SUMMARY
Background: In patients with bipolar disorder, medication is effective in preventing relapses. Unfortunately, adherence to treatment in bipolar disorder, as in other chronic or recurrent conditions, is not optimal. Estimates of nonadherence to prescribed treatment range from 30% to 60% in epidemiological studies, and are at around 30% in clinical trials. Adherence to treatment is a potent predictor of effectiveness, both in clinical trials and cohort studies, therefore is a very relevant area of investigation. This study will try to show a picture of the real life care where adherence is influenced by a wide range of variables. Methods: Prospective, observational, multicenter study in 650 adult patients with bipolar disorder, who had to initiate or change their treatment regimen, observed for 1 year. Adherence was measured by the Simplified Medication Adherence Questionnaire (SMAQ). Additional variables: Symptom severity, Montgomery‐Åsberg Depression Rating Scale (MADRS), Young Mania Rating Scale (YMRS), Clinical Global Impression–Bipolar Disorder (CGI‐BD), the Drug Attitude Inventory score (DAI‐30), and quality of life (EuroQoL 5 Dimensions). The variables were recorded every 3 months for the next year. Results: Most subjects were out‐patients (77.1%), female (58.8%), aged 31–50 years (50.1%) and overweight (41.8%) or obese (28.7%); 67.4% had type I bipolar disorder and 66.8% had depressive or mixed symptoms. Adherence was 39.9% at baseline (and increased up to 67.0% at completion. The main predictors of nonadherence were alcohol consumption, severe bipolar symptoms, young age at time of first treatment, negative attitude towards treatment. Conclusions: The patient population of this observational trial was representative of the patients changing their therapy for bipolar disorder seen in clinical practice in Italy. Lack of adherence to pharmacotherapy for bipolar disorder is a serious issue, which is more likely to arise in alcohol users and patients with severe symptoms, negative attitude towards medication and/or initiation of treatment early in life. The findings could lead to a more adequate approach of adherence in patients with bipolar disorders.
Keywords: Adherence, Bipolar disorder, Observational trial, Predictors
Introduction
Bipolar illness is a severe, chronic and recurrent condition that represents a major health problem which includes both a great economic burden [1] and high mortality rates [2].
In research settings, prophylaxis with mood stabilizers reduces the risk of relapse by about 50%. Unfortunately, in day to day practice, the benefits of prophylaxis are less impressive [3]. A key contributor to the efficacy‐effectiveness gap is medication nonadherence [4]. On average, nearly half of the patients seen by a clinician for pharmacological treatment will not be fully adherent with prescribed medication.
Reported nonadherence rates for long‐term prophylactic pharmacotherapy range from 20% to 66%, with a median prevalence of 41%[5]. In bipolar disorders, a number of researchers highlight the risk of early recurrence of mania if long‐term treatments are discontinued [6]. Keck et al. [7] reported that 60% of patients admitted with an acute episode of mania had failed to adhere to medication in the month prior to hospitalization. Scott [5] showed that, over 1 year, the risk of hospitalization was four times higher in subjects who were nonadherent as compared with adherent with mood stabilizers. Worryingly, in a large‐scale study of over 1,500 patients, Johnson and McFarland [8]) reported that the median duration of continuous use of lithium after it was first prescribed was only 76 days. Later nonadherence is also an issue. Jamison et al. [9] also noted that over a 2‐year follow‐up period, use of prophylactic medication may be intermittent, with 50% of patients with bipolar disorders stopping and restarting their lithium at least once and 30% at least twice.
The increased ability to treat the acute phase of mood disorders brought clinical and academic interest to the maintenance phase of the treatment with more emphasis on risk reduction, disease prevention, lifestyle change, and adherence.
Total adherence and total nonadherence are rare. As noted by Thompson et al. [10] adherence is best viewed as “continuously distributed rather than naturally dichotomous.” Using this model, it is clear we need to research factors that increase or decrease levels of adherence within this continuum and then use these data to inform clinical strategies that enhance adherence. At this moment, the main clinical imperative is to raise professional awareness of the size of the problem and help clinicians to engage in a collaborative process with their patients to allow them to make informed choices about treatment regimens [11].
A study, which looked, in a 2‐years naturalistic follow‐up, at clinical factors associated with treatment nonadherence in euthymic bipolar patientsfound 60.5% to have good adherence, 39.5% to have partial or poor adherence among their study population [12]. In another study, 51% of bipolar patients were partially or totally nonadherent with pharmacologic maintenance treatment during the 1‐year follow‐up period [13]. Treatment discontinuation is the most important predictor of relapse and poor outcome for bipolar patients [6, 14]. There is evidence that mortality rates among noncompliant bipolar patients are much higher than among compliant patients [15, 16].
Psychiatric researchers have attempted to delineate predictors of nonadherence specific to unipolar and bipolar disorders. Jamison [9, 17, 18] suggested that it is useful to consider four interacting sets of variables, which combine to form the framework for our understanding of nonadherence issues. These are variables unique to the disorder (type of affective disorder and phase of illness); treatment issues (e.g., treatment regimen, reported side‐effects); patient factors (demography, attitudes to medication, and illness); and physician factors (attitudes to the illness, treatment, and their interaction with the patient).
Although there are a number of studies that have assessed the long‐term treatment of bipolar disorder in clinical trial setting, there has not yet been a study to broadly and prospectively evaluate in real life practice the long‐term adherence, evaluating the usefulness of interventions applied to different phases of illness, stages of treatment and people with diverse presentations of bipolar disorder, e.g., bipolar II, mixed presentations and rapid cycling, temperamental factors, and comorbid disorders. The promotion of treatment adherence may be integrated into the collaborative management of medication in the treatment alliance to assist in diminishing the efficacy effectiveness gap and reducing the morbidity and mortality associated with this chronic illness.
The measurement of adherence faces at least two methodological problems. First of all adherence seems to be larger in clinical studies than in clinical practice and this aspect limits the possibility to generalize the results. Secondly, it has been noted that in the adherence field, simple measure are not accurate, and accurate measures are not simple. Both patients and providers are prone to overestimate adherence and physician are particularly inaccurate at predicting it. A 100% reliable method does not exist [19].
In this study will be used a modified version of the Simplified Medication Adherence Questionnaire (SMAQ) to evaluate the level of adherence. The SMAQ was developed to evaluate a low cost, reliable and easily applicable instrument for measuring adherence in chronic condition. It has been decided to use this questionnaire in consideration of the fact that treatment adherence in psychiatric patients seems comparable to other patient population [19].
Knobel et al. [20] found sensitivity to be 72%, specificity 91%, positive predictive value 87%, and a positive likelihood ratio of 7.94. The results suggest that the SMAQ is a valid indicator of a patient's nonadherence to treatment, although in different chronic population. SMAQ has been used in a clinical study performed on adults with Attention‐Deficit Hyperactivity Disorder (ADHD) to evaluate medication adherence [21]. Results from the study supported the use of this instrument in a psychiatric population.
This paper presents results from the Evaluation of Pharmacotherapy Adherence in Bipolar Disorder (EPHAR) Study addressing the need for further information from everyday clinical practice regarding the treatment adherence patterns of patients treated for a diagnosis of Bipolar Disorder associated with clinical, functional and economic outcomes.
The EPHAR study was a large observational trial primarily designed to observe and measure adherence to pharmacotherapy in bipolar patients within a clinical practice setting for 1 year. Adherence was measured using a modified version of the SMAQ [20].
The secondary objectives of the study were as follows:
-
•
Describe the relationship between variables (e.g., adherence, index episode, severity of illness, treatment prescription, and treatment combinations) at baseline and after 3, 6, 9, and 12 months (after new treatment has been established or changed).
-
•
Describe the impact that baseline factors (e.g., index episode, severity of episode) and treatment choice (treatment prescription, and treatment combinations) have on outcomes (MADRS, YMRS, CGI‐BD, and EUROQol‐5D) after 3, 6, 9, and 12 months
Materials and Methods
Study Design
This was a prospective, observational, longitudinal, noninterventional multicenter, cohort study involving 42 Italian psychiatric centers, where a total of 686 patients with bipolar disorder were screened between January 2006 and February 2007. Data collection was completed 1 year later and 650 patients were enrolled in the study.
Investigators and sites were selected on the basis of representativeness of the wide range of treatment services, facilities and locations (e.g., urban and rural, or Northern, Central and Southern Italy).
Patient Population
For the study, investigators invited the participation of in or out patients who were at least 18 years old, who, in accordance with the decision of the treating psychiatrist, had to initiate a new therapy for the treatment of bipolar disorder as defined by DSM‐IV criteria [22] or, in any phase of their illness, changed an existing therapy, because of side‐effects or lack of efficacy (excluding dose change). Patients were not participating in a separate study that had an interventional design.
The participating physician, at his/her own discretion, decided to initiate or change pharmacological treatment for bipolar disorder. The decision to initiate or change medication and the type of medication selected were completely independent of the study, which only observed treatment choices and outcomes, rather than directing treatment. Psychiatric and treatment histories of each patient were recorded.
-
•
The participating physician observed and recorded data on a regular basis (at baseline, at 3, at 6, at 9, and at 12 months after baseline). Patients were not excluded from the study even if any of the scheduled visit was missed; this was to preserve the naturalistic nature of the study.
Treatment for bipolar disorder was prescribed in the usual standard of care and were not provided by the study sponsor. Patients provided informed consent as required by local laws and regulations.
Patients who had medication treatment changed or terminated at any time after the baseline observation were able to remain in the study, as discontinuation of medication was not a criterion for study discontinuation. Medication use patterns were collected throughout the course of the study.
Patients were observed throughout 1 year of treatment indicated for a bipolar disorder. For each patient entered in the study, the participating investigator or designee completed an electronic data form via a dedicated, secure website. The data collected included the following:
-
•
Socio‐demographic: age, gender, educational status, marital status, weight, and height.
-
•
Psychiatric history: onset of first affective symptoms, first contact with psychiatric services, first hospital stay as inpatient because of psychiatric symptoms, current alcohol use or abuse (as judged by the investigators), current drug consumption, past and current smoking habits; frequency of any mood episodes (manic, mixed, and depressive) during the previous 12 months; frequency and duration of inpatient previous admissions because of, bipolar disorder or any other psychiatric or not diagnoses, prevalence of type of mood episode in the disease history.
Functional status: living conditions, dependent care. and social activity pattern.
-
•
Information on treatment medication for bipolar disorder (antipsychotics, anticonvulsants, lithium as well as antidepressants), their respective doses and mode of intake as well as information on concomitant medication (benzodiazepines, hypnotics. and anticholinergics) was collected both upon presentation and following the change in oral medication at baseline.
-
•
Primary clinical assessment scales: Montgomery‐Åsberg Depression Rating Scale (MADRS) [23], Young Mania Rating Scale (YMRS) [24]), Clinical Global Impression–Bipolar Disorder (CGI‐BD) [25].
-
•
Treatment adherence assessment scales: the SMAQ questionnaire and the Drug Attitude Inventory (DAI).
SMAQ questionnaire we used was based on the Morisky scale [26]) modified by Knobel [20]. It included both qualitative and quantitative questions as follows:
Qualitative questions: (1) “Do you ever forget to take your medicine?”; (2) “Are you careless at times about taking your medicine?”; (3) “When you feel better, do you sometimes stop taking your medicine?”; (4) “If at times you feel worse, do you stop taking your medicine?’. (6) “Did you not take any of your medicine over the last weekend?”
Quantitative questions: (5) “Thinking about the last week, how often have you not taken your medicine?”; (7) “Over the past 3 months, how many days have you not taken any medicine at all?”
We chose this modified version of the SMAQ questionnaire [20] to calculate adherence to treatment. Although this questionnaire is not specific for psychiatric conditions, it has proved to be a valid indicator of patient nonadherence to treatment during chronic diseases: Knobel et al. [20] found, albeit in a different patient population (HIV‐infected patients, where adherence to long term treatment is crucial for patient's outcome), that its sensitivity was 72%, its specificity 91%, its positive predictive value 87%, and its positive likelihood ratio was 7.94. The SMAQ was considered “positive” when a nonadherence patient was detected, that was, when: there was a positive response (yes) to any of the qualitative questions (1, 2, 3, 4, and 6); or more than two doses missed over the past week; or over 2 days of total nonmedication during the past 3 months.
The Drug Attitude Inventory score DAI [27] has been used to examine how the attitude of patients towards their medications may affect adherence. The scale has shown to have a excellent reliability and validity with a good correlation between short term clinical improvement and DAI scores [28]. The DAI has been demonstrated to be associated with degree of adherence with psychotropic medication among individuals with serious mental illness [29], and it is known to be relatively unaffected by psychiatric symptom severity [30]. Its total score comprises values from −10 to 10, with higher scores indicating more positive attitude towards medication. The DAI scale has been intensively used to investigate patient attitudes for oral antipsychotics [31, 32, 33, 34, 35].
-
•
Measure of Quality of Life:
-
•
EuroQuality of Life – 5 Dimensions Visual Analog Scale (EQ‐5D) [36]. EQ‐5D is a standardised instrument designed for self‐completion by respondents, for use as a measure of health outcome, that provides a single index value for health status based on evaluation of five dimensions (mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression). EQ‐VAS is a standard vertical 20 cm visual analogue scale for recording an individual's rating for patients current health‐related quality of life. It has been used and showed to have acceptable validity and reliability in a wide range of health conditions and treatments, including psychiatric disorders [37, 38, 39, 40].
Following enrolment, patients were observed for 1 year with a total of 5 visits, at 3‐month intervals (±1 month).
Sample Size
Considering a simple random sampling for a percentage estimation, with a population of at least 500,000 units and accepting a maximum distance of 4% from the confidence limit to the point estimate for a confidence interval of 95%, it was necessary to observe 625 subjects, assuming that the prevalence of bipolar disorder affects ∼1% of the population [41]—the generally accepted estimate at the time the study was planned.
Statistical Analysis
All patients who provided consent to release information and who fulfilled the study entry criteria were included in the analyses.
For patients who were lost to follow‐up, or who dropped out of the study, the analyses included all data up to the point of their last data collection. No adjustments were made for missing data.
No drop‐out rate was considered. In case a subject left the observation he was classified into the nonadherence group.
Data summarization and statistical analyses were performed using the SAS System, Version 9.1.3 (TS1M3) for PC. All recorded variables were tabulated using summary statistics (number of patients, mean, standard deviation) for continuous variables and frequency tables (absolute and relative) for categorical variables. The distribution of categorical variables was compared by using the chi‐square test. Continuous variables were compared by using the t‐test. All confidence intervals were calculated at the 95% level.
A logit‐link function [42] was chosen to relate the dichotomous response variable of nonadherence to the potential prognostic (predictor) baseline and post‐baseline variables. The β‐coefficients were estimated from the data set using Generalized Estimating Equations (GEE), using the Proc GENMOD module of the Statistical Analysis System (SAS) version 9.1.3. As suggested by Hosmer D.W. and Lemeshow S. [42] the binary responses for individual patients were assumed to be equally correlated, implying an exchangeable correlation structure. First, all variables whose univariable test had a P‐value <0.25 were entered into the model. Then the least significant variable was dropped, as long as it was not significant at our chosen critical level (P‐value 0.05). The process continued by successively refitting reduced models and applying the same rule until all remaining variables were statistically significant. In a modified version of a forward step, variables with P‐values between 0.05 and 0.25 were individually added to a model that included all variables previously significant at the 5% level to see whether the newly added variable contributed further. Once the best main effect model was fitted, interaction between each couple of variables were tested adding the appropriate term into the model. As measure of association between the response variable and the independent variables, the odds ratios (ORs) with the relative 95% confidence interval as well as the P‐value were reported.
Results
Patient Disposition
Out of the 686 patients screened, 650 met the criteria and were included in the full analysis set. Of the patients enrolled, a total of 578 patients (88.9%) were observed for 1 year as planned, because 46 patients were lost to follow‐up, 12 withdrew their informed consent and the remaining 14 dropped out for a variety of reasons (Figure 1).
Figure 1.
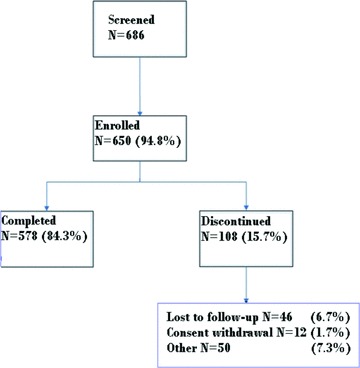
Patients disposition.
Demographics, Lifestyle and Psychiatric History
Patients were mostly outpatients (77.1%), female (58.8%), aged 31–50 years (50.1%), and overweight (41.8%), or obese (28.7%). Out of the obese patients, 18.1% had a body mass index (BMI) within the 30–34.9 range, 8.3% were within the 35–40 range and 2.3% had a BMI ≥40. Most patients denied illegal substance use (88.3%), smoking (52.7%–8.6% were ex‐smokers), and alcohol consumption (55.3%). Only 8.9% of patients reported alcohol abuse and only 1.7% of patients were drug addicts or ex‐drug addicts (0.5% and 1.2%, respectively). One concomitant disease was in 15.7% of patients, mainly metabolic/endocrine disorders (15.8%), gastrointestinal disorders (4.9%), musculoskeletal disorders (4.8%), and/or genitourinary disorders (3.2%).
The demographic data are shown by gender in Table 1. Women were significantly (P < 0.0001) older than men and twice as many men were working full time and were unmarried.
Table 1.
Demographic characteristics
| Parameter | Males N = 268 (41.2%) | Females N = 382 (58.8%) |
|---|---|---|
| Age (yrs) Mean ± SD | 44.6 ± 13.1 | 49.4 ± 12.7 |
| Age distribution; n (%) | ||
| 18–30 yrs | 43 (16.0) | 19 (5.0) |
| 31–40 | 69 (25.7) | 86 (22.5) |
| 41–50 | 60 (22.4) | 111 (29.1) |
| 51–65 | 84 (31.3) | 118 (30.9) |
| ≥66 | 12 (4.5) | 48 (12.6) |
| Weight (kg) Mean ± SD | 84.7 ± 17.3 | 73.1 ± 14.7 |
| BMI Mean ± SD | 28.1 ± 5.2 | 27.8 ± 5.5 |
| Education; n (%) | ||
| None | 3 (1.1) | 8 (2.1) |
| Primary school | 35 (13.1) | 96 (25.1) |
| Jun high school | 100 (37.3) | 136 (35.6) |
| High school | 105 (39.2) | 117 (30.6) |
| University | 25 (9.3) | 25 (6.5) |
| Marital status; n (%) | ||
| Unmarried | 145 (54.1) | 99 (25.9) |
| Married | 120 (44.8) | 235 (61.5) |
| Widowed | 3 (1.1) | 48 (12.6) |
| Occupation; n (%) | ||
| Full‐time | 82 (30.6) | 60 (15.7) |
| Part‐time | 18 (6.7) | 45 (11.8) |
| Occasional | 32 (11.9) | 17 (4.5) |
| Unemployed | 38 (14.2) | 56 (14.7) |
| Student | 10 (3.7) | 3 (0.8) |
| Retired | 82 (30.6) | 131 (34.3) |
| Does not work | 6 (2.2) | 70 (18.3) |
| Out‐pts; n (%) | 199 (74.3) | 302 (79.1) |
Pts, patients; yrs, years; SD, standard deviation.
The psychiatric history of patients is shown in Table 2. Most patients (67.4%) had type I bipolar disorder and were experiencing, above all, depressive (40.6%) or mixed (26.2%) symptoms. Almost one fourth of patients (26.8%) showed concomitant psychotic symptoms, congruent with bipolar symptoms in the vast majority of cases (77.0%). Nearly all patients were not at their first episode (96.6%) and had received psychiatric treatment in the last 12 months (94%), especially mood stabilizers (69.8%) and/or antipsychotics (61.8%); during the last 12 months most patients had experienced one to three episodes (89.1%). Most patients had been hospitalized in the past because of their bipolar disorder (75.1%).
Table 2.
Psychiatric history
| Parameter | Patients n (%) |
|---|---|
| Bipolar disorder diagnosis | |
| Type 1 | 438 (67.4) |
| Type 2 | 211 (32.5) |
| Symptoms at enrollment | |
| Depressive | 264 (40.6) |
| Mixed | 170 (26.2) |
| Manic | 66 (10.2) |
| Hypomanic | 123 (18.9) |
| Other | 27 (4.2) |
| Psychotic symptoms | 174 (26.8) |
| Mood congruent | 134 (77.0) |
| Mood incongruent | 40 (23.0) |
| Prevalent mood during bipolar disorder | |
| Depression | 243 (39.8) |
| Mixed | 187 (30.6) |
| Mania | 181 (29.6) |
| First episode | 22 (3.4) |
| Number of episodes in the last 12 months | |
| 1 | 240 (42.1) |
| 2–3 | 268 (47.0) |
| 4 | 25 (4.4) |
| >4 | 37 (6.5) |
| Lithium at baseline | 228(35.1) |
| Psychiatric therapy last 12 months | 611 (94.0) |
| Nonpharmacological therapy | 118 (18.4) |
| Antipsychotics | 402 (61.8) |
| Antidepressants | 294 (45.2) |
| Mood stabilizers | 454 (69.8) |
| Tranquill/hypnotics | 187 (28.8 |
| Hospitalization due to BD (% pts) | 476 (75.1) |
| Mean ± SD | |
| Age at first diagnosis (years) | 31.5 ± 11.7 |
| Age at first treatment (years) | 32.0 ± 11.3 |
| Duration present episode (days) | 31.5 ± 41.6 |
| Interval last‐present episode (months) | 10.4 ± 11.6 |
| Hospitalizations due to BD | 5.4 ± 7.4 |
| Mean MADRS total score at baseline | 19.6 ± 11.5 |
| Mean YMRS total score at baseline | 11.7 ± 10.9 |
Pts, patients; BD, bipolar disorder; MADRS, Montgomery‐Åsberg Depression Rating Scale; YMRS,Young Mania Rating Scale.
At baseline, the illness was fairly well controlled by treatment, as symptoms at baseline were generally mild: mean ± SD MADRS total score was 19.6 ± 11.5 and mean ± SD YMRS total score was 11.7 ± 10.9.
The CGI‐BD mania, depression and overall bipolar illness scores were 3.5 (1.6), 3.6 (1.5), and 4.4 (1.2), respectively.
Adherence
The adherence rate increased from 39.9% at baseline up to a maximum of 68.7% after 9 months and remained stable thereafter, being 67% at the end of the study (Figure 2).
Figure 2.
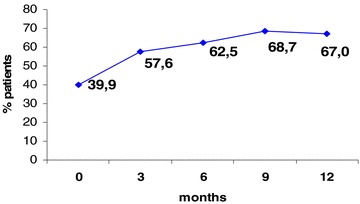
Percentage of patients adherent to prescribed pharmacological treatment for bipolar disorder measured by SMAQ.
Twenty‐one variables with a P‐value of the univariate test <0.25 were introduced into the multivariate model for the identification of predictors: sex, alcohol (yes vs. no), civil status, smoking habits (yes vs. no), substance use (yes vs. no), patient setting, living condition, marital status, employment, type of bipolar disorder, type of present symptoms, type of mood disorder episodes prevalent during the illness, mood episodes satisfying criteria for depressive, manic\mixed, hypomanic episode, concomitant psychiatric illnesses, hospitalizations due to concomitant psychiatric illnesses, CGI‐BD: overall bipolar illness, YMRS: total score, MADRS total score, EQ‐5D VAS, patient's age at first treatment for bipolar disorder, lapse of time between previous and present episode score.
Due to the high frequency of missing data for the DAI total score variable two separate models were built.
According to the first model, which excluded DAI total score, performed in 532 patients for which all needed data were available, the following five variables, listed in order of importance, were found being predictors of nonadherence:
-
•
Alcohol consumption. Alcohol users had a higher risk to be not adherent than nonalcohol users (OR: 1.53, 95% CI: 1.18, 2.00; P‐value = 0.0014), holding CGI‐BD: overall bipolar illness, YMRS total score, MADRS total score and patient's age at first treatment constant.
-
•
Increase in CGI‐BD score by 1 point (OR 1.14 95% CI: 1.04, 1.24; P‐value = 0.0045), after adjusting for YMRS total score, MADRS total score and patient's age at first treatment.
-
•
Increase in YMRS total score by 5 points (OR 1.22 95% CI: 1.13, 1.32; P‐value <0.0001), after adjusting for CGI‐BD: overall bipolar illness, MADRS total score and patient's age at first treatment.
-
•
Increase in MADRS total score by 5 points (OR 1.15 times with 95% CI: 1.09, 1.21; P‐value <0.0001), after adjusting for CGI‐BD: overall bipolar illness, YMRS total score and patient's age at first treatment.
-
•
Increase in age by 10 years at first treatment for bipolar disorder (OR 0.83 95% CI: 0.74, 0.93; P‐value = 0.0018), after adjusting for CGI‐BD: overall bipolar illness, YMRS total score and MADRS total score.
When the DAI total score was considered as a candidate variable for the construction of the multivariate model, once again five variables showed an association with the state of nonadherence: an increase in the DAI total score by 1 point, which was the most important, and the same variables (use of alcohol (yes vs. no), YMRS total score, MADRS total score and the patient's age at first treatment for bipolar disorder) as in the first model, with the sole exception of CGI‐BD score (Table 3).
Table 3.
Pharmacotherapy adherence in bipolar patients according to SMAQ – Multivariate statistical model including DAI total score variable Total number of observation used 1,854 on 497 patients
| Variable | No. obs. used (No. patients)a | Effects | _Odds ratio estimates_ | |||
|---|---|---|---|---|---|---|
| Point estimate | 95% Lower limit | 95% Upper limit | p‐value | |||
| Use of alcohol (Recoded) | 2,552 (588) | Yes versus no | 1.70 | 1.28 | 2.26 | 0.0003 |
| YMRS: Total score | 2,504 (588) | OR for an increase by 5 points | 1.17 | 1.08 | 1.27 | 0.0001 |
| MADRS: Total score | 2,539 (589) | OR for an increase by 5 points | 1.15 | 1.09 | 1.21 | <.0001 |
| Age at first treatment for bipolar disorder | 2,355 (533) | OR for an increase by 10 years | 0.82 | 0.72 | 0.93 | 0.0023 |
| DAI: total score | 2,012 (557) | OR for an increase by 5 points | 0.79 | 0.74 | 0.84 | <.0001 |
Odds ratios estimates based on GEE approach. These observations were the ones for which all variables included in the GEE model were available.
aNumber of observations and number of patients for the single variable.
Discussion
Nonadherence to pharmacological treatment is a serious issue in many disorders. It is particularly pronounced in bipolar disorder, in which medications can be life‐saving in view of their ability to reduce the risk of suicide [43] and in which the symptoms of the disorder itself promote nonadherence [44]. The objective to increase the level of medication adherence during the long‐term treatment is a well worth effort considering that its final result is associated with a better patients outcomes, clinical status and risk of hospitalization [45]. This prospective, observational study has provided further evidence that nearly a quarter of patients (39.9%) adhere to medications prescribed for bipolar disorder in daily clinical practice and suggests that the best predictors of nonadherence are alcohol consumption, severity of symptoms, a negative attitude towards medication (DAI‐30 score) and younger age at first treatment of bipolar disorder. All these characteristics related with adherence should be considered every time a long‐term treatment is needed by the patients. Clinicians will have to work to find specific strategies to deal with them and minimize their negative impact.
This was a large, nonrandomized, multicenter study carried out enrolling 686 patients, in which the management of psychiatric patients was observed in the setting of standard medical care without any formal intervention. The patients’ characteristics confirm that a nonselected study population was enrolled, as there was a balanced representation of the suspected markers of reduced adherence, such as age, sex, drinking/smoking habits, among others. Moreover, the EPHAR patient population characteristics resemble those of the patient population recruited in the only other observational trial performed in bipolar patients in the same country—although in a different subset. The European Mania in Bipolar Longitudinal Evaluation of Medication (EMBLEM) study [46], was designed to assess the management of bipolar mania in clinical practice, including only those patients who needed change the type of therapy during a manic or mixed episode. Both studies included mostly female (58.8% vs. 54.1%) out‐patients (77.1% vs. 66.8%), who were on average 31–32 years of age at diagnosis and first treatment, and were on average overweight (females 26.5 ± 5.5 vs. 27.8 ± 5.5 kg/m2; males 27.0 ± 5.2 vs. 28.1 ± 5.2 kg/m2). The proportion of alcohol abusers was similar (8.9% vs. 7.6%) and so was the proportion of substance abusers (1.7% vs. 1.8% if one does not consider cannabis abusers 2.4%, a piece of information not collected in this study). Thus, the advantage is that its results should be representative of, and reflect actual clinical practice, although the conditions of a clinical study may have per se a favorable influence on the patients’ adherence to therapy. Indeed, adherence improved up to 67% at the end of the study.
The downside is that nonrandomization sets should be associated with a theoretical methodological limit to the quality of results. However, confounding factors were explored using multivariate analyses.
Protocol violations in the screening phase leading to patient loss in the analysis were 5%. A 15% drop‐out rate was reported for various reasons, including patients lost to follow‐up. Overall, the data loss can be considered acceptable and it did not affect the consistency of results because of the observational nature of the study and the inclusion in the analysis of all data up to the point of their last data collection, in case of drop‐out. However, it is not known whether the reason for having lost patients to follow‐up may have been related to poor medication adherence.
We took advantage of the prospective design of the study to collect also additional data that could influence adherence and that can be collected only prospectively, namely the Drug Attitude Inventory and the EuroQuality of Life – EQ‐5D. The former provides additional information on how patients feel about the medication [27] rather than what they believe about it, i.e., an irrational attitude that requires a more subtle intervention than the routine hand‐out with additional information. The latter was used to evaluate patient's quality of life [36]. This quality of life scale has proved to be useful in bipolar disorder, and seems to be the strongest predictors of general health and well being in this patient population [47, 48].
The study may show a limit in the reliance on self‐reported measures of adherence (SMAQ), particularly because patients tend to overestimate adherence. However, the scope of the study was to use standard tools of clinical evaluation and give insight into the reasons behind nonadherence.
The study did not include the determination of plasma levels of psychiatric drugs, as an index to measure adherence, because the naturalistic design of the study considering that blood dosage of most psychiatric drugs is not performed in clinical practice in Italy. In addition, individuals may adhere poorly to a regimen of mood stabilizers but still have plasma levels within the therapeutic range, or have subtherapeutic plasma levels determined by physiological variability.
The predictors that were identified in this observational trial in a clinical practice setting, namely alcohol consumption, symptom severity, a negative attitude towards medication (DAI‐30 score) and younger age at first treatment of bipolar disorder, have all already been identified in clinical trials. Nonadherent subjects are known from the literature to be more likely to be younger, unmarried, homeless (determining lack of access to medication), to show concurrent alcohol or drug abuse, to have a poor relationship with the psychiatrist and lack of awareness of illness [19, 49, 50]. The results from this study are consistent with previous findings both including or excluding DAI total score from the statistical model. Attitudes toward medication and insight have also been reported as highly relevant to medication adherence [51]. No improvement in symptoms is also an obvious cause of poor adherence. Confusion and depression are other factors known to contribute to medication nonadherence in individuals with bipolar disorder. In this study, at baseline, 40.6% of patients reported depressive symptoms (severe depression in 32.3% according to the CGI‐BD scale) and about half had mixed, hypomanic or manic symptoms; psychotic symptoms were present in 26% of patients. On the contrary, medication side effects, although widely believed to be the most important reason for medication nonadherence in general, are a less important reason compared to the other factors cited.
At baseline, 60.1% of study patients were not adherent to therapy, which confirms the relevance of the study results. This figure is consistent with the finding of a medication possession ratio of less than 50% in 61.7% of 7,769 patients with bipolar disorder in a recent large retrospective study that analyzed the claims data from commercial healthcare plans [52]. On the contrary, it compares unfavorably with estimates of nonadherence to prescribed treatment, ranging from 30% to 60% in other epidemiological studies, and around 30% in clinical trials [50, 53, 54].
Study patient adherence to the prescribed therapy improved by almost 20% over the 12 months of observation, with adherence rates escalating from 39.9% at baseline up to 57.6% at 3 months, 62.5% at 6 months, 68.7% at 9 months, 67.0% at 12 months. The progressive improvement in the adherence rate was kept up to the 9th month and was most evident at the first visits, then the probability of switching from the nonadherent to the adherent state reduced over time and reached a plateau at 9th month until the end of the study.
It should be observed that adherence to therapy in this study was recorded as a dichotomous variable, without categories of intermediate level of adherence. This approach may have affected the final outcome compared to the results of other trials.
Adherence appears to reflect a complex interaction of influences, which may change over time. As it is recognized that total adherence to therapy and total nonadherence are rare, the clinical question is identifying the conditions that have an impact on adherence and try to apply strategies to enhance the adherence to therapy. The analysis of variables independently associated with nonadherence to therapy confirmed the significance of a number of interactions at multivariate analysis, as known from the literature. In particular, the multivariate analysis identified associations between level of adherence and treatment, patient characteristics and disease characteristics: severity of bipolar disorder and alcohol consumption were strongly related to low adherence, while an older age at the start of disease and a good attitude to the therapy were predictive of high adherence.
It's important to note that given the correlational nature of results, we cannot identify a principle of causality. The correlation between alcohol abuse, treatments discontinuation and severity of disease should be considered not as a causal association. Is likely that the severity of the disease facilitates alcohol abuse, and at the same time that alcohol abuse worsens the course of the disease.
An important finding related to the patient population is the high hospitalization rate for bipolar disorder in the past (75.1%). As the risk of hospitalization is related to adherence to medication [52], this finding shows how important it is to monitor adherence and to identify predictors of nonadherence in order to avoid, or at least reduce, hospital stays. This is an additional advantage in addition to the containment of the drop‐out rate and the improvement in the quality of interventions in the management of patients with bipolar disorder.
Another important issue is that the advantage of the naturalistic study was also pointed out that in Italy the specific well structured treatment for the bipolar disorder including specific treatment for substance abuse, psychoeducational interventions or psychotherapies focused on adherence are not available from mental health services. This study confirms even more the importance of complete evaluation in early stage of assessment and to focus attention on specific programs for alcohol abuse [55]. These programs could increase, the rate of adherence, reduce drop out and, probably, rate of morbidity, and suicide.
In conclusion, the patient population of this observational trial was representative of the patients changing their therapy for bipolar disorder seen in clinical practice in Italy. Lack of adherence to pharmacotherapy for bipolar disorder is a serious issue, which is more likely to arise in alcohol users and patients with severe symptoms, negative attitude towards medication and/or initiation of treatment early in life.
Funding sources:
The study was fully sponsored by Eli Lilly Italia.
Conflict of Interest
Alessandra Barraco and Andrea Rossi are employees of Eli Lilly Italia.
Acknowledgments
The author thank Dr P. Serra in his support in statistical analysis and Dr J. Hartwig for her support in drafting this manuscript. The authors thank Francesca Mancini and Hyperphar for study management. The following centers have collaborated to the study (EPHAR study group): Dr. Nicolò Baldini Rossi, Villa Baruzziana, Bologna; Dr. Gerardo Bertolazzi, Ospedale Isola Della Scala, Verona; Dr. Nunzio Bucci, Csm Asl Taranto; Dr. Lodovico Cappellari Dipartimento Di Salute Mentale C/O Ospedale Camposampiero‐ Azienda Ulss 15 Alta Padovana; Dr. Renato Cardelli Servizio Psichiatrico Territoriale, Ferrara; Dr. Lino Carfagna Csm Asl Latina; Dr. Giorgio D’allio Dipartimento Di Salute Mentale (Dsm) Asl 21 Piemonte; Dr. Aldo D’arco, Unita’ Funzionale Di Salute Mentale Asl 8 Val Di Chiana; Dr. Vincenzo Del Curatolo, CSM Barletta; Dr. Giuseppe De Paoli Cps Di Voghera‐Stradella Dr. Ernesto Destro Dipartimento Di Salute Az. Usl 19Dr. Sandro Domenichetti Asl Firenze Modulo Operativo Multidisciplinare Salute Mentale Adulti; Prof. Arcadio Erlicher Centro Psicosociale Milano; Dr. Ezio Ferilli Unita’ Operativa Psichiatrica Complessa/Servizio Psichiatrico Diagnosi E Cura/Ospedale Civile, Brindisi; Dr.Ssa Tiziana Ferrario Cps Unita’ Operativa Menaggio, Lario Occidentale – Ossuccio; Dr. Andrea Ferrero Centro Salute Mentale Dip. Asl 7 Piemonte, Chivasso; Dr. Roberto Ferrua Centro Di Salute Mentale Asl 8, Chieri; Dr. Paolo Fontana Ospedale Privato Villa Rosa, Modena; Dr. Giorgio Francobandiera Spac Morbegno – Ospedale Di Morbegno; Dr. Franco Garonna Centro di Igiene Mentale ASL 12 Mestre (VE) Dr. Mario Fuzzi Dsm – Aus 2 Perugia, Perugia; Dr. Paolo Tito Struttura Complessa Di Psichiatria, Ospedale San Bassiano,Bassano Del Grappa; Dr. Carlo Gianfelice Dipartimento Di Salute Mentale, Presso Osp. Fabriano 60100, Ancona; Dr. Nicolò Governanti Dipartimento Di Salute Mentale, Azienda 6 Palermo Palermo; Dr. Luigi Grassi Sezione Di Clinica Psichiatrica – Universita’ Di Ferrara Ferrara; Dr. Fabrizio Lazzerini Dipartimento Di Salute Mentale Via Marina Vecchia, 74 54100, Massa; Dr. Gianfranco Nuvoli U.O. Salite Mentale, 5/Centro Salute Mentale Struppa, Genova; Dr. Piero Antonio Magnani Centro Psicosociale, Mantova; Dr.Ssa Paola Mancosu Centro Salute Mentale Distretto Di Guspini Via Dante 0937, Sangavino Monreale; Dr. Mauro Mauri Clinica Pediatrica (Dipartimento Di Psichiatria Neurobiologia, Farmacologia E Biotecnologia) Universita’ Di Pisa Presso Az. Osp. S. Chiara Pisa; Prof. Marco Meduri Unita’ Operativa Complessa Di Psichiatria, Policlinico Universitario Gazzi, Messina; Dr. Antonio Morlicchio Unita’ Operativa Di Salute Mentale Pompei; Dr. Berardo Di Giuseppe Dipartimento Di Salute Mentale Centro Diurno 64100, Teramo; Dr. Domenico Nano Dipartimento Di Salute Mentale Asl 13, Novara; Dr. Giuseppe Nicolo’ Csm Boccea, Roma; Dr. Mario Nicotera Csm Di Soverato – G. Falco Sede Montepaone, Catanzaro; Dr. Ugo Lancia Casa Di Cura San Valentino, 119 00123, Roma; Dr.Ssa Giuseppina Rizzo Centro Salute Mentale, Enna; Dr. Francesco Saviotti Centro Psicosociale Di Leno; Dr. Alfredo Sgaramella Centro Di Salute Mentale N. 06 Asl Bari, Dr. Maria Cesarano Casa Di Cura Villa Degli Ulivi San Leucio Di Caserta; Dr. Giancarlo Tribastone Servizio Di Psichiatria Ospedale Maria Paterno, Ragusa; Dr. Carlo Vittorio Valenti Unita’ Operativa Assistenza Territoriale Asl 2, Savona; Dr. Franco Veltro Dipartimento Di Salute Mentale, Presso Osp. Cardelli, Campobasso; Dr. Marco Venuta Unita’ Operativa Di Psichiatria Policlinico Di Modena; Dr. Gaetano Vivona Centro Salute Mentale Azienda Usl 9, Trapani, Erice Casa Santa; Dr. Attilio Randone Dipartimento Di Salute Mentale 5a, Rigoli; Dr. Mario Serrano Uf Salute Mentale Adulti, Csm Poggiali – Asl N.6 Livorno Livorno; and Dr. Matteo Zarrillo Centro Salute Mentale Ambulatorio Viale Kennedy 84121, Salerno.
References
- 1. Goetzel RZ, Hawkins K, Ozminkowski RJ, Wang S. The health and productivity cost burden of the “top 10” physical and mental health conditions affecting six large U.S. employers in 1999. J Occup Environ Med 2003;45:5–14. [DOI] [PubMed] [Google Scholar]
- 2. Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: Follow‐up over 34–38 years. J Affect Disord 2002;68:167–181. [DOI] [PubMed] [Google Scholar]
- 3. Schou M. The combat of non‐compliance during prophylactic lithium treatment. Acta Psychiatr Scand 1997;95:361–363. [DOI] [PubMed] [Google Scholar]
- 4. Guscott R, Taylor R. Lithium prophylaxis in recurrent affective illness. Efficacy, effectiveness and efficiency. Br J Psychiatry 1994;164:741–746. [DOI] [PubMed] [Google Scholar]
- 5. Scott J. Predicting medication non‐adherence in severe affective disorders. Acta Neurolopsychiatr 2000;12:128–130. [DOI] [PubMed] [Google Scholar]
- 6. Suppes T, Baldessarini RJ, Faedda GL, Tohen M. Risk of recurrence following discontinuation of lithium treatment in bipolar disorder. Arch Gen Psychiatry 1991;48:1082–1088. [DOI] [PubMed] [Google Scholar]
- 7. Keck PE, McElroy S, Strakowski SM, et al Factors associated with pharmacologic non‐compliance in patients with mania. J Clin Psychiatry 1996;57:292–297. [PubMed] [Google Scholar]
- 8. Jonhson R, McFarland B. Lithium use and discontinuation in a health maintenance organisation. Am J Psychiatry 1998;153:993–1000. [DOI] [PubMed] [Google Scholar]
- 9. Jamison KR, et al, Patient and physician attitudes toward lithium: Relationship to compliance. Arch Gen Psychiatry 1979;36:866–869. [DOI] [PubMed] [Google Scholar]
- 10. Thomson C, Peveler RC, Stephenson D, MC Kendrik J. Compliance with antidepressant medication in the treatment of major depressive disorder in primary care: A randomized comparison of fluoxetine and a tricyclic anti‐depressant. Am J Psychiatry 2000;157:338–343. [DOI] [PubMed] [Google Scholar]
- 11. Lingam R & Scott J. Treatment non‐adherence in affective disorders. Acta Psychiatr Scand 2002;105:164–172. [DOI] [PubMed] [Google Scholar]
- 12. Colom F, Vieta E, Martinez‐Aran A, Reinares M, Benabarre A, Gast G. Clinical Factors Associated with treatment Noncompliance in Euthymic bipolar Patients. J Clin Psychiatry 2000;61:549–555. [DOI] [PubMed] [Google Scholar]
- 13. Keck PE, McElroy SL, Strakowski SM, Balistreri TM, Kizer DL. Factors associated with maintenance antipsychotic treatment of patients with bipolar disorder. J Clin Psychiatry 1996;57:147–151. [PubMed] [Google Scholar]
- 14. Strakowski SM, Keck PE, Mc Elroy SL. Twelve‐month outcome after a first hospitalization for affective psychosis. Arch Gen Psychiatry 1988;55:49–55. [DOI] [PubMed] [Google Scholar]
- 15. Coppen A, Standish‐Barry H, Bailey J, Houston G, Silcocks P, Hermon C. Does Lithium reduce the mortality of recurrent mood disorders? J Affect Disord 1991;23:1–7. [DOI] [PubMed] [Google Scholar]
- 16. Isometsä E, Henriksson M, Lönnqvist J. Completed suicide and recent lithium treatment. J Affect Disord 1992;2:101–103. [DOI] [PubMed] [Google Scholar]
- 17. Jamison KR, Akiskal HS. Medication compliance in patients with bipolar disorder. Psychiatr Clin. North Am 1983;6:175–192. [PubMed] [Google Scholar]
- 18. Jamison KR. Medication compliace In: Goodwin FK, Jamison KR. editors. Manic depressive illness. Oxford : Oxford University Press, 1990;746–762. [Google Scholar]
- 19. Demyttenaere K. Compliance during treatment with antidepressants. J Affect Disord 1997;43:27–39. [DOI] [PubMed] [Google Scholar]
- 20. Knobel H, Alonso J, Casado JL, et al Validation of a simplified medication adherence questionnaire in a large cohort of HIV‐infected patients: The GEEMA Study. AIDS 2002;16:605–613. [DOI] [PubMed] [Google Scholar]
- 21. Ramos‐Quiroga JA, Bosch R, Castells X, et al Effect of switching drug formulations from immediate‐release to extended‐release OROS methylphenidate: A chart review of Spanish adults with attention‐deficit hyperactivity disorder. CNS Drugs 2008;22:603–611. [DOI] [PubMed] [Google Scholar]
- 22. American Psychiatric Association . Diagnostic and statistical manual of mental disorders, 4th edn., Washington : American Psychiatric Publishing, 1994. [Google Scholar]
- 23. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382–389. [DOI] [PubMed] [Google Scholar]
- 24. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 25. Spearing MK, Post RM, Leverich GS, Brandt D, Nolen W. Modification of the Clinical Global Impression (CGI) Scale for use in bipolar illness (BP): The CGI‐BP. Psychiatry Res 1997;73:159–171. [DOI] [PubMed] [Google Scholar]
- 26. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self‐reported measure of medication adherence. Med Care 1986;24:67–74. [DOI] [PubMed] [Google Scholar]
- 27. Hogan TP, Awad AG, Eastwood R. A self‐report scale predictive of drug compliance in schizophrenics: Reliability and discriminative validity. Psychol Med 1983;13:177–183. [DOI] [PubMed] [Google Scholar]
- 28. Hogan TP, Awad AG. Subjective response to neuroleptics and outcome in schizophrenia: A re‐examination comparing two measures. Psychol Med 1992;22:347–352. [DOI] [PubMed] [Google Scholar]
- 29. Awad AG. Subjective response to neuroleptics in schizophrenia. Schizophr Bull 1993;19:609–618. [DOI] [PubMed] [Google Scholar]
- 30. Sajatovic M, Rosch DS, Sivec HJ, et al Insight into illness and attitudes toward medications among inpatients with schizophrenia. Psychiatr Serv 2002;53:1319–1321. [DOI] [PubMed] [Google Scholar]
- 31. Hofer A, Kemmler G, Eder U, Honeder M, Hummer M, Fleischhacker WW. Attitudes toward antipsychotics among outpatient clinic attendees with schizophrenia. J. Clin. Psychiatry 2002;63:49–53. [DOI] [PubMed] [Google Scholar]
- 32. Freudenreich O, Cather C, Evins AE, Henderson DC, Goff DC. Attitudes of schizophrenia outpatients toward psychiatric medications: Relationship to clinical variables and insight. J Clin Psychiatry 2004;65:1372–1376. [DOI] [PubMed] [Google Scholar]
- 33. Day JC, Bentall RP, Roberts C, et al Attitudes toward antipsychotic medication: The impact of clinical variables and relationships with health professionals. Arch Gen Psychiatry 2005;62:717–724 [DOI] [PubMed] [Google Scholar]
- 34. Adewuya AO, Ola BA, Mosaku SK, Fatoye FO, Eegunranti AB. Attitude towards antipsychotics among out‐patients with schizophrenia in Nigeria. Acta Psychiatr Scand 2006;113:207–211. [DOI] [PubMed] [Google Scholar]
- 35. Muller MJ. Attitudes toward different formulations of psychotropic drugs: The view of patients and healthcare providers. Am J Drug Deliv 2006;4:33–41. [Google Scholar]
- 36. The EuroQol Group . EuroQol‐a new facility for the measurement of health‐related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 37. Brooks R, Rabin R, de Charro F. The measurement and valuation of health status using EQ‐5D: A european perspective evidence from the EuroQol BIO MED research programme. Denmark: Kluwer Academic Publishers, 2003. [Google Scholar]
- 38. König HH, Roick C, Angermeyer MC. Validity of the EQ‐5D in assessing and valuing health status in patients with schizophrenic, schizotypal or delusional disorders. Eur Psychiatry 2007;22:177–187. [DOI] [PubMed] [Google Scholar]
- 39. Günther O, Roick C, Angermeyer MC, König HH. The EQ‐5D in alcohol dependent patients: Relationships among health‐related quality of life, psychopathology and social functioning. Drug Alcohol Depend 2007;86:253–264. [DOI] [PubMed] [Google Scholar]
- 40. Supina AL, Johnson JA, Patten SB, Williams JV., Maxwell CJ. The usefulness of the EQ‐5D in differentiating among persons with major depressive episode and anxiety. Qual Life Res 2007;16:749–754. [DOI] [PubMed] [Google Scholar]
- 41. Carta MG, Angst J. Epidemiological and clinical aspects of bipolar disorders: Controversies or a common need to redefine the aims and methodological aspects of surveys. Clin Pract Epidemiol Ment Health 2005;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hosmer DW, Lemeshow S. Applied logistic regression, UK: Wiley Series in probability and statistics, Wiley & Sons, Inc, 2000. [Google Scholar]
- 43. Baldessarini RJ, Tondo L, Davis P, Pompili M, Goodwin FK, Hennen J. Decreased risk of suicides and attempts during long‐term lithium treatment: A meta‐analytic review. Bipolar Disord 2006;8:625–639. [DOI] [PubMed] [Google Scholar]
- 44. Buckley PF, Correll CU. Strategies for dosing and switching antipsychotic for optimal clinical management. J Clin Psychiatry 2008;69(Suppl 1):4–17. [PubMed] [Google Scholar]
- 45. Hassan M, Lage MJ. Risk of rehospitalization among bipolar disorder patients who are nonadherent to antipsychotic therapy after hospital discharge. Am J Health Syst Pharm 2009;66:358–365. [DOI] [PubMed] [Google Scholar]
- 46. Bellantuono C, Barraco A, Rossi A, Goetz I. The management of bipolar mania: A national survey of baseline data from the EMBLEM study in Italy. BMC Psychiatry 2007;7:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hayhurst H, Palmer S, Abbott R, Johnson T, Scott J. Measuring health‐related quality of life in bipolar disorder: Relationship of the EuroQol (EQ‐5D) to condition‐specific measures. Qual Life Res 2006;15:1271–1280. [DOI] [PubMed] [Google Scholar]
- 48. Kessing LV, Hansen HV, Bech P. General health and well‐being in outpatients with depressive and bipolar disorders. Nord J Psychiatry 2006;60:150–156. [DOI] [PubMed] [Google Scholar]
- 49. Lacro JP, Dunn LB, Dolder CR, Leckband SG, Jeste DV. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: A comprehensive review of recent literature. J Clin Psychiatry 2002;63:892–909. [DOI] [PubMed] [Google Scholar]
- 50. Sajatovic M, Valenstein M, Blow F, Ganoczy D, Ignacio R. Treatment adherence with lithium and anticonvulsant medications among patients with bipolar disorder. Psychiatr Serv 2007;58:855–863. [DOI] [PubMed] [Google Scholar]
- 51. Mutsatsa SH, Joyce EM, Hutton SB, Webb E, Gibbins H, Paul S, Barnes TR. Clinical correlates of early medication adherence: West London first episode schizophrenia study. Acta Psychiatr Scand 2003;108:439–446. [DOI] [PubMed] [Google Scholar]
- 52. Lage MJ, Hassan MK. The relationship between antipsychotic medication adherence and patient outcomes among individuals diagnosed with bipolar disorder: A retrospective study. Ann Gen Psychiatry 2009;18:7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pampallona S, Bollini P, Tibaldi G, Kupelnick B, Munizza C. Patient adherence in the treatment of depression. Br J Psychiatry 2002;180:104–109. [DOI] [PubMed] [Google Scholar]
- 54. Calabrese JR, Shelton MD, Rapport DJ, et al A 20‐month, double‐blind, maintenance trial of lithium versus divalproex in rapid‐cycling bipolar disorder. Am J Psychiatry 2005;162:2152–2162. [DOI] [PubMed] [Google Scholar]
- 55. Velligan DI, Weiden PJ, Sajatovic M, Scott J, Carpenter D, Ross R, Docherty JP. Strategies for addressing adherence problems in patients with serious and persistent mental illness: Recommendations from the expert consensus guidelines. J Psychiatr Pract 2010;16:306–324. [DOI] [PubMed] [Google Scholar]


