Summary
Heparin‐binding epidermal growth factor‐like growth factor (HB‐EGF) is a member of the EGF family of growth factors, which interacts with the EGF receptor to exert mitogenic activity for various types of cells. Through its interactions with various molecules, it is involved in diverse biological processes, including wound healing, blast implantation, and tumor formation. At the same time, HB‐EGF is widely expressed in the central nervous system, including the hippocampus and cerebral cortex, and is considered to play pivotal roles in the developing and adult nervous system. Because HB‐EGF protein levels in the brain are much higher than those of TGF‐α and EGF, it is possible that HB‐EGF serves as a major physiologic ligand for the EGF receptor (ErbB1) within the central nervous system. Recent studies indicate that HB‐EGF contributes to the neuronal survival and proliferation of glial/stem cells. HB‐EGF also promotes the survival of dopaminergic neurons, an action mediated by mitogen‐activated protein kinase (MAPK) as well as by the Akt signaling pathway. In this review, we discuss recent findings on the implications of HB‐EGF in higher brain functions of the central nervous system.
Keywords: Central nervous system, Epidermal growth factor, ErbB, Heparin‐binding epidermal growth factor, Transforming growth factor‐α
Introduction
Heparin‐binding epidermal growth factor‐like growth factor (HB‐EGF) is a member of the EGF family of growth factors, which includes EGF, transforming growth factor (TGF)‐α, amphiregulin, betacelulin, and neuregulin 1, 2, 3. HB‐EGF is a 22‐kDa, O‐glycosylated protein, which was isolated from the conditioned medium of a human macrophage‐like cell line in 1991 2. HB‐EGF and amphiregulin can be distinguished from other members of the EGF family by the presence of a heparin‐binding domain, which interacts with membrane‐bound heparin sulfate proteoglycans and may thereby regulate EGF receptor activation. HB‐EGF binds to and activates the EGF receptor (EGF receptor/ErbB1) 2 and ErbB4 4 to exert mitogenic, chemoattractant, and cell survival activities. Among the members of the EGF family, HB‐EGF knockout (KO) mice die shortly after birth 5, 6, whereas KO mice for the other EGF family growth factors, such as EGF, TGF‐α, amphiregulin, and epiregulin, do not die in that manner and have different phenotypes 7, 8, 9. These results suggest that of all the members of the EGF family, HB‐EGF plays the most roles in growth and development.
Heparin‐binding epidermal growth factor is initially synthesized as a membrane‐anchored precursor protein (proHB‐EGF), similar to other EGFR ligands 2. HB‐EGF and amphiregulin could be distinguished from other members of the EGF family by the presence of a heparin‐binding domain, which interacts with membrane‐bound heparin sulfate proteoglycans and may thereby regulate EGF receptor activation. ProHB‐EGF is cleaved by metalloproteases, known as a disintegrin and metalloproteases (ADAMs), to yield the soluble form of HB‐EGF (sHB‐EGF), through a process known as ectodomain shedding 2, 10. sHB‐EGF binds to and activates the EGF receptor (ErbB1) 2 and ErbB4 4 to exert a potent mitogen and chemoattractant for a number of different cell types 3. On the other hand, the carboxyl terminal fragment of HB‐EGF (HB‐EGF‐CTF) generated by ectodomain shedding translocates to the nucleus by endocytosis. Subsequently, HB‐EGF‐CTF exerts effects on the regulation of cell proliferation by binding the nuclear promyelocytic leukemia zinc finger (PLZF) protein, a transcriptional repressor, causing its nuclear export 11. PLZF exerts transcriptional repression through decreased expression of cyclin A. Through these two different pathways, HB‐EGF exerts its physiological functions. Furthermore, forms of proHB‐EGF associate with several molecules, such as CD9 integrin α3β1 and heparan‐sulfate proteoglycan to modulate their biological activity 12. In addition, proHB‐EGF functions as the sole receptor for the diphtheria toxin in humans, mediating the entry of the diphtheria toxin into the cytoplasm 13, 14. The diphtheria toxin binds to human HB‐EGF, but not to mouse HB‐EGF, because of the difference of amino acid sequence between human and mouse in the EGF‐like domain, which is responsible for the binding of diphtheria toxin with HB‐EGF 15.
Many studies have demonstrated the importance of HB‐EGF in diverse biological processes, including wound healing 16, 17, 18, blast implantation 19, atherosclerosis 20, heart development 5, 6, 21, and tumor formation 22, through the activation of the signaling molecules downstream of ErbB receptors and interactions with molecules associated with HB‐EGF. In particular, HB‐EGF has been implicated in tumor progression because of its altered expression in many tumors, including hepatocarcinoma 23, colon 24, breast 25, 26, prostate 27, and bladder tumors 28, 29. Cross‐reacting material 197 (CRM197), a specific inhibitor of HB‐EGF, showed a remarkable suppression of tumor growth 30, and a clinical trial of CRM197 in patients with advanced cancer also showed a promising antitumor effect 31, 32. These results suggest that HB‐EGF would be a likely target for cancer therapy.
Meanwhile, HB‐EGF is widely expressed in the central nervous system, including the hippocampus and cerebral cortex, and is considered to play pivotal roles in the developing and adult nervous system 33, 34, 35. Previous studies indicate that HB‐EGF contributes to the neuronal survival and glial/stem cells proliferation 34, 36, 37. HB‐EGF also promotes the survival of dopaminergic neurons, an action mediated by MAPK as well as by the Akt signaling pathway 37. In this review, we discuss recent findings on the implication of HB‐EGF in higher brain functions in the central nervous system. In particular, we focused on the biological functions of HB‐EGF on psychomotor behavior, cerebral ischemia, nigrostriatal dopaminergic neuron, memory, and synaptic plasticity (Figure 1 and Table 1).
Figure 1.
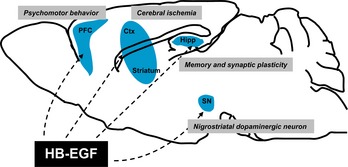
Roles of heparin‐binding epidermal growth factor (HB‐EGF) in the central nervous system and higher brain function. HB‐EGF is widely expressed throughout the brain, in areas such as the cerebral cortex, hippocampus, thalamus, hypothalamus, basal ganglia, midbrain, olfactory bulb, and so on. In this review, we discuss recent findings on the implications of HB‐EGF on psychomotor behavior, cerebral ischemia, nigrostriatal dopaminergic neuron, memory, and synaptic plasticity. PFC, prefrontal cortex; Hipp, hippocampus; SN, substantia nigra; Ctx, cortex.
Table 1.
Biological function of heparin‐binding epidermal growth factor (HB‐EGF) in several pathological conditions
| Condition | Species | Experimental findings | References |
|---|---|---|---|
| Psychiatric disorder | Mice | Forebrain specific HB‐EGF knockout (KO) mice exhibited several behavioral abnormalities | 51 |
| Memory | Mice | Forebrain specific HB‐EGF KO mice impaired memory formation and hippocampal long‐term potentiation | 66 |
| Cerebral ischemia | Rat/Mice | HB‐EGF expression was increased after focal cerebral ischemia | 81,84 |
| Rat | Intraventricular administration of HB‐EGF reduced infarct size after focal cerebral ischemia | 80 | |
| Rat | Gene transfer of HB‐EGF enhanced neurogenesis and angiogenesis after focal cerebral ischemia | 93 | |
| Mice | Forebrain specific HB‐EGF KO mice show exacerbated ischemia and reperfusion injury | 81 | |
| Parkinson's disease | Rat | Intracerebral injection of HB‐EGF protected the nigrostriatal dopaminergic system in an in vivo adult rat model of Parkinson's disease | 109 |
| Human | HB‐EGF level was not significantly altered in the patients of Parkinson's disease | 108 |
Expression of HB‐EGF in the Central Nervous System
In the central nervous system, HB‐EGF is highly expressed in neurons, as well as astrocytes and oligodendrocytes 34. In situ hybridization and immunohistochemical analysis reveals that HB‐EGF mRNA and proteins are distributed throughout the brain, in sites such as the cerebral cortex, hippocampus, thalamus, hypothalamus, basal ganglia, midbrain, olfactory bulb, and so on 34. The level of HB‐EGF expression is much higher in brains of young animals than in adult brains, which implies its marked function during early developmental stages 38. On the other hand, the expression of EGF receptors (ErbB1, ErbB2, ErbB3, and ErbB4) varies among different types of neurons, widely distributed in the brain, and play a major role under both physiological and pathological conditions 39. ErbB1 is enriched in the neocortex, striatum, and hippocampus and has been proposed to contribute to the development of the central nervous system 40. ErbB4 receptor mRNA also includes the cortex, amygdala, hippocampus, medial habenula, reticular thalamic nucleus, several hypothalamic nuclei, subthalamic nucleus, substantia nigra pars compacta, and the ventral tegmental area (VTA) 41. Also, astrocytes express HER1/ErbB1 and maybe small amounts of HER4/ErbB4 36, 42. HER4/ErbB4 has been detected at high levels in the postsynaptic density, indicating the possibility of its involvement in synaptic plasticity 43.
Among ErbB1 ligands, HB‐EGF protein levels were much higher in several brain regions, such as the cerebral cortex, striatum, thalamus, and so on than those of the TGF‐α and those of EGF were the lowest 44. It is possible that HB‐EGF serves as a major physiologic ligand for the EGF receptor within the central nervous system. These localizations of the HB‐EGF and EGF receptors indicate that the HB‐EGF signal may be an important trophic factor in the developing central nervous system and a contributor to higher brain functions.
Activity‐dependent shedding of HB‐EGF was also observed in neuronal cells, as in other types of cells 45. Several neurotransmitters, such as glutamate, kainate, and N‐methyl‐d‐aspartate (NMDA), trigger the ectodomain shedding of proHB‐EGF in neuron‐enriched cortical and hippocampal cultures 45. Metalloproteinases in the ADAM family play prominent roles in the shedding of proHB‐EGF induced by these neurotransmitters in neurons and glia 45. Individual ADAM isoforms showed strikingly different expression in the central nervous system 45, 46. For instance, ADAM10 and ADAM 17 (TACE) were expressed in the hippocampus, neocortex, and cerebellum 46, while ADAM11 mRNA was present throughout the forebrain 47. Accordingly, the region‐specific expression pattern of ADAM isoforms might precisely regulate the action of HB‐EGF and other EGF families.
Roles of HB‐EGF in Psychomotor Behavior and Neuronal Transmission
Recently, neurotrophic and growth factor have been shown to be associated in psychiatric disorders, such as schizophrenia, bipolar disorder, and depression 48, 49. Abnormal development of the brain is implicated in the etiology and/or pathology of various psychiatric diseases. Similarly, psychiatric patients can display abnormalities in the expression of cytokines and neurotrophic factors. EGF protein levels have been found to be lower in the prefrontal cortex and striatum of schizophrenic patients 50. Serum EGF levels were also lower in these patients, whereas EGF receptor expression in the prefrontal cortex was elevated 50. These reports suggest that EGF signals may be associated with the pathogenesis of psychiatric disorders.
Ventral forebrain‐specific HB‐EGF conditional KO mice exhibited some behavioral abnormalities, such as increased locomotor activity, decreased social interaction behavior, impairment of prepulse inhibition (PPI), and deficit of object recognition and short‐term memory 51. Additionally, treatments with either a typical or an atypical antipsychotic drug ameliorated these behavioral impairments in HB‐EGF KO mice. A typical antipsychotic (haloperidol) reduced the locomotor activity of HB‐EGF KO mice, but not the social withdrawal or the deficit of PPI 51. In contrast, atypical antipsychotics (clozapine and/or risperidone) ameliorated the impairments of social interaction and PPI. Generally, in animal models of psychiatric disorders, abnormal behaviors are accompanied by altered monoamine levels. In the prefrontal cortex, dopamine, 5‐HT, and its metabolite 5‐hydroxyindole acetic acid (5‐HIAA) levels were decreased in HB‐EGF KO mice. Similarly, various monoamine contents were also changed in other brain regions of HB‐EGF KO mice, such as the striatum, cerebellum, and thalamus 51. The dopamine hypothesis of schizophrenia has proposed that hyperactivity of dopamine transmission in the striatal dopamine system is responsible for the positive symptoms 52, while a deficit in dopamine transmission in the prefrontal cortex might be implicated in the cognitive impairments and negative symptoms of schizophrenia 53, 54. Abnormal development of the brain is implicated in the etiology and/or pathology of various psychiatric disorders. In schizophrenic patients, spine density in deep layer 3 prefrontal cortical pyramidal neurons is decreased compared with normal controls 55. Behavioral deficits of HB‐EGF KO mice are also accompanied with decreased spine density on the apical dendrites of the layer III pyramidal neurons (Figure 2) and NR1 protein of the NMDA receptor in the prefrontal cortex 51. On the other hand, activation of Ca2+/calmodulin‐dependent protein kinase II (CaMKII) and p21‐activated kinase (PAK) was markedly reduced in the prefrontal cortex of HB‐EGF KO mice 51. Overall, HB‐EGF KO mice exhibited the behavioral abnormalities reflected in a comprehensive spectrum of psychomotor and cognitive dysfunctions, similar to many psychiatric disorders. HB‐EGF KO mice also showed some neurological and neurochemical changes, which were relevant to the pathogenesis of schizophrenia. Meanwhile, genetically modified mouse for other EGF receptor ligands, such as EGF and TGF‐α, have not been reported to show phenotypes of psychiatric disorders.
Figure 2.
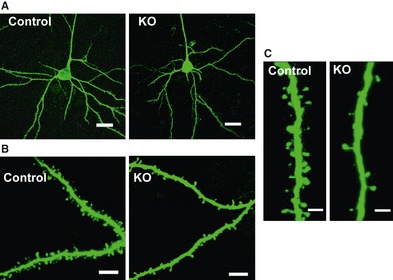
Morphological changes in the prefrontal cortex of ventral forebrain‐specific heparin‐binding epidermal growth factor (HB‐EGF) knockout (KO) mice. (A) Representative photomicrographs showing morphology of pyramidal neurons in cortical layer III of the prefrontal cortex from wild‐type control (left) and KO (right) mice. Scale bar = 20 μm. (B) Representative photomicrographs of apical dendritic segments from wild‐type control (left) and KO (right) mice. Scale bar = 8 μm. (C) High‐magnification images of apical dendritic segments from wild‐type control (left) and KO (right) mice. Scale bar = 2 μm. These figures were reproduced from Oyagi et al. 51.
Contrary to the change of EGF expression in schizophrenia patients, there have been no reports about the relationship of psychiatric disease and the Hb‐egf gene. Typically, HB‐EGF is processed from its precursor protein, pro‐HB‐EGF, which is susceptible to proteolytic cleavage, namely ectodomain shedding, and is converted to the mature secreted factor 56. Ectodomain shedding of HB‐EGF, regulated by ADAM, is critical for its function. According to the recent report, the density of ADAM12 immunoreactive oligodendrocytes were decreased in the white matter of the anterior cingulate cortex of schizophrenic patients 18. The ADAM12 gene is located on human chromosome 10q26.3, which has been linked to schizophrenia. These decreased expressions of ADAM family kinase might affect the activity of HB‐EGF in the brains of schizophrenia patients and the alterations affecting HB‐EGF signaling could comprise a contributing factor to psychiatric disorders. Meanwhile, EGF administration to neonatal rat induced some behavioral abnormalities in PPI, latent inhibition of learning, social interaction, and methamphetamine sensitivity 57. All of these findings indicate that an abnormal EGF receptor signal on the neonatal stage may contribute to the pathology of a number of psychiatric diseases, partly through perturbing normal brain development.
On the other hand, neuregulin‐1 (NRG‐1), another member of the EGF family, has been shown to be associated with schizophrenia in several different populations 58, 59. NRG‐1 binds to the ErbB family of tyrosine kinase transmembrane receptors (ErbB4). Dysfunction of NRG‐1 and ErbB4 signaling has been shown to participate in the pathophysiology of schizophrenia, partly through the NMDA receptor hypofunction 60, 61. HB‐EGF also binds to the ErbB4 type of EGF receptor, but little is known about the function of HB‐EGF and ErbB4 signaling in the central nervous system. Further studies, focused on the role of HB‐EGF in the ErbB4 receptor in the central nervous system, could provide the proof for a causal link between HB‐EGF and schizophrenia.
Roles of HB‐EGF in Neuronal Growth and Synaptic Plasticity in the Hippocampus
In the brain, EGF family members act as neurotrophic molecules, serving to enhance stem cell proliferation and neural differentiation, and they also influence synaptic plasticity 62, 63. HB‐EGF has been implicated in neuronal survival, glial/stem cell proliferation, and differentiation. HB‐EGF also enhances the neurite outgrowth and neuroprotection against ischemic injury in PC12 cells by activation of the MAPK signal pathway 64. In the hippocampus of the postnatal early stage (from P0 to P7), HB‐EGF mRNA is distributed within all principle cell layers of the hippocampus, including stratum pyramidal fields CA1–CA3 and the stratum granulosum of the dentate gyrus, whereas by P14 and through adulthood, the expression in the pyramidal cell layer (CA1–3) was decreased substantially, but in the dentate gyrus, the layer remained higher 38. Another report also suggests that HB‐EGF contributes to adult neurogenesis in the dentate gyrus of the hippocampus 65. These reports suggest that HB‐EGF may be one of the important contributors to neuronal development and synaptic plasticity in the hippocampus.
The HB‐EGF mRNA level was elevated in the hippocampus after kainate‐induced excitotoxic seizures. In these conditions, HB‐EGF protects the neurons against kainate toxicity in hippocampal cell cultures without affecting intracellular Ca2+ concentration 38. Our group of previously revealed ventral forebrain‐specific HB‐EGF KO mice were impaired by long‐term potentiation (LTP) induced by a high‐frequency stimulation in hippocampus CA1 neurons using slice preparations 66. HB‐EGF KO mice also showed reduction in the activities of CaMKII and GluR1 66. CaMKII has been implicated as a key molecule in the induction of LTP 67. Phosphorylation of AMPA receptors by CaMKII is reported to be particularly important for LTP induction 68, 69. The efficiency of neuronal transmission, known as synaptic plasticity, forms the cellular basis for learning and memory. HB‐EGF KO mice were impaired in spatial memory in the Morris water maze and in fear learning in a passive avoidance test 66. These reports suggest that HB‐EGF plays a significant, but yet to be fully identified, role in synaptic plasticity and memory formation.
Here, it is worth noting that neurotrophic factors and cytokines display profound neuromodulatory functions and are involved in the survival and homeostatic maintenance of the central nervous system through regulation of each other's expression. Disruption of the neurotrophin balance has been associated with the pathogenesis of various neurological diseases, such as schizophrenia, amyotrophic lateral sclerosis, and Alzheimer's disease 70, 71, 72. Region‐specific HB‐EGF deletion altered the levels of various neurotrophic factors in several brain regions in HB‐EGF KO mice. In particular, nerve growth factor, neurotrophin‐3 (NT‐3), or brain‐derived neurotrophic factor (BDNF) levels were upregulated in the hippocampus and/or cortex of HB‐EGF KO mice, compared with WT mice 66. Because HB‐EGF itself has a neurotrophic effect, the absence of HB‐EGF may secondarily alter the expression of neurotrophins. Taken together, these findings suggest that the induction of these growth factors compensates for the deficit in HB‐EGF and that the imbalance of neurotrophic and growth factors might partly associate with impaired memory function and synaptic plasticity in HB‐EGF KO mice. On the other hand, there are no significant differences between controls and HB‐EGF KO mice regarding the relative expression of other EGF family growth factors, such as EGF, TGF‐α, and betacelulin in the prefrontal cortex, using real‐time PCR 51.
On the other hand, EGF also promoted the hippocampal LTP in rat 63, 73. Furthermore, EGF and TGF‐α increased the paired pulse ration, which represents a reduction in local inhibitory strength 74. Meanwhile, EGF treatment also diminished the amplitude of excitatory postsynaptic currents in the GABAergic neurons 74. Given all these reports, EGF and TGF‐α, as well as HB‐EGF, may be important for the synaptic plasticity and neuronal transmission in the hippocampus.
Roles of HB‐EGF in Ischemia‐Induced Brain Damage
Neurotrophic and growth factors, such as EGF 75, fibroblast growth factor‐2 (FGF‐2) 75, 76, and BDNF 77 have been implicated in neurogenesis as well as in in vivo neuroprotection. For this reason, recent studies have focused on the ability of these factors to promote endogenous neurogenesis as a novel therapeutic strategy against ischemic stroke 78, 79. Focused on HB‐EGF, intraventricular injection of HB‐EGF into rats also reduced infarct size after focal cerebral ischemia 80. We recently reported that ventral forebrain‐specific HB‐EGF KO mice show exacerbated ischemia and reperfusion injury 81. Similarly, EGF family growth factors, such as EGF and TGF‐α, have also been reported to exert protective effects in rodent models of ischemic brain injury 36, 82, 83. The level of HB‐EGF mRNA in the cortex and hippocampus was increased after cerebral ischemia and reperfusion injury in a time‐dependent manner 81, 84. On the other hand, members of the EGF family are differentially expressed and regulated after ischemic brain injury. The time course changes after cerebral ischemia for other major EGF receptor ligands, such as EGF and TGF‐α, were investigated, but no changes in EGF mRNA expression were detected, while TGF‐α mRNA was increased only at 24 h after middle cerebral artery occlusion (MCAO) and reperfusion 81.
Many studies have shown that acute central nervous system insults, such as seizure, oxidative damage, lesion, traumatic injury, and global and focal ischemia, significantly increase progenitor cell proliferation in the adult brain 85, 86, 87, 88. These newly proliferated cells migrate to the damaged areas of the brain, particularly following cerebral ischemia 89, 90, 91, 92. The major function of neurogenesis in the adult brain seems to generate replacements for the neurons that die regularly in certain brain areas. In normal adult rats, the intracerebroventricular administration of HB‐EGF increased bromodeoxyuridine (BrdU)‐positive cells in the subventricular zone and in the subgranular zone of the dentate gyrus 80. HB‐EGF stimulates neurogenesis in mouse cerebral cortical cultures in vitro 65. Furthermore, adenovirus‐mediated gene transfer of HB‐EGF promoted neurogenesis and angiogenesis in the striatum after focal ischemia 93. Similarly, fewer BrdU positive cells were found in the subventricular zone in HB‐EGF KO mice after focal cerebral ischemia 81. HB‐EGF is also associated with ischemia and reperfusion injury in various organs other than the brain, such as the intestine and kidney 94, 95, 96, 97. Similarly, HB‐EGF expression was also upregulated after intestinal and renal ischemia and reperfusion injuries 95, 98.
The activation of the apoptotic pathway following cerebral ischemia and reperfusion is one of the major processes that lead to cell death 99, 100. Generation of excessive reactive oxygen species (ROS) during reperfusion is known to play a pivotal role in brain injury associated with stroke. These free radicals cause oxidative damage to brain lipids, proteins, and DNA, leading to brain dysfunction and cell death 101. In the KO mice study for HB‐EGF, the numbers of 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG)‐positive cells were significantly increased in the cortex of HB‐EGF KO mice compared with the WT mice 81. Previous studies have also shown that HB‐EGF decreases both in vitro and in vivo production of oxygen free radicals 102. Conversely, oxidative stress increases gene expression of HB‐EGF in various types of cells 103, 104. Therefore, HB‐EGF may exert its protective role at least in part by preventing oxidation of DNA by the ROS, which increases after transient ischemia.
Taken together, the recent studies indicate that HB‐EGF may play a pivotal role in reducing ischemia and reperfusion injury. Endogenously synthesized HB‐EGF would appear to exert a neuroprotective effect and also modify the neurogenesis after ischemic brain injury.
Roles of HB‐EGF in Nigrostriatal Dopaminergic Neurons
The EGF receptor mRNA was distributed throughout the ventral mesencephalon within cells of the substantia nigra pars compacta and VTA 44, 105. The expression of EGF receptor in nigral dopaminergic neurons favors the direct actions of EGF receptor ligands on this cell population 106. Several ligands for ErbB receptors have neurotrophic effects on dopaminergic neurons in in vivo and in vitro 37, 107. ErbB1 ligands also protect the dopaminergic neurons from neurotoxin‐induced degeneration and promote the morphological and biochemical differentiation of immature dopaminergic neurons 108, 109. Indeed, mice lacking an ErbB1 ligand and ErbB1‐deficient midbrain cell cultures show abnormalities in the development of dopaminergic neurons 107, 110. HB‐EGF also enhances the survival of midbrain dopaminergic neurons by activation of the MAPK and the Akt signal pathway 37. Furthermore, intracerebral administration of recombinant human HB‐EGF protects the nigrostriatal dopaminergic system in 6‐hydroxydopamine (6‐OHDA)‐induced rat Parkinson's disease model 109. HB‐EGF and EGF, as well as GDNF, enhance dopamine uptake in mesencephalic cultures 107. On the other hand, among various neurotransmitters examined, such as dopamine, 5‐HT, acetylcholine, and glutamate, only dopamine triggered the release of EGF from neuron‐enriched striatal cultures, but not HB‐EGF or TGF‐α 111.
Dopamine receptors belong to the G protein‐coupled receptor superfamily and can activate matrix metalloproteinases (MMPs) and ADAMs, which are responsible for ectodomain shedding 112, 113. The cellular mechanism suggests that once dopamine is released and bound to dopamine D1‐like receptors on striatal neurons, the activated MMP/ADAM enzymes shed the membrane‐anchored EGF precursors and lets soluble EGF be released to dopaminergic afferent terminals and this promotes dopaminergic development or dopamine release 108, 114. But HB‐EGF and TGF‐α might be differently regulated in striatal dopaminergic neurons, or these releases would be carried by the MMP/ADAM enzymes uncoupled with dopamine receptors 111.
The degeneration of nigrostriatal dopaminergic neurons is associated with the pathogenesis of some kinds of neurodegenerative disease, such as Parkinson's disease 115. A previous study that investigated the EGF family expression in Parkinson's disease revealed protein levels of EGF and tyrosine hydroxylase were decreased in the prefrontal cortex and striatum of patients 108. On the other hand, HB‐EGF or TGF‐α levels were not significantly altered in either region of patients 108. The expression of EGF receptors, such as ErbB1 and ErbB2, were also down‐regulated significantly in the striatum and prefrontal cortex of patients, but not other EGF receptors, such as ErbB3 or ErbB4 108.
These findings suggest that EGF receptor ligands, such as HB‐EGF, EGF, and TGF‐α, affect the development and function of nigrostriatal dopaminergic neurons, and further studies using KO mice would elucidate potential effects of HB‐EGF on these neurons.
Conclusion
As described in this review, a number of studies indicate that HB‐EGF would be an important trophic factor in the developing central nervous system and a contributor for higher brain functions. HB‐EGF is widely expressed in the central nervous system, including the hippocampus and cerebral cortex, and exerts significant action on the developing and adult nervous system. (1) HB‐EGF signaling may play a pivotal role in psychomotor behavior and neuronal transmission, and alterations affecting HB‐EGF signaling could comprise a contributing factor in psychiatric disorders. (2) HB‐EGF would be important for the synaptic plasticity and neuronal transmission in the hippocampus. (3) HB‐EGF, as well as other EGF receptor ligands, affects the development and function of nigrostriatal dopaminergic neurons. (4) Endogenously synthesized HB‐EGF would appear to exert a neuroprotective effect and also modify the neurogenesis after ischemic brain injury.
Although some action of HB‐EGF overlaps with other ErbB1 ligands, such as EGF and TGF‐α, much higher expression of HB‐EGF within the brain suggests that HB‐EGF serves as a major physiologic ligand for the EGF receptor (ErbB1) within the central nervous system. HB‐EGF plays essential roles in the central nervous system. Furthermore, HB‐EGF and careful regulation of its activity will be strong targets for treating a number of neurological diseases of the central nervous system.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1. Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res 2003;284: 2–13. [DOI] [PubMed] [Google Scholar]
- 2. Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M. A heparin‐binding growth factor secreted by macrophage‐like cells that is related to EGF. Science 1991;251: 936–939. [DOI] [PubMed] [Google Scholar]
- 3. Raab G, Klagsbrun M. Heparin‐binding EGF‐like growth factor. Biochim Biophys Acta 1997;1333: F179–F199. [DOI] [PubMed] [Google Scholar]
- 4. Elenius K, Paul S, Allison G, Sun J, Klagsbrun M. Activation of HER4 by heparin‐binding EGF‐like growth factor stimulates chemotaxis but not proliferation. EMBO J 1997;16: 1268–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iwamoto R, Mekada E. ErbB and HB‐EGF signaling in heart development and function. Cell Struct Funct 2006;31: 1–14. [DOI] [PubMed] [Google Scholar]
- 6. Iwamoto R, Yamazaki S, Asakura M, et al. Heparin‐binding EGF‐like growth factor and ErbB signaling is essential for heart function. Proc Natl Acad Sci USA 2003;100: 3221–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, Lee DC. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development 1999;126: 2739–2750. [DOI] [PubMed] [Google Scholar]
- 8. Shirasawa S, Sugiyama S, Baba I, et al. Dermatitis due to epiregulin deficiency and a critical role of epiregulin in immune‐related responses of keratinocyte and macrophage. Proc Natl Acad Sci USA 2004;101: 13921–13926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee D, Pearsall RS, Das S, Dey SK, Godfrey VL, Threadgill DW. Epiregulin is not essential for development of intestinal tumors but is required for protection from intestinal damage. Mol Cell Biol 2004;24: 8907–8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Higashiyama S, Iwabuki H, Morimoto C, Hieda M, Inoue H, Matsushita N. Membrane‐anchored growth factors, the epidermal growth factor family: Beyond receptor ligands. Cancer Sci 2008;99: 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nanba D, Mammoto A, Hashimoto K, Higashiyama S. Proteolytic release of the carboxy‐terminal fragment of proHB‐EGF causes nuclear export of PLZF. J Cell Biol 2003;163: 489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iwamoto R, Mekada E. Heparin‐binding EGF‐like growth factor: A juxtacrine growth factor. Cytokine Growth Factor Rev 2000;11: 335–344. [DOI] [PubMed] [Google Scholar]
- 13. Naglich JG, Metherall JE, Russell DW, Eidels L. Expression cloning of a diphtheria toxin receptor: Identity with a heparin‐binding EGF‐like growth factor precursor. Cell 1992;69: 1051–1061. [DOI] [PubMed] [Google Scholar]
- 14. Iwamoto R, Higashiyama S, Mitamura T, Taniguchi N, Klagsbrun M, Mekada E. Heparin‐binding EGF‐like growth factor, which acts as the diphtheria toxin receptor, forms a complex with membrane protein DRAP27/CD9, which up‐regulates functional receptors and diphtheria toxin sensitivity. EMBO J 1994;13: 2322–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mitamura T, Higashiyama S, Taniguchi N, Klagsbrun M, Mekada E. Diphtheria toxin binds to the epidermal growth factor (EGF)‐like domain of human heparin‐binding EGF‐like growth factor/diphtheria toxin receptor and inhibits specifically its mitogenic activity. J Biol Chem 1995;270: 1015–1019. [DOI] [PubMed] [Google Scholar]
- 16. Shirakata Y, Kimura R, Nanba D, et al. Heparin‐binding EGF‐like growth factor accelerates keratinocyte migration and skin wound healing. J Cell Sci 2005;118: 2363–2370. [DOI] [PubMed] [Google Scholar]
- 17. Tolino MA, Block ER, Klarlund JK. Brief treatment with heparin‐binding EGF‐like growth factor, but not with EGF, is sufficient to accelerate epithelial wound healing. Biochim Biophys Acta 2011;1810: 875–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farkas N, Lendeckel U, Dobrowolny H, et al. Reduced density of ADAM 12‐immunoreactive oligodendrocytes in the anterior cingulate white matter of patients with schizophrenia. World J Biol Psychiatry 2010;11: 556–566. [DOI] [PubMed] [Google Scholar]
- 19. Paria BC, Elenius K, Klagsbrun M, Dey SK. Heparin‐binding EGF‐like growth factor interacts with mouse blastocysts independently of ErbB1: A possible role for heparan sulfate proteoglycans and ErbB4 in blastocyst implantation. Development 1999;126: 1997–2005. [DOI] [PubMed] [Google Scholar]
- 20. Miyagawa J, Higashiyama S, Kawata S, et al. Localization of heparin‐binding EGF‐like growth factor in the smooth muscle cells and macrophages of human atherosclerotic plaques. J Clin Invest 1995;95: 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jackson LF, Qiu TH, Sunnarborg SW, Chang A, Zhang C, Patterson C, Lee DC. Defective valvulogenesis in HB‐EGF and TACE‐null mice is associated with aberrant BMP signaling. EMBO J 2003;22: 2704–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miyamoto S, Yagi H, Yotsumoto F, Kawarabayashi T, Mekada E. Heparin‐binding epidermal growth factor‐like growth factor as a novel targeting molecule for cancer therapy. Cancer Sci 2006;97: 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inui Y, Higashiyama S, Kawata S, Tamura S, Miyagawa J, Taniguchi N, Matsuzawa Y. Expression of heparin‐binding epidermal growth factor in human hepatocellular carcinoma. Gastroenterology 1994;107: 1799–1804. [DOI] [PubMed] [Google Scholar]
- 24. Ito Y, Higashiyama S, Takeda T, Okada M, Matsuura N. Bimodal expression of heparin‐binding EGF‐like growth factor in colonic neoplasms. Anticancer Res 2001;21: 1391–1394. [PubMed] [Google Scholar]
- 25. Ito Y, Takeda T, Higashiyama S, Noguchi S, Matsuura N. Expression of heparin‐binding epidermal growth factor‐like growth factor in breast carcinoma. Breast Cancer Res Treat 2001;67: 81–85. [DOI] [PubMed] [Google Scholar]
- 26. Yotsumoto F, Oki E, Tokunaga E, Maehara Y, Kuroki M, Miyamoto S. HB‐EGF orchestrates the complex signals involved in triple‐negative and trastuzumab‐resistant breast cancer. Int J Cancer 2010;127: 2707–2717. [DOI] [PubMed] [Google Scholar]
- 27. Freeman MR, Paul S, Kaefer M, et al. Heparin‐binding EGF‐like growth factor in the human prostate: Synthesis predominantly by interstitial and vascular smooth muscle cells and action as a carcinoma cell mitogen. J Cell Biochem 1998;68: 328–338. [DOI] [PubMed] [Google Scholar]
- 28. Adam RM, Danciu T, McLellan DL, et al. A nuclear form of the heparin‐binding epidermal growth factor‐like growth factor precursor is a feature of aggressive transitional cell carcinoma. Cancer Res 2003;63: 484–490. [PubMed] [Google Scholar]
- 29. Kramer C, Klasmeyer K, Bojar H, Schulz WA, Ackermann R, Grimm MO. Heparin‐binding epidermal growth factor‐like growth factor isoforms and epidermal growth factor receptor/ErbB1 expression in bladder cancer and their relation to clinical outcome. Cancer 2007;109: 2016–2024. [DOI] [PubMed] [Google Scholar]
- 30. Miyamoto S, Hirata M, Yamazaki A, et al. Heparin‐binding EGF‐like growth factor is a promising target for ovarian cancer therapy. Cancer Res 2004;64: 5720–5727. [DOI] [PubMed] [Google Scholar]
- 31. Buzzi S, Rubboli D, Buzzi G, Buzzi AM, Morisi C, Pironi F. CRM197 (nontoxic diphtheria toxin): Effects on advanced cancer patients. Cancer Immunol Immunother 2004;53: 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martarelli D, Pompei P, Mazzoni G. Inhibition of adrenocortical carcinoma by diphtheria toxin mutant CRM197. Chemotherapy 2009;55: 425–432. [DOI] [PubMed] [Google Scholar]
- 33. Mishima K, Higashiyama S, Nagashima Y, et al. Regional distribution of heparin‐binding epidermal growth factor‐like growth factor mRNA and protein in adult rat forebrain. Neurosci Lett 1996;213: 153–156. [DOI] [PubMed] [Google Scholar]
- 34. Nakagawa T, Sasahara M, Hayase Y, et al. Neuronal and glial expression of heparin‐binding EGF‐like growth factor in central nervous system of prenatal and early‐postnatal rat. Brain Res Dev Brain Res 1998;108: 263–272. [DOI] [PubMed] [Google Scholar]
- 35. Hayase Y, Higashiyama S, Sasahara M, Amano S, Nakagawa T, Taniguchi N, Hazama F. Expression of heparin‐binding epidermal growth factor‐like growth factor in rat brain. Brain Res 1998;784: 163–178. [DOI] [PubMed] [Google Scholar]
- 36. Kornblum HI, Zurcher SD, Werb Z, Derynck R, Seroogy KB. Multiple trophic actions of heparin‐binding epidermal growth factor (HB‐EGF) in the central nervous system. Eur J Neurosci 1999;11: 3236–3246. [DOI] [PubMed] [Google Scholar]
- 37. Farkas LM, Krieglstein K. Heparin‐binding epidermal growth factor‐like growth factor (HB‐EGF) regulates survival of midbrain dopaminergic neurons. J Neural Transm 2002;109: 267–277. [DOI] [PubMed] [Google Scholar]
- 38. Opanashuk LA, Mark RJ, Porter J, Damm D, Mattson MP, Seroogy KB. Heparin‐binding epidermal growth factor‐like growth factor in hippocampus: Modulation of expression by seizures and anti‐excitotoxic action. J Neurosci 1999;19: 133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gerecke KM, Wyss JM, Karavanova I, Buonanno A, Carroll SL. ErbB transmembrane tyrosine kinase receptors are differentially expressed throughout the adult rat central nervous system. J Comp Neurol 2001;433: 86–100. [DOI] [PubMed] [Google Scholar]
- 40. Ayuso‐Sacido A, Moliterno JA, Kratovac S, et al. Activated EGFR signaling increases proliferation, survival, and migration and blocks neuronal differentiation in post‐natal neural stem cells. J Neurooncol 2010;97: 323–337. [DOI] [PubMed] [Google Scholar]
- 41. Steiner H, Blum M, Kitai ST, Fedi P. Differential expression of ErbB3 and ErbB4 neuregulin receptors in dopamine neurons and forebrain areas of the adult rat. Exp Neurol 1999;159: 494–503. [DOI] [PubMed] [Google Scholar]
- 42. Pinkas‐Kramarski R, Eilam R, Alroy I, Levkowitz G, Lonai P, Yarden Y. Differential expression of NDF/neuregulin receptors ErbB‐3 and ErbB‐4 and involvement in inhibition of neuronal differentiation. Oncogene 1997;15: 2803–2815. [DOI] [PubMed] [Google Scholar]
- 43. Huang YZ, Wang Q, Xiong WC, Mei L. Erbin is a protein concentrated at postsynaptic membranes that interacts with PSD‐95. J Biol Chem 2001;276: 19318–19326. [DOI] [PubMed] [Google Scholar]
- 44. Piao YS, Iwakura Y, Takei N, Nawa H. Differential distributions of peptides in the epidermal growth factor family and phosphorylation of ErbB 1 receptor in adult rat brain. Neurosci Lett 2005;390: 21–24. [DOI] [PubMed] [Google Scholar]
- 45. Shishido Y, Tanaka T, Piao YS, Araki K, Takei N, Higashiyama S, Nawa H. Activity‐dependent shedding of heparin‐binding EGF‐like growth factor in brain neurons. Biochem Biophys Res Commun 2006;348: 963–970. [DOI] [PubMed] [Google Scholar]
- 46. Karkkainen I, Rybnikova E, Pelto‐Huikko M, Huovila AP. Metalloprotease‐disintegrin (ADAM) genes are widely and differentially expressed in the adult CNS. Mol Cell Neurosci 2000;15: 547–560. [DOI] [PubMed] [Google Scholar]
- 47. Rybnikova E, Karkkainen I, Pelto‐Huikko M, Huovila AP. Developmental regulation and neuronal expression of the cellular disintegrin ADAM11 gene in mouse nervous system. Neuroscience 2002;112: 921–934. [DOI] [PubMed] [Google Scholar]
- 48. Nawa H, Futamura T, Mizuno M, Takahashi M, Toyooka K, Someya T. [Contribution of neurotrophic factors and cytokines to schizophrenia]. Nihon Rinsho 2003;61: 521–528. [PubMed] [Google Scholar]
- 49. Voleti B, Duman RS. The roles of neurotrophic factor and wnt signaling in depression. Clin Pharmacol Ther 2012;91: 333–338. [DOI] [PubMed] [Google Scholar]
- 50. Futamura T, Toyooka K, Iritani S, et al. Abnormal expression of epidermal growth factor and its receptor in the forebrain and serum of schizophrenic patients. Mol Psychiatry 2002;7: 673–682. [DOI] [PubMed] [Google Scholar]
- 51. Oyagi A, Oida Y, Kakefuda K, et al. Generation and characterization of conditional heparin‐binding EGF‐like growth factor knockout mice. PLoS ONE 2009;4: e7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Carlsson A, Lindqvist M. Effect of chlorpromazine or haloperidol on formation of 3methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol Toxicol (Copenh) 1963;20: 140–144. [DOI] [PubMed] [Google Scholar]
- 53. Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: A review and reconceptualization. Am J Psychiatry 1991;148: 1474–1486. [DOI] [PubMed] [Google Scholar]
- 54. Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987;44: 660–669. [DOI] [PubMed] [Google Scholar]
- 55. Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry 2000;57: 65–73. [DOI] [PubMed] [Google Scholar]
- 56. Goishi K, Higashiyama S, Klagsbrun M, et al. Phorbol ester induces the rapid processing of cell surface heparin‐binding EGF‐like growth factor: Conversion from juxtacrine to paracrine growth factor activity. Mol Biol Cell 1995;6: 967–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Futamura T, Kakita A, Tohmi M, Sotoyama H, Takahashi H, Nawa H. Neonatal perturbation of neurotrophic signaling results in abnormal sensorimotor gating and social interaction in adults: Implication for epidermal growth factor in cognitive development. Mol Psychiatry 2003;8: 19–29. [DOI] [PubMed] [Google Scholar]
- 58. Zhao X, Shi Y, Tang J, et al. A case control and family based association study of the neuregulin1 gene and schizophrenia. J Med Genet 2004;41: 31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stefansson H, Sigurdsson E, Steinthorsdottir V, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet 2002;71: 877–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hahn CG, Wang HY, Cho DS, et al. Altered neuregulin 1‐erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med 2006;12: 824–828. [DOI] [PubMed] [Google Scholar]
- 61. Banerjee A, Macdonald ML, Borgmann‐Winter KE, Hahn CG. Neuregulin 1‐erbB4 pathway in schizophrenia: From genes to an interactome. Brain Res Bull 2010;83: 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ferrari G, Toffano G, Skaper SD. Epidermal growth factor exerts neuronotrophic effects on dopaminergic and GABAergic CNS neurons: Comparison with basic fibroblast growth factor. J Neurosci Res 1991;30: 493–497. [DOI] [PubMed] [Google Scholar]
- 63. Ishiyama J, Saito H, Abe K. Epidermal growth factor and basic fibroblast growth factor promote the generation of long‐term potentiation in the dentate gyrus of anaesthetized rats. Neurosci Res 1991;12: 403–411. [DOI] [PubMed] [Google Scholar]
- 64. Zhou Y, Besner GE. Heparin‐binding epidermal growth factor‐like growth factor is a potent neurotrophic factor for PC12 cells. Neurosignals 2010;18: 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jin K, Mao XO, Sun Y, et al. Heparin‐binding epidermal growth factor‐like growth factor: Hypoxia‐inducible expression in vitro and stimulation of neurogenesis in vitro and in vivo. J Neurosci 2002;22: 5365–5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Oyagi A, Moriguchi S, Nitta A, et al. Heparin‐binding EGF‐like growth factor is required for synaptic plasticity and memory formation. Brain Res 2011;1419: 97–104. [DOI] [PubMed] [Google Scholar]
- 67. Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci 2002;3: 175–190. [DOI] [PubMed] [Google Scholar]
- 68. Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA‐type glutamate receptors by CaM‐KII during long‐term potentiation. Science 1997;276: 2042–2045. [DOI] [PubMed] [Google Scholar]
- 69. Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci 2007;8: 101–113. [DOI] [PubMed] [Google Scholar]
- 70. Narisawa‐Saito M, Wakabayashi K, Tsuji S, Takahashi H, Nawa H. Regional specificity of alterations in NGF, BDNF and NT‐3 levels in Alzheimer's disease. NeuroReport 1996;7: 2925–2928. [DOI] [PubMed] [Google Scholar]
- 71. Schulte‐Herbruggen O, Braun A, Rochlitzer S, Jockers‐Scherubl MC, Hellweg R. Neurotrophic factors–a tool for therapeutic strategies in neurological, neuropsychiatric and neuroimmunological diseases? Curr Med Chem 2007;14: 2318–2329. [DOI] [PubMed] [Google Scholar]
- 72. Takahashi M, Shirakawa O, Toyooka K, et al. Abnormal expression of brain‐derived neurotrophic factor and its receptor in the corticolimbic system of schizophrenic patients. Mol Psychiatry 2000;5: 293–300. [DOI] [PubMed] [Google Scholar]
- 73. Abe K, Ishiyama J, Saito H. Effects of epidermal growth factor and basic fibroblast growth factor on generation of long‐term potentiation in the dentate gyrus of fimbria‐fornix‐lesioned rats. Brain Res 1992;593: 335–338. [DOI] [PubMed] [Google Scholar]
- 74. Abe Y, Nawa H, Namba H. Activation of epidermal growth factor receptor ErbB1 attenuates inhibitory synaptic development in mouse dentate gyrus. Neurosci Res 2009;63: 138–148. [DOI] [PubMed] [Google Scholar]
- 75. Tureyen K, Vemuganti R, Bowen KK, Sailor KA, Dempsey RJ. EGF and FGF‐2 infusion increases post‐ischemic neural progenitor cell proliferation in the adult rat brain. Neurosurgery 2005;57: 1254–1263; discussion ‐63. [DOI] [PubMed] [Google Scholar]
- 76. Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor‐2 have different effects on neural progenitors in the adult rat brain. J Neurosci 1997;17: 5820–5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain‐derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci 2001;21: 6706–6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nakatomi H, Kuriu T, Okabe S, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 2002;110: 429–441. [DOI] [PubMed] [Google Scholar]
- 79. Teramoto T, Qiu J, Plumier JC, Moskowitz MA. EGF amplifies the replacement of parvalbumin‐expressing striatal interneurons after ischemia. J Clin Invest 2003;111: 1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jin K, Sun Y, Xie L, Childs J, Mao XO, Greenberg DA. Post‐ischemic administration of heparin‐binding epidermal growth factor‐like growth factor (HB‐EGF) reduces infarct size and modifies neurogenesis after focal cerebral ischemia in the rat. J Cereb Blood Flow Metab 2004;24: 399–408. [DOI] [PubMed] [Google Scholar]
- 81. Oyagi A, Morimoto N, Hamanaka J, Ishiguro M, Tsuruma K, Shimazawa M, Hara H. Forebrain specific heparin‐binding epidermal growth factor‐like growth factor knockout mice show exacerbated ischemia and reperfusion injury. Neuroscience 2011;185: 116–124. [DOI] [PubMed] [Google Scholar]
- 82. Peng H, Wen TC, Tanaka J, et al. Epidermal growth factor protects neuronal cells in vivo and in vitro against transient forebrain ischemia‐ and free radical‐induced injuries. J Cereb Blood Flow Metab 1998;18: 349–360. [DOI] [PubMed] [Google Scholar]
- 83. Justicia C, Planas AM. Transforming growth factor‐alpha acting at the epidermal growth factor receptor reduces infarct volume after permanent middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab 1999;19: 128–132. [DOI] [PubMed] [Google Scholar]
- 84. Kawahara N, Mishima K, Higashiyama S, Taniguchi N, Tamura A, Kirino T. The gene for heparin‐binding epidermal growth factor‐like growth factor is stress‐inducible: Its role in cerebral ischemia. J Cereb Blood Flow Metab 1999;19: 307–320. [DOI] [PubMed] [Google Scholar]
- 85. Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci 1997;17: 2492–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci 1998;18: 7768–7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dash PK, Mach SA, Moore AN. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J Neurosci Res 2001;63: 313–319. [DOI] [PubMed] [Google Scholar]
- 88. Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol 2002;52: 802–813. [DOI] [PubMed] [Google Scholar]
- 89. Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature 2000;405: 951–955. [DOI] [PubMed] [Google Scholar]
- 90. Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 2002;8: 963–970. [DOI] [PubMed] [Google Scholar]
- 91. Parent JM, Valentin VV, Lowenstein DH. Prolonged seizures increase proliferating neuroblasts in the adult rat subventricular zone‐olfactory bulb pathway. J Neurosci 2002;22: 3174–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hicks AU, Hewlett K, Windle V, et al. Enriched environment enhances transplanted subventricular zone stem cell migration and functional recovery after stroke. Neuroscience 2007;146: 31–40. [DOI] [PubMed] [Google Scholar]
- 93. Sugiura S, Kitagawa K, Tanaka S, et al. Adenovirus‐mediated gene transfer of heparin‐binding epidermal growth factor‐like growth factor enhances neurogenesis and angiogenesis after focal cerebral ischemia in rats. Stroke 2005;36: 859–864. [DOI] [PubMed] [Google Scholar]
- 94. Pillai SB, Hinman CE, Luquette MH, Nowicki PT, Besner GE. Heparin‐binding epidermal growth factor‐like growth factor protects rat intestine from ischemia/reperfusion injury. J Surg Res 1999;87: 225–231. [DOI] [PubMed] [Google Scholar]
- 95. Mulder GM, Nijboer WN, Seelen MA, et al. Heparin binding epidermal growth factor in renal ischaemia/reperfusion injury. J Pathol 2010;221: 183–192. [DOI] [PubMed] [Google Scholar]
- 96. El‐Assal ON, Paddock H, Marquez A, Besner GE. Heparin‐binding epidermal growth factor‐like growth factor gene disruption is associated with delayed intestinal restitution, impaired angiogenesis, and poor survival after intestinal ischemia in mice. J Pediatr Surg 2008;43: 1182–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sakai M, Zhang M, Homma T, Garrick B, Abraham JA, McKanna JA, Harris RC. Production of heparin binding epidermal growth factor‐like growth factor in the early phase of regeneration after acute renal injury. Isolation and localization of bioactive molecules. J Clin Invest 1997;99: 2128–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Xia G, Rachfal AW, Martin AE, Besner GE. Upregulation of endogenous heparin‐binding EGF‐like growth factor (HB‐EGF) expression after intestinal ischemia/reperfusion injury. J Invest Surg 2003;16: 57–63. [PubMed] [Google Scholar]
- 99. Mattson MP, Duan W, Pedersen WA, Culmsee C. Neurodegenerative disorders and ischemic brain diseases. Apoptosis 2001;6: 69–81. [DOI] [PubMed] [Google Scholar]
- 100. Xing B, Chen H, Zhang M, Zhao D, Jiang R, Liu X, Zhang S. Ischemic postconditioning inhibits apoptosis after focal cerebral ischemia/reperfusion injury in the rat. Stroke 2008;39: 2362–2369. [DOI] [PubMed] [Google Scholar]
- 101. Chan PH. Role of oxidants in ischemic brain damage. Stroke 1996;27: 1124–1129. [DOI] [PubMed] [Google Scholar]
- 102. Kuhn MA, Xia G, Mehta VB, Glenn S, Michalsky MP, Besner GE. Heparin‐binding EGF‐like growth factor (HB‐EGF) decreases oxygen free radical production in vitro and in vivo. Antioxid Redox Signal 2002;4: 639–646. [DOI] [PubMed] [Google Scholar]
- 103. Miyazaki Y, Shinomura Y, Tsutsui S, et al. Oxidative stress increases gene expression of heparin‐binding EGF‐like growth factor and amphiregulin in cultured rat gastric epithelial cells. Biochem Biophys Res Commun 1996;226: 542–546. [DOI] [PubMed] [Google Scholar]
- 104. Sakai M, Tsukada T, Harris RC. Oxidant stress activates AP‐1 and heparin‐binding epidermal growth factor‐like growth factor transcription in renal epithelial cells. Exp Nephrol 2001;9: 28–39. [DOI] [PubMed] [Google Scholar]
- 105. Seroogy KB, Numan S, Gall CM, Lee DC, Kornblum HI. Expression of EGF receptor mRNA in rat nigrostriatal system. NeuroReport 1994;6: 105–108. [DOI] [PubMed] [Google Scholar]
- 106. Abe Y, Namba H, Zheng Y, Nawa H. In situ hybridization reveals developmental regulation of ErbB1‐4 mRNA expression in mouse midbrain: Implication of ErbB receptors for dopaminergic neurons. Neuroscience 2009;161: 95–110. [DOI] [PubMed] [Google Scholar]
- 107. Iwakura Y, Zheng Y, Sibilia M, et al. Qualitative and quantitative re‐evaluation of epidermal growth factor‐ErbB1 action on developing midbrain dopaminergic neurons in vivo and in vitro: Target‐derived neurotrophic signaling (Part 1). J Neurochem 2011;118: 45–56. [DOI] [PubMed] [Google Scholar]
- 108. Iwakura Y, Piao YS, Mizuno M, Takei N, Kakita A, Takahashi H, Nawa H. Influences of dopaminergic lesion on epidermal growth factor‐ErbB signals in Parkinson's disease and its model: Neurotrophic implication in nigrostriatal neurons. J Neurochem 2005;93: 974–983. [DOI] [PubMed] [Google Scholar]
- 109. Hanke M, Farkas LM, Jakob M, Ries R, Pohl J, Sullivan AM. Heparin‐binding epidermal growth factor‐like growth factor: A component in chromaffin granules which promotes the survival of nigrostriatal dopaminergic neurones in vitro and in vivo. Neuroscience 2004;124: 757–766. [DOI] [PubMed] [Google Scholar]
- 110. Blum M. A null mutation in TGF‐alpha leads to a reduction in midbrain dopaminergic neurons in the substantia nigra. Nat Neurosci 1998;1: 374–377. [DOI] [PubMed] [Google Scholar]
- 111. Iwakura Y, Wang R, Abe Y, et al. Dopamine‐dependent ectodomain shedding and release of epidermal growth factor in developing striatum: Target‐derived neurotrophic signaling (Part 2). J Neurochem 2011;118: 57–68. [DOI] [PubMed] [Google Scholar]
- 112. Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF receptor transactivation by G‐protein‐coupled receptors requires metalloproteinase cleavage of proHB‐EGF. Nature 1999;402: 884–888. [DOI] [PubMed] [Google Scholar]
- 113. Yan Y, Shirakabe K, Werb Z. The metalloprotease Kuzbanian (ADAM10) mediates the transactivation of EGF receptor by G protein‐coupled receptors. J Cell Biol 2002;158: 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Mizuno M, Sotoyama H, Narita E, et al. A cyclooxygenase‐2 inhibitor ameliorates behavioral impairments induced by striatal administration of epidermal growth factor. J Neurosci 2007;27: 10116–10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Dauer W, Przedborski S. Parkinson's disease: Mechanisms and models. Neuron 2003;39: 889–909. [DOI] [PubMed] [Google Scholar]


