Neuropathic pain is a type of pathological pain caused by nerve injury or diseases, which is characterized as allodynia and hyperalgesia. Because of the resistance of neuropathic pain to standard analgesics and the unknown underlying molecular mechanisms, effective remedies based on novel therapeutic strategies need to be established 1.
Dexmedetomidine, a highly selective alpha2‐adrenoceptor (α2‐AR) agonist, has been used in human as a medication for analgesia, sedation in the intensive care unit (ICU), and an adjunct for anesthesia 2. It has been shown that dexmedetomidine can stimulate α2‐ARs in the spinal cord to enhance analgesia 3. There are three α2‐AR subtypes, α2A, α2B, and α2C. However, it has been assessed that α2A‐AR is the predominant subtype involved in the mediation of the antinociceptive action of dexmedetomidine 4. Increasing evidence demonstrates that systemic or intrathecal application of dexmedetomidine produces significant antinociceptive effects in various rodent models of chronic pain 3, 5. However, the signaling pathway involved in the analgesic effect of dexmedetomidine remains unclear.
Emerging evidence indicates that protein kinase B (PKB)/Akt is usually implicated in a variety of cellular processes, including glucose metabolism, transcription, apoptosis, proliferation, migration, and angiogenesis 6. Recently, it has been found that stimulation of the PI3K/Akt signaling pathway by opioid receptors directly blocked inflammatory hyperalgesia 7, 8. Moreover, a previous report showed that dexmedetomidine likely activated PKB/Akt signaling via α2 adrenoceptors to reduce cell death and to provide renoprotection, suggesting a link between α2 adrenoceptors and PKB/Akt pathway 9. In light of previous reports, the present study aimed to explore the potential role of spinal PKB/Akt in the analgesic effect of dexmedetomidine on the neuropathic pain induced by sciatic nerve chronic constriction injury (CCI).
First, we investigated the time course of paw withdrawal threshold (PWT) in the ipsilateral hind paw of CCI rats. CCI rats were produced by loosely ligating the left sciatic nerve with 4.0 chromic gut in three regions at about 1–2 mm intervals. The same surgical procedure was made in the rats of the sham‐operated group except for nerve ligation. Mechanical allodynia was assessed by measuring the PWT in response to a calibrated series of von Frey hairs (Stoelting, Wood Dale, IL, USA). The PWT was defined as the lowest hair force in gram that elicited positive response. Compared with sham group, we found that the mechanical tactile allodynia of CCI rats occurred on day 3, peaked on day 7, and persisted for at least 21 days during the observation period (Figure 1A). After behavioral test, CCI rats were deeply anesthetized by overdose of urethane (2 g/kg, i.p.) and decapitated at the defined time points (1, 3, 5, 7, 14, 21 days). The dorsal quadrants of the L4 and L5 spinal cord segments were dissected and homogenized in a lysis buffer for Western blot. Blots were incubated overnight at 4°C with rabbit anti‐α2A‐AR (1:1000; Sigma, St. Louis, MO, USA) or rabbit anti‐pAkt (1:1000; Cell Signaling, Beverly, MA, USA) primary antibody, followed by donkey anti‐rabbit secondary antibody (1:4000; Pierce, Rockford, IL, USA). Compared with the sham group, the level of α2A‐AR was significantly increased on day 3 after CCI, reached a peak on day 7, and maintained to day 21 after operation (Figure 1B). As shown in Figure 1, the expression of pAkt in ipsilateral spinal cord dorsal horn began to significantly increased on the 3rd day after surgery and reached a peak on the 7th day and maintained to the 14th day after operation (Figure 1C).
Figure 1.
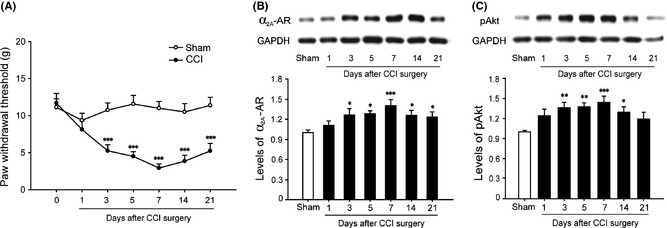
Chronic constriction injury (CCI)‐induced time course of paw withdrawal threshold (PWT) and changes of α2A‐AR and pAkt protein expression. (A) Time course of PWT in ipsilateral hind paw following CCI/sham surgery. (B, C) Western blot shows the changes of α2A‐AR and pAkt protein expression in ipsilateral spinal dorsal horn at different time points after CCI operation. The data are presented as mean ± SEM of six to eight rats per group. *P < 0.05, **P < 0.01, ***P < 0.001 compared with sham group by two‐way RM ANOVA (A) or one‐way ANOVA (B, C).
Based on our previous experiments, we examined the effects of intrathecal administration of dexmedetomidine (2.5 μg; Jiangsu Hengrui Medicine Co., Ltd, Lianyungang, China) on the CCI‐induced mechanical allodynia on day 7 after CCI surgery when the pain behaviors were established. A significant antiallodynic effect was observed at 30, 60, 90, and 120 min after administration of dexmedetomidine on CCI‐induced mechanical allodynia (Figure 2A). We found that the antiallodynic effect was most prominent at 60 min after injection of dexmedetomidine. Then, CCI rats were euthanized 60 min after intrathecal administration of vehicle and dexmedetomidine for Western blot. In parallel with the changes of behavioral results, Western blot showed that dexmedetomidine induced an apparent upregulation of pAkt expression in the ipsilateral lumbar spinal dorsal horn compared with the vehicle control group (Figure 2B).
Figure 2.
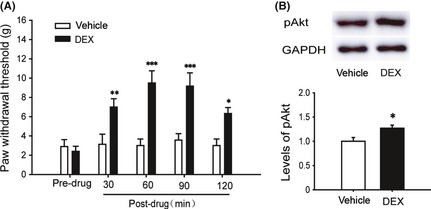
Effects of intrathecal dexmedetomidine (DEX) on chronic constriction injury (CCI)‐induced pain behaviors and expression of pAkt in ipsilateral spinal dorsal horn. DEX was given at day 7 after CCI surgery. (A) Time course of paw withdrawal threshold (PWT) to stimulation in ipsilateral hind paw before and 30, 60, 90, and 120 min after intrathecal DEX/vehicle. (B) Western blot shows the expression of pAkt in ipsilateral spinal dorsal horn at 60 min after intrathecal DEX/vehicle. The data are presented as mean ± SEM of six to eight rats per group. *P < 0.05, **P < 0.01, ***P < 0.001 compared with vehicle group by two‐way RM ANOVA (A) or t‐test (B).
Our data showed that the extent of activation of α2A‐AR and PKB/Akt in ipsilateral lumbar spinal dorsal horn was significantly correlated with injury‐induced increases in mechanical sensitivity in the CCI rats, suggesting that increased expression of α2A‐AR and PKB/Akt might contribute to the neuropathic pain state. Our previous study demonstrated that α2A‐AR was diffusely distributed in the primary afferents and dorsal horn neurons 5; thus, presynaptic and postsynaptic α2A‐AR expressions in the spinal dorsal horn might both upregulate in CCI rats and contribute to dexmedetomidine‐induced antiallodynia.
Growing evidence indicates that PKB/Akt signaling pathway is involved in pain regulation. Consistent with our present result that CCI significantly upregulated pAkt level in the ipsilateral spinal dorsal horn, Xu et al. 10 observed an increased expression of pAkt in the L5 spinal dorsal horn following L5 spinal nerve ligation (SNL). Furthermore, Xu et al. 10 found that inhibition of PKB/Akt activation in the spinal cord decreased abnormal pain behaviors, suggesting that PKB/Akt signal pathway activation might contribute to the development of neuropathic pain. However, the results from Ferreira's laboratory showed that blockade of PI3K/Akt pathway prevented opioids‐induced antinociception 7, 8. Similarly, we revealed that intrathecal dexmedetomidine upregulated spinal pAkt expression and alleviated mechanical allodynia in CCI rats, suggesting that PKB/Akt signaling activation might mediate dexmedetomidine‐induced antiallodynia. Anyway, at present, it still remains unknown whether the α2A‐AR and PKB/Akt activation happens in the same cell. Further investigation will be required.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (NSFC, 81070895).
References
- 1. Calo’ G, Rizzi A, Cifani C, et al. UFP‐112 a potent and long‐lasting agonist selective for the Nociceptin/Orphanin FQ receptor. CNS Neurosci Ther 2011;17:178–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paris A, Tonner PH. Dexmedetomidine in anaesthesia. Curr Opin Anesthesiol 2005;18:412–418. [DOI] [PubMed] [Google Scholar]
- 3. Asano T, Dohi S, Ohta S, Shimonaka H, Iida H. Antinociception by epidural and systemic a(2)‐adrenoceptor agonists and their binding affinity in rat spinal cord and brain. Anesth Analg 2000;90:400–407. [DOI] [PubMed] [Google Scholar]
- 4. Hunter JC, Fontana DJ, Hedley LR, et al. Assessment of the role of alpha2‐adrenoceptor subtypes in the antinociceptive, sedative and hypothermic action of dexmedetomidine in transgenic mice. Br J Pharmacol 1997;122:1339–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu B, Zhang WS, Yang JL, et al. Evidence for suppression of spinal glial activation by dexmedetomidine in a rat model of monoarthritis. Clin Exp Pharmacol Physiol 2010;37:e158–e166. [DOI] [PubMed] [Google Scholar]
- 6. Michell RH, Heath VL, Lemmon MA, Dove SK. Phosphatidylinositol 3,5‐bisphosphate: metabolism and cellular functions. Trends Biochem Sci 2006;31:52–63. [DOI] [PubMed] [Google Scholar]
- 7. Cunha TM, Souza GR, Domingues AC, et al. Stimulation of peripheral kappa opioid receptors inhibits inflammatory hyperalgesia via activation of the PI3Kγ/AKT/nNOS/NO signaling pathway. Mol Pain 2012;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cunha TM, Roman‐Campos D, Lotufo CM, et al. Morphine peripheral analgesia depends on activation of the PI3Kgamma/AKT/nNOS/NO/KATP signaling pathway. Proc Natl Acad Sci USA 2010;107:4442–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gu J, Sun P, Zhao H, et al. Dexmedetomidine provides renoprotection against ischemia‐reperfusion injury in mice. Crit Care 2011;15:R153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu JT, Tu HY, Xin WJ, Liu XG, Zhang CH. Activation of phosphatidylinositol 3‐kinase and protein kinase B/Akt in dorsal root ganglia and spinal cord contributes to the neuropathic pain induced by spinal nerve ligation in rats. Exp Neurol 2007;206:269–279. [DOI] [PubMed] [Google Scholar]


