Danshen, the dried roots of Salvia miltiorrhiza, is a well‐known traditional Chinese medicine used for the treatment of stroke since 1970. A similar plant called Salvia columbariae was also used by Californian Indians to treat people suffering from stroke [1]. Pharmacological studies indicated that their active ingredients, tanshinones, and salvianolic acids exhibited anticoagulant, vasodilatory, anti‐inflammatory, antioxidant, neuroprotective, and other activities underlying the therapeutic effect [2].
Thrombosis secondary to atherosclerosis is a major cause of stroke and coronary artery disease. Inhibition of clot formation and potential clot dissolution has been demonstrated in many clinical trials of S. miltiorrhiza[1]. Early in vitro studies found that S. miltiorrhiza extract can increase the proteolysis of fibrinogen to fibrinogen degradation products [3], suggesting a unique mechanism of antithrombosis comparing to other anticoagulant drugs that prevent clot formation by interfering with platelet aggregation, thrombin activity or vitamin K function. However, the molecular basis of the fibrinolytic action of S. miltiorrhiza remains unknown. Plasminogen activator inhibitor‐1 (PAI‐1), the primary inhibitor of both tissue type plasminogen activator (tPA) and urokinase type plasminogen activator (uPA), is a key physiological regulator of the fibrinolytic system and is implicated in thrombotic pathophysiology in ischemic stroke and coronary artery disease [4, 5]. Pharmacological inhibition of PAI‐1 showed antithrombotic benefits devoid of bleeding effect in rodents and monkey [6, 7]. In the present study, we tested the hypothesis that S. miltiorrhiza contains active compounds that inhibit PAI‐1 activity.
Using a chromogenic substrate‐based PAI‐1 activity assay kit (Milllipore, MA, USA), we analyzed the PAI‐1 inhibitory effect of seven natural compounds from S. miltiorrhiza: Tanshinone I, Tanshinone IIA, Sodium tanshinone IIA sulfonate, Cryptotanshinone, Sodium Danshensu, Protocatechuic aldehyde and β‐sitosterol (National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China). The purity of the compounds was determined to be >98% by high‐performance liquid chromatography analysis. For the uPA‐mediated assay, PAI‐1 (Molecular Innovations, MI, USA) was incubated with compounds at 23°C for 15 min, then PAI‐1 activity was assayed according to the protocol of the kit. For the tPA‐mediated PAI‐1 activity assay, uPA and its chromogenic substrate in the kit was replaced by tPA (Molecular Innovations, MI, USA) and its substrate S‐2288 (Chromogenix, NC, USA). As shown in Figure 1(A), sodium tanshinone IIA sulfonate and cryptotanshinone inhibited PAI‐1 activity in a dose‐dependent manner. The IC50 values for sodium tanshinone IIA sulfonate and cryptotanshinone are 41 and 98 μM, respectively, in uPA/PAI‐1 assay and 59 and 152 μM, respectively, in tPA/PAI‐1 assay. Tanshinone IIA exhibited much weaker effect than its salt form derivative sodium tanshinone IIA sulfonate, likely due to its lower solubility.
Figure 1.
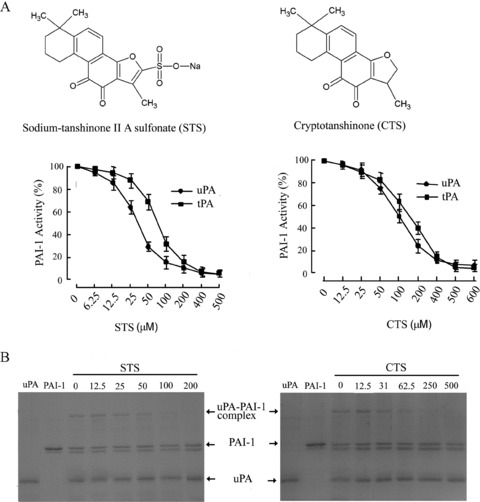
Identification of tanshinone compounds as PAI‐1 inhibitors. (A) Structures and PAI‐1 inhibitory activity of sodium tanshinone IIA sulfonate (STS, left) and cryptotanshinone (CTS, right). (B) Inhibition of PAI‐1/uPA complex formation by STS (left) and CTS (right).
To further confirm the PAI‐1 inhibitory activity by the tanshinone compounds, we performed biochemical analysis to determine if sodium tanshinone IIA sulfonate and cryptotanshinone can block the formation of PAI‐1/uPA complex. In a 25 μL reaction volume in tris‐buffered saline buffer, recombinant human PAI‐1 (Molecular Innovations, MI, USA) at 1 μM final concentration was first incubated with various concentrations of sodium tanshinone IIA sulfonate and cryptotanshinone for 15 min at room temperature. Then uPA (Molecular Innovations, MI, USA) was added to a final concentration of 0.8 μM and incubated for 10 min at 37°C. The samples were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis on a 10% gel and stained with Coomassie blue. As shown in Figure 1(B), both sodium tanshinone IIA sulfonate and cryptotanshinone dose dependently prevented the formation of PAI‐1/uPA complex. The results of enzymatic and biochemical analyses clearly identified the two tanshinones from S. miltiorrhiza as PAI‐1 inhibitors.
The fibrinolytic activity of the two tanshinone compounds was analyzed using an uPA‐mediated clot lysis assay performed in 96‐well microplate. Pooled human plasma (50 μL) was added to 65 μL assay buffer containing 150 mM NaCl, 2 mM CaCl2, 20 mM HEPES, pH 7.4, 4 μL human thrombin (75 NIH IU/μL), (Sigma, Shanghai, China) and incubated at 37°C for 10 min to form fibrin clots that usually remain stable at 37°C for at least 2 h. In another set of reaction in 50 μL reaction volume, PAI‐1 at 5 nM final concentration was first incubated with various concentrations of sodium tanshinone IIA sulfonate or cryptotanshinone for 15 min at room temperature, then uPA was added to a final concentration of 4 nM. After incubation at 37°C for 10 min, the reaction was transferred to the fibrin clots plate and incubated for 120 min. The lysis of fibrin clots was monitored by optical absorbance at 405 nm in a Varioskan Flash plate reader (Thermo Scientific, NY, USA). The results demonstrated that sodium tanshinone IIA sulfonate and cryptotanshinone dose dependently accelerated the lysis of fibrin clots (Figure 2A), which correlates with increased uPA activity in the reaction system (Figure 2B).
Figure 2.
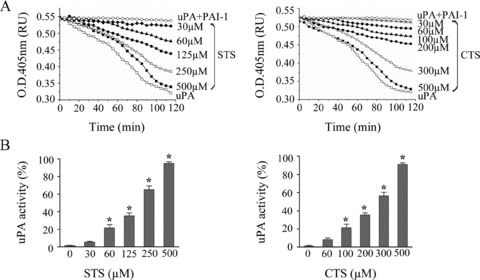
Fibrinolytic effect of PAI‐1 inhibitory tanshinones. (A) STS and CTS dose dependently enhance uPA‐mediated lysis of fibrin clots. (B) Increase of uPA activity in response to various concentrations of STS and CTS. Data were presented as mean±S.E.M, n = 4, *p < 0.01 vs. blank.
Taken together, our data identified two tanshinone compounds from S. miltiorrhiza as PAI‐1 inhibitors. The active compounds inhibited the activity of PAI‐1, prevented the formation of PAI‐1/uPA complex, and enhanced uPA‐mediated lysis of fibrin clots. The study uncovered a novel mechanism underlying the antithrombotic effect of S. miltiorrhiza in treating ischemic stroke and coronary artery disease. In addition, because PAI‐1 is involved in the pathogenesis of a variety of other pathological processes including organ fibrosis, inflammation, malignancy, and metabolic disorders [8, 9, 10], this study also provided a new rationale for development of tanshinone compounds to treat PAI‐1 related diseases other than thrombotic disorders.
This study was supported by grants from the National Basic Research Program of China (973) (No. 2009CB521908) and the National Natural Science Fund (No. 81173109).
References
- 1. Adams JD, Wang R, Yang J, Lien EJ. Preclinical and clinical examinations of salvia miltiorrhiza and its tanshinones in ischemic conditions. Chin Med 2006;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou L, Zuo Z, Chow MS. Danshen: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol 2005;45:1345–1359. [DOI] [PubMed] [Google Scholar]
- 3. Wang CS, Chen CS, Yang TT, Luo FC. Invitro root of Salvia miltiorrhza action on blood anticoagulation and fibrinolysis. Chin Med J 1978;4:123–126. [PubMed] [Google Scholar]
- 4. Zorio E, Gilabert‐Estellés J, España F, Ramón LA, Cosín R, Estellés A. Fibrinolysis: The key to new pathogenetic mechanisms. Curr Med Chem 2008;15:923–929. [DOI] [PubMed] [Google Scholar]
- 5. Westrick RJ, Eitzman DT. Plasminogen activator inhibitor‐1 in vascular thrombosis. Curr Drug Targets 2007;8:966–1002. [DOI] [PubMed] [Google Scholar]
- 6. Suzuki J, Ogawa M, Muto S, Yamaguchi Y, Itai A, Isobe M. The effects of pharmacological PAI‐1 inhibition on thrombus formation and neointima formation after arterial injury. Expert Opin Ther Targets 2008;12:783–794. [DOI] [PubMed] [Google Scholar]
- 7. Izuhara Y, Yamaoka N, Kodama H, Dan T, Takizawa S, Hirayama N, Meguro K, van Ypersele de Strihou C, Miyata T. A novel inhibitor of plasminogen activator inhibitor‐1 provides antithrombotic benefits devoid of bleeding effect in nonhuman primates. J Cereb Blood Flow Metab 2010;30:904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown NJ. Therapeutic potential of plasminogen activator inhibitor‐1 inhibitors. Ther Adv Cardiovasc Dis 2010;4:315–324. [DOI] [PubMed] [Google Scholar]
- 9. Ghosh AK, Vaughan DE. PAI‐1 in tissue fibrosis. J Cell Physiol 2012;227:493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oishi K. Plasminogen activator inhibitor‐1 and the circadian clock in metabolic disorders. Clin Exp Hypertens 2009;31:208–219. [DOI] [PubMed] [Google Scholar]


