP subcutaneously immunized mice and the MOG/MBP antigen cocktail. The second included naive spleen cells, immune spleen cells from MOG/MBP subcutaneously immunized mice, and the MOG/MBP antigen cocktail. Both controls were deficient in MOG/MBP‐specific regulatory splenocytes. A negative control group included naïve spleen cells and the MOG/MBP antigen cocktail injected into the ear pinna of naive mice. Another negative control involved the injection of the MOG/MBP antigen cocktail. P values < 0.05 were considered significant (*).
LAT Assay 2: In Vivo‐Generated Putative Regulatory Splenic T Cells of Mice That Received a Coinjection of MOG/MBP in the AC Suppress MOG/MBP‐Induced DTH Responses
In vivo‐generated splenic T cells isolated from whole spleen cells of AC‐injected mice were used in a LAT assay, as described above. Here, we sought to see whether T cells within the whole spleen cell compartment of MOG/MBP AC‐injected mice exhibited regulatory functions when mixed with immune spleen cells (Figure 4). T cells were separated based on the expression of CD90.2 by the incubation of spleen cells with CD90.2 microBeads. The CD90.2+ splenic T cells containing the putative regulatory cells were used to perform the LAT assay. Similar positive and negative control groups were included as in LAT assay #1 (above). As expected, the positive control groups showed extensive ear swelling responses, indicating their inability to suppress DTH responses induced by primed immune effector cells after 24 and 48 h (Figure 4). Conversely, splenic T cells in mice that received MOG/MBP via the AC caused significant reduction of ear swelling responses as compared with the positive controls (Figure 4). The statistical significance of ear swelling reductions obtained in this experiment were as follows: AC‐injected mice versus positive control mice (no regulatory splenocytes), *P = 0.00008 (24 h); *P = 0.0027 (48 h). AC‐injected mice versus positive control mice (nonspecific regulatory splenocytes), *P = 0.002 (24 h); *P = 0.0002 (48 h). It is noteworthy that the non‐T‐cell population did not exhibit any appreciable suppressive functions (data not shown). This suggests that the ear swelling DTH responses induced by primed immune cells were directly suppressed by the splenic T‐cell population of AC‐induced mice, suggesting that the T‐cell population in the spleen exhibited regulatory activities. In other ACAID models, efferent CD8+ Tregs were shown to suppress DTH responses 8, 9, 10, 26, 27. Hence, we hypothesized that CD8+ efferent Tregs within the whole splenic T‐cell population are solely responsible for the DTH regulatory activities and the induction of MOG/MBP‐specific peripheral tolerance.
Figure 4.
In vivo‐generated putative regulatory splenic T cells of mice that received a coinjection of MOG/MBP in the AC inhibit MOG/MBP‐induced DTH responses. On day 14, splenic T cells (CD90.2 microBeads) were isolated from Balb/c mice that received a coinjection of MOG/MBP in the AC on day 0 and a s.c. immunization of MOG/MBP on day 7. These putative regulatory splenic T cells were mixed with immune spleen cells (isolated from MOG/MBP subcutaneously immunized donors) and the MOG/MBP antigen cocktail. The suspension was injected into the left ear pinna of naïve mice to test for regulatory functions of the putative suppressor cells by monitoring the reduction in ear swelling responses after 24 (A) and 48 h (B). Two positive controls were included: The first included OVA‐specific regulatory spleen cells isolated from mice that received AC‐injected OVA mixed with immune spleen cells from MOG/MBP subcutaneously immunized mice and the MOG/MBP antigen cocktail. The second included naive spleen cells, immune spleen cells from MOG/MBP subcutaneously immunized mice, and the MOG/MBP antigen cocktail. A negative control group included naïve spleen cells and the MOG/MBP antigen cocktail injected into the ear pinna of naive mice. Another negative control involved the injection of the MOG/MBP antigen cocktail. P values < 0.05 were considered significant (*).
LAT Assay 3: In Vivo‐Generated Putative Regulatory Splenic CD8+ T Cells of Mice that Received a Coinjection of MOG/MBP in the AC Suppress MOG/MBP‐Induced DTH Responses
The splenic CD8+ T‐cell population in the AC‐induced mice was isolated using CD8 microbeads, and a LAT assay was conducted. Positive control mice developed substantial ear swelling responses. The splenic CD8+ T cells in mice that received MOG/MBP via the AC caused significant reduction of ear swelling responses as compared with the positive controls (Figure 5). Statistical significance observed between the groups was as follows: AC‐injected mice versus positive control (no regulatory splenocytes), *P = 0.00007 (24 h); *P = 0.0013 (48 h). AC‐injected mice versus positive control (nonspecific regulatory splenocytes), *P = 0.0004 (24 h); *P = 0.0016 (48 h). Although, the non‐CD8+ T‐cell population did not cause any remarkable inhibition (data not shown), the splenic CD8+ T cells caused significant suppression (Figure 5), suggesting that CD8+ T cells could solely be responsible for the efferent suppression of DTH responses in this model.
Figure 5.
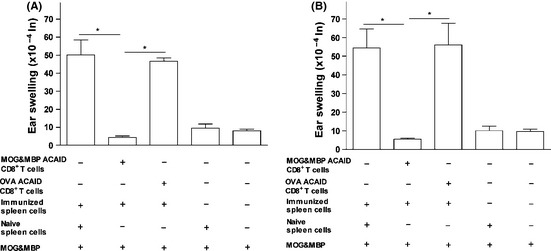
In vivo‐generated putative regulatory splenic CD8+ T cells of mice that received a coinjection of MOG/MBP in the AC inhibit MOG/MBP‐induced DTH responses. On day 14, splenic CD8+ T cells (CD8+ microBeads) were isolated from Balb/c mice that received a coinjection of MOG/MBP in the AC on day 0 and a s.c. immunization of MOG/MBP on day 7. These putative regulatory splenic CD8+ T cells were mixed with immune spleen cells (isolated from MOG/MBP subcutaneously immunized donors) and the MOG/MBP antigen cocktail. The suspension was injected into the left ear pinna of naïve mice to test for regulatory functions of the putative suppressor cells by monitoring the reduction in ear swelling responses after 24 (A) and 48 h (B). Two positive controls were included: The first included OVA‐specific regulatory spleen cells isolated from mice that received AC‐injected OVA mixed with immune spleen cells from MOG/MBP subcutaneously immunized mice and the MOG/MBP antigen cocktail. The second included naive spleen cells, immune spleen cells from MOG/MBP subcutaneously immunized mice, and the MOG/MBP antigen cocktail. A negative control group included naïve spleen cells and the MOG/MBP antigen cocktail injected into the ear pinna of naive mice. Another negative control involved the injection of the MOG/MBP antigen cocktail. P values < 0.05 were considered significant (*).
Discussion
Injection of antigens into the AC of the eye leads to specific inhibition of cell‐mediated immune responses against the AC‐injected antigens 9, 27, 28. Circulating ocular F4/80+ macrophages capture the AC‐injected antigen and migrate into the spleen to participate in the activation of NKT cells and marginal zone B cells. These cells together with other immune cells and cytokines stimulate antigen‐specific CD4+ and CD8+ Treg expansion 9, 27, 28, 29. We and others previously reported that splenic B cells, γδ T cells, and NK T cells play critical roles in the induction of ACAID via the generation of both CD4+ and CD8+ Tregs 5, 6 30. AC‐induced Tregs were shown to possess the ability to inhibit antigen‐induced DTH responses 10, 28, 31.
To the best of our knowledge, the current investigation is the first to report that the AC injection of MBP alone or its coinjection with MOG into the AC of the eye induces ACAID. This was proven via DTH and LAT assays (Figures 1 and 2). In addition, we sought to examine whether multiple myelin antigens could effectively induce peripheral tolerance. The coadministration of MOG/MB‐induced MOG/MBP‐specific ACAID, MOG‐specific ACAID, and MBP‐specific ACAID (Figure 2). We also showed that the antigen‐specific spleen cells, whole T cells, and CD8+ T cells from the AC MOG/MBP‐injected mice exhibited suppressive properties (Figures 3, 4, 5). Other studies demonstrated the crucial functions of Tregs in the induction of ACAID‐mediated immune tolerance 32, 33, 34. The immunoregulatory roles of efferent CD8+ Tregs in these models were antigen specific 10, 35, 36.
The efficiency and therapeutic potentials of CD4+ and CD8+ Tregs have been demonstrated in several studies with autoimmune diseases 37, 38 (including hepatitis 39, 40, nephritis 41, myositis 42, EAE 23, 24, 43, 44, rheumatoid arthritis 45, 46, 47, lupus 48, and diabetes mellitus type 1 49), pulmonary inflammation 35, transplantation 50, and inflammatory bowel disease 51. Hence, we propose that ACAID CD8+ Tregs could be used as potential immunotherapeutic tools in autoimmune diseases. It remains to be seen whether MOG or MBP could drive similar in vivo immuno‐modulatory responses in autoimmune disease animal models. MOG‐specific CD4+ Tregs were shown to ameliorate the initiation stage of EAE disease induction, whereas MOG‐specific CD8+ Tregs restricted disease progression at the effector phase 23.
MS is dependent on multiple mylein proteins including MOG 13, 14, MBP 15, 16, PLP 15, 17, MOBP 18, 19, and OSP 20, 21. Other key antigens associated with MS pathogenesis remain unknown. Autoreactivity against myelin proteins is implicated in the pathogenesis as well as the clinical and pathological manifestations of EAE in susceptible animals. It is necessary to establish the roles of multiple myelin proteins in the pathogenesis of MS and to develop strategies for specific immune tolerance induction in the context of MS. ACAID is a potential tool that could be utilized in this regard. In the present study, we showed that ACAID‐mediated tolerance to multiple myelin antigens was effectively induced, as evidenced by the suppression of DTH responses to MOG and MBP alone or in combination (Figure 2).
This study indicated that specific immune tolerance to multiple antigens could be induced in a one‐step process, in which these antigens are coinjected into the AC of the eye of experimental mice. This could also be potentially performed in humans or primates, in which ACAID has been shown to be functional. Multiple encephalitogenic myelin antigens have long been implicated in the pathogenesis of MS and EAE as potential prime target antigens. Thus, previous attempts to treat EAE involved the administration of a chimeric fusion protein of PLP and MBP (MP4) 52, the administration of an engineered “multiantigen/multiepitope” (containing epitopes of PLP, MBP, and MOG) 53, and the administration of multiple myelin peptides 54. Another study has employed cocktails of peptides (PLP, MBP, and MOG) in the Ethylenecarbodiimide (ECDI) coupled‐ Antigen presenting cell tolerance system and inhibited the initiation of active EAE induction by preventing activation of autoreactive Th1 cells and the subsequent infiltration of inflammatory cells into the CNS 55. A promising future area of research is to investigate the therapeutic potential of the coadministration of different combinations of antigens implicated in MS pathogenesis such as MOG, MBP, PLP, MOBP, and OSP into the AC of the eye. This is supposed to induce ACAID‐mediated specific peripheral tolerance that could be tested in different MS autoimmune disease settings.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1. Taylor AW. Ocular immune privilege. Eye (Lond) 2009;23:1885–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Medawar PB. A second study of the behaviour and fate of skin homografts in rabbits: a report to the war wounds committee of the medical research council. J Anat 1945;79:157–176, 154. [PubMed] [Google Scholar]
- 3. Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- 4. Forrester JV, Xu H, Lambe T, Cornall R. Immune privilege or privileged immunity? Mucosal Immunol 2008;1:372–381. [DOI] [PubMed] [Google Scholar]
- 5. Ashour HM, Niederkorn JY. Gammadelta t cells promote anterior chamber‐associated immune deviation and immune privilege through their production of il‐10. J Immunol 2006;177:8331–8337. [DOI] [PubMed] [Google Scholar]
- 6. Ashour HM, Niederkorn JY. Peripheral tolerance via the anterior chamber of the eye: role of b cells in mhc class i and ii antigen presentation. J Immunol 2006;176:5950–5957. [DOI] [PubMed] [Google Scholar]
- 7. Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol 2003;3:879–889. [DOI] [PubMed] [Google Scholar]
- 8. Niederkorn JY. Regulatory t cells and the eye. Chem Immunol Allergy 2007;92:131–139. [DOI] [PubMed] [Google Scholar]
- 9. Niederkorn JY. The induction of anterior chamber‐associated immune deviation. Chem Immunol Allergy 2007;92:27–35. [DOI] [PubMed] [Google Scholar]
- 10. Wilbanks GA, Streilein JW. Characterization of suppressor cells in anterior chamber‐associated immune deviation (acaid) induced by soluble antigen. Evidence of two functionally and phenotypically distinct t‐suppressor cell populations. Immunology 1990;71:383–389. [PMC free article] [PubMed] [Google Scholar]
- 11. Kuerten S, Angelov DN. Comparing the cns morphology and immunobiology of different eae models in c57bl/6 mice ‐ a step towards understanding the complexity of multiple sclerosis. Ann Anat 2008;190:1–15. [DOI] [PubMed] [Google Scholar]
- 12. Kaushansky N, Eisenstein M, Zilkha‐Falb R, Ben‐Nun A. The myelin‐associated oligodendrocytic basic protein (mobp) as a relevant primary target autoantigen in multiple sclerosis. Autoimmun Rev 2010;9:233–236. [DOI] [PubMed] [Google Scholar]
- 13. Li J, Zhao X, Skoff R, Shaw MK, Tse HY. Differential levels of resistance to disease induction and development of relapsing experimental autoimmune encephalomyelitis in two h‐2b‐restricted mouse strains. J Neuroimmunol 2011;234:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo X, Harada C, Namekata K, et al. Delayed onset of experimental autoimmune encephalomyelitis in olig1 deficient mice. PLoS ONE 2010;5:e13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuerten S, Schickel A, Kerkloh C, et al. Tertiary lymphoid organ development coincides with determinant spreading of the myelin‐specific t cell response. Acta Neuropathol 2012;124:861–873. [DOI] [PubMed] [Google Scholar]
- 16. Yin LL, Lin LL, Zhang L, Li L. Epimedium flavonoids ameliorate experimental autoimmune encephalomyelitis in rats by modulating neuroinflammatory and neurotrophic responses. Neuropharmacology 2012;63:851–862. [DOI] [PubMed] [Google Scholar]
- 17. Forghani R, Wojtkiewicz GR, Zhang Y, et al. Demyelinating diseases: myeloperoxidase as an imaging biomarker and therapeutic target. Radiology 2012;263:451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaushansky N, Zilkha‐Falb R, Hemo R, et al. Pathogenic t cells in mobp‐induced murine eae are predominantly focused to recognition of mobp21f and mobp27p epitopic residues. Eur J Immunol 2007;37:3281–3292. [DOI] [PubMed] [Google Scholar]
- 19. de Rosbo NK, Kaye JF, Eisenstein M, et al. The myelin‐associated oligodendrocytic basic protein region mobp15‐36 encompasses the immunodominant major encephalitogenic epitope(s) for sjl/j mice and predicted epitope(s) for multiple sclerosis‐associated hla‐drb1*1501. J Immunol 2004;173:1426–1435. [DOI] [PubMed] [Google Scholar]
- 20. Kaushansky N, Eisenstein M, Oved JH, Ben‐Nun A. Activation and control of pathogenic t cells in osp/claudin‐11‐induced eae in sjl/j mice are dominated by their focused recognition of a single epitopic residue (osp58 m). Int Immunol 2008;20:1439–1449. [DOI] [PubMed] [Google Scholar]
- 21. Kaushansky N, Hemo R, Eisenstein M, Ben‐Nun A. Osp/claudin‐11‐induced eae in mice is mediated by pathogenic t cells primarily governed by osp192y residue of major encephalitogenic region osp179‐207. Eur J Immunol 2007;37:2018–2031. [DOI] [PubMed] [Google Scholar]
- 22. Lu L, Cantor H. Generation and regulation of cd8(+) regulatory t cells. Cell Mol Immunol 2008;5:401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bhowmick S, Clark RB, Brocke S, Cone RE. Antigen‐specific splenic cd4+ and cd8+ regulatory t cells generated via the eye, suppress experimental autoimmune encephalomyelitis either at the priming or at the effector phase. Int Immunol 2011;23:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Faunce DE, Terajewicz A, Stein‐Streilein J. Cutting edge: in vitro‐generated tolerogenic apc induce cd8+ t regulatory cells that can suppress ongoing experimental autoimmune encephalomyelitis. J Immunol 2004;172:1991–1995. [DOI] [PubMed] [Google Scholar]
- 25. Ashour HM, Niederkorn JY. Expansion of b cells is necessary for the induction of t‐cell tolerance elicited through the anterior chamber of the eye. Int Arch Allergy Immunol 2007;144:343–346. [DOI] [PubMed] [Google Scholar]
- 26. Konya C, Goronzy JJ, Weyand CM. Treating autoimmune disease by targeting cd8(+) t suppressor cells. Expert Opin Biol Ther 2009;9:951–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cone RE, Chattopadhyay S, O'Rourke J. Control of delayed‐type hypersensitivity by ocular‐ induced cd8+ regulatory t cells. Chem Immunol Allergy 2008;94:138–149. [DOI] [PubMed] [Google Scholar]
- 28. Niederkorn JY. Mechanisms of immune privilege in the eye and hair follicle. J Invest Dermatol 2003;8:168–172. [DOI] [PubMed] [Google Scholar]
- 29. Li X, Shen S, Urso D, et al. Phenotypic and immunoregulatory characteristics of monocytic iris cells. Immunology 2006;117:566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Faunce DE, Sonoda KH, Stein‐Streilein J. Mip‐2 recruits nkt cells to the spleen during tolerance induction. J Immunol 2001;166:313–321. [DOI] [PubMed] [Google Scholar]
- 31. Keino H, Takeuchi M, Kezuka T, et al. Induction of eye‐derived tolerance does not depend on naturally occurring cd4+ cd25+ t regulatory cells. Invest Ophthalmol Vis Sci 2006;47:1047–1055. [DOI] [PubMed] [Google Scholar]
- 32. Lin HH, Faunce DE, Stacey M, et al. The macrophage f4/80 receptor is required for the induction of antigen‐specific efferent regulatory t cells in peripheral tolerance. J Exp Med 2005;201:1615–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sonoda KH, Stein‐Streilein J. Cd1d on antigen‐transporting apc and splenic marginal zone b cells promotes nkt cell‐dependent tolerance. Eur J Immunol 2002;32:848–857. [DOI] [PubMed] [Google Scholar]
- 34. Skelsey ME, Mayhew E, Niederkorn JY. Cd25+, interleukin‐10‐producing cd4+ t cells are required for suppressor cell production and immune privilege in the anterior chamber of the eye. Immunology 2003;110:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Katagiri K, Zhang‐Hoover J, Mo JS, Stein‐Streilein J, Streilein JW. Using tolerance induced via the anterior chamber of the eye to inhibit th2‐dependent pulmonary pathology. J Immunol 2002;169:84–89. [DOI] [PubMed] [Google Scholar]
- 36. Streilein JW, Takeuchi M, Taylor AW. Immune privilege, t‐cell tolerance, and tissue‐restricted autoimmunity. Hum Immunol 1997;52:138–143. [DOI] [PubMed] [Google Scholar]
- 37. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self‐tolerance maintained by activated t cells expressing il‐2 receptor alpha‐chains (cd25). Breakdown of a single mechanism of self‐tolerance causes various autoimmune diseases. J Immunol 1995;155:1151–1164. [PubMed] [Google Scholar]
- 38. Stern ME, Schaumburg CS, Dana R, Calonge M, Niederkorn JY, Pflugfelder SC. Autoimmunity at the ocular surface: pathogenesis and regulation. Mucosal Immunol 2010;3:425–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Longhi MS, Hussain MJ, Mitry RR, et al. Functional study of cd4+ cd25+ regulatory t cells in health and autoimmune hepatitis. J Immunol 2006;176:4484–4491. [DOI] [PubMed] [Google Scholar]
- 40. Ferri S, Longhi MS, De Molo C, et al. A multifaceted imbalance of t cells with regulatory function characterizes type 1 autoimmune hepatitis. Hepatology 2010;52:999–1007. [DOI] [PubMed] [Google Scholar]
- 41. Wang YM, Zhang GY, Hu M, et al. Cd8+ regulatory t cells induced by t cell vaccination protect against autoimmune nephritis. J Am Soc Nephrol 2012;23:1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Allenbach Y, Solly S, Gregoire S, et al. Role of regulatory t cells in a new mouse model of experimental autoimmune myositis. Am J Pathol 2009;174:989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Berthelot L, Laplaud DA, Pettre S, et al. Blood cd8+ t cell responses against myelin determinants in multiple sclerosis and healthy individuals. Eur J Immunol 2008;38:1889–1899. [DOI] [PubMed] [Google Scholar]
- 44. Correale J, Villa A. Isolation and characterization of cd8+ regulatory t cells in multiple sclerosis. J Neuroimmunol 2008;195:121–134. [DOI] [PubMed] [Google Scholar]
- 45. van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. Cd4(+)cd25(+) regulatory t cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum 2004;50:2775–2785. [DOI] [PubMed] [Google Scholar]
- 46. Jung S, Park YK, Lee H, Shin JH, Lee GR, Park SH. Tgf‐beta‐treated antigen presenting cells suppress collagen‐ induced arthritis through the promotion of th2 responses. Exp Mol Med 2010;42:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Davila E, Kang YM, Park YW, et al. Cell‐based immunotherapy with suppressor cd8+ t cells in rheumatoid arthritis. J Immunol 2005;174:7292–7301. [DOI] [PubMed] [Google Scholar]
- 48. Singh RP, La Cava A, Wong M, Ebling F, Hahn BH. Cd8+ t cell‐mediated suppression of autoimmunity in a murine lupus model of peptide‐induced immune tolerance depends on foxp3 expression. J Immunol 2007;178:7649–7657. [DOI] [PubMed] [Google Scholar]
- 49. Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC. Tcr stimulation with modified anti‐cd3 mab expands cd8+ t cell population and induces cd8+ cd25+ tregs. J Clin Invest 2005;115:2904–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cunnusamy K, Chen PW, Niederkorn JY. Il‐17a‐dependent cd4+ cd25+ regulatory t cells promote immune privilege of corneal allografts. J Immunol 2011;186:6737–6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Menager‐Marcq I, Pomie C, Romagnoli P, van Meerwijk JP. Cd8+ cd28‐ regulatory t lymphocytes prevent experimental inflammatory bowel disease in mice. Gastroenterology 2006;131:1775–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Elliott EA, McFarland HI, Nye SH, et al. Treatment of experimental encephalomyelitis with a novel chimeric fusion protein of myelin basic protein and proteolipid protein. J Clin Invest 1996;98:1602–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhong MC. Kerlero de Rosbo N, Ben‐Nun A. Multiantigen/multiepitope‐directed immune‐specific suppression of “complex autoimmune encephalomyelitis” by a novel protein product of a synthetic gene. J Clin Invest 2002;110:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Leadbetter EA, Bourque CR, Devaux B, et al. Experimental autoimmune encephalomyelitis induced with a combination of myelin basic protein and myelin oligodendrocyte glycoprotein is ameliorated by administration of a single myelin basic protein peptide. J Immunol 1998;161:504–512. [PubMed] [Google Scholar]
- 55. Smith CE, Miller SD. Multi‐peptide coupled‐cell tolerance ameliorates ongoing relapsing eae associated with multiple pathogenic autoreactivities. J Autoimmun 2006;27:218–231. [DOI] [PMC free article] [PubMed] [Google Scholar]


