Summary
Aims
To establish a radiation‐induced neural injury model using C17.2 neural stem cells (NSCs) and to investigate whether basic fibroblast growth factor (bFGF) can protect the radiation‐induced injury of C17.2 NSCs. Furthermore, we aim to identify the possible mechanisms involved in this model.
Methods
C17.2 NSCs received a single exposure (3, 6, and 9 Gy, respectively) at a dose rate of 300 cGy/min with a control group receiving 0 Gy. Different concentrations of bFGF were added for 24 h, 5 min postirradiation. The MTS assay and flow cytometry were used to detect cytotoxicity and apoptosis. Expression of FGFR1, ERK1/2, and p‐ERK1/2 proteins was detected with or without U0126 was pretreated prior to C17.2 NSCs receiving irradiation.
Results
C17.2 NSCs showed a dose‐dependent cell death as the dose of radiation was increased. Additionally, the rate of apoptosis in the C17.2 NSCs reached 31.2 ± 1.23% in the 6 Gy irradiation group, which was the most significant when compared to the other irradiation treated groups. bFGF showed protective effect on cell apoptosis in a dose‐dependent manner. The mean percentage of apoptotic cells decreased to 7.83 ± 1.75% when 100 ng/mL bFGF was given. Furthermore, U0126 could block the protective effect of bFGF by inhibiting the phosphorylation of ERK1/2.
Conclusions
An in vitro cellular model of radiation‐induced apoptosis of NSCs, in C17.2 NSCs, was developed successfully. Additionally, bFGF can protect neurons from radiation injury in vitro via the ERK1/2 signal transduction pathway.
Keywords: apoptosis, basic fibroblast growth factor, C17.2 neural stem cells, ERK1/2, radiation‐induced neural injury
Introduction
Radiation therapy is an important treatment for tumors located close to, or within, the central nervous system (CNS) 1, 2. As many as 200,000 patients receive partial large‐field or whole‐brain irradiation every year, and the population of long‐term cancer survivors continues to grow. However, while radiation therapy has caused improved survival rates and longevity for cancer patients, there are also debilitating, late onset effects for brain health that are associated with radiation therapy 3. Following radiation therapy in the CNS, in vitro and in vivo, anatomical, pathophysiological, and clinical injuries were observed 4, 5, 6. Radiation‐induced brain injury may involve the loss of neural precursor cells and alterations in new cell production (neurogenesis). Hellström et al. 7 reported that 9 weeks after irradiation treatment in young rats, hippocampal neurogenesis was reduced to 5% of control levels, and olfactory bulb neurogenesis was reduced to 40% of control levels. Radiation‐induced apoptosis of precursor cells have also been reported 8. These evidences indicate a particular vulnerability of progenitor and stem cells in the CNS after irradiation. The understanding of the mechanisms underlying the radiation‐induced injury of NSCs is the basis for improving therapies and prophylaxes; however, it is currently poorly understood. This lack of understanding is why it is important to establish a suitable model that will allow for detailed examination of the possible mechanisms involved.
Basic fibroblast growth factor (bFGF) is expressed in neurons and glia cells throughout the mammalian nervous system and is now recognized as a multifunctional growth factor with notable actions in neuronal cells 9. bFGF was shown to act on angiogenesis, cell proliferation, and differentiation 10. Furthermore, bFGF is a well‐known mitogen for proliferating NSCs. NSCs isolated from the embryonic rat telencephalon can predominantly express fibroblast growth factor receptors and self‐renew in bFGF‐containing medium 11. Jordan et al. 12 found that NSCs could generate cholinergic neurons with specific properties when treated with bFGF within a specific time window.
Additionally, bFGF has been reported to be protective in radiotherapy. Administration of physiological concentrations of bFGF prior to, and immediately after, irradiation decreased the apoptotic response in rat salivary glands in vitro 13. Moreover, bFGF protects intestinal stem cells from radiation‐induced apoptosis 14. However, there has been little work performed of the protective effects of bFGF in radiation‐induced neural injury, and the underlying mechanism remains uncertain. Therefore, in our present study, our aims were to (1) establish a novel radiation‐induced model, in vitro by using C17.2 NSCs, for studying the unknown mechanism and therapeutic potential of neural injury with radiotherapy; (2) observe the neural protective effects of bFGF against irradiation; and (3) investigate the mechanism involved in the neural protective process of bFGF.
Materials and Methods
Materials
Fetal bovine serum (FBS) was obtained from Atlanta Biologicals (Norcross, GA, USA). Other routine cell culture supplies and reagents were purchased from Sigma, Invitrogen, and Fisher. The nonradioactive cell proliferation assay kit, bFGF, and U0126 were obtained from Promega (Madison, WI, USA). The primary antibodies used for immunocytochemistry and Western blotting were antifibroblast growth factor receptor l (FGFR1), antiexternal‐signal‐regulated kinase 1/2 (ERK1/2), and anti‐pERK1/2; all antibodies were received from Santa Cruz (Santa Cruz, CA, USA).
C17.2 Cell Culture
C17.2 NSCs were cultured in Dulbecco's modified Eagle medium: Nutrient Mixture F‐12 (DMEM/F‐12) media supplied with 10% FBS, 100 ng/mL streptomycin, and 100 units/mL penicillin, as previously described 15. Cells were passaged after 80% fusion.
Protocol of Irradiation and Treatment
C17.2 NSCs received different doses of irradiation treatment by a linear accelerator (SIEMENS‐Primus Linear Accelerator, Marietta, GA, USA). For each experimental group, a single exposure (3, 6, and 9 Gy, respectively) at a dose rate of 300 cGy/min was given. Cells in the sham control group received a 0 Gy dose of irradiation, but underwent the same stresses as the other groups. For bFGF treatment, different concentrations of bFGF (0, 25, 50, and 100 ng/mL) were added 5 min postirradiation, for 24 h, to the appropriate dosage groups according to preliminary experimental data.
To investigate possible signaling pathways involved in radiation‐induced neural injury, a MEK 1/2 inhibitor, 1,4‐diamino‐2,3‐ dicyano‐1,4‐bis [2‐ aminophenylthio] butadiene (U0126), was added 1 h before C17.2 cells received irradiation with appropriate bFGF treatment.
Cytotoxicity Assays
Cytotoxicity/cell viability in response to different treatments was evaluated using both the MTS assay and Hoechst 33342 discrimination staining. For the MTS assay, the CellTiter 96® AQueous Assay Kit (Promega) was used, and the data were expressed as a percentage of untreated cells.
In parallel to the MTS assay, Hoechst 33342 staining in living cells was performed to discriminate apoptotic/necrotic cell death induced by the treatments. Hoechst 33342 can be transported through the cell membrane where it binds to nuclear DNA and emits blue fluorescence. Highly condensed, marginalized, or fragmented chromatins in apoptotic cells are stained bright blue with Hoechst 33342 16.
Flow Cytometric Analysis Using Annexin V and Propidium Iodide (PI)
The percentage of apoptotic cells in culture was evaluated by flow cytometric analysis using annexin V–FITC and propidium iodide (PI) fluorescence. Annexin V–FITC conjugates allow for the identification and qualification of cell surface changes that occur early during the apoptotic process when flow cytometry is used. These conjugates facilitate the rapid fluorometric quantification of apoptotic cells. By simultaneously staining cells with annexin V–FITC and the nonvital dye, PI, it is possible to distinguish between intact cells (FITC−, PI−), early apoptotic (FITC+, PI−), and late apoptotic or necrotic cells (FITC+, PI+).
Cells were seeded in six‐well plates and treated with or without different concentrations of bFGF for 24 h as described above. And then they were centrifuged to remove the medium, washed twice with 1× phosphate buffered saline (PBS), and stained with annexin V‐FITC and PI in binding buffer (10 mM Hepes, 140 mM NaCl, and 2.5 mM CaCl2). Ten thousand events were collected for each sample. Stained cells were analyzed using Cell‐Quent software in the FL1‐H and FL2‐H channels.
Immunocytochemistry
Immunochemical characterization of the C17.2 cells was performed as previously described 17, with the exception that a different primary antibody, FGFR1 diluted 1:100, was used in this study.
Western Blot
C17.2 neural stem cells (NSCs) were lysed with the appropriate amount of boiling, denaturing lysate buffer (1% SDS, 1 mM sodium orthovanadate, 10 mM Tris–Cl, pH 7.4) supplemented with a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA). The total protein quantification and Western blot procedures were performed routinely as previously described 18, with the exception that the dilution rates for the primary antibodies used were different: FGFR1 (1:200), ERK1/2 (1:200), and p‐ERK1/2 and ß‐actin (1:5000).
Statistics
Quantitative data are expressed as the mean ± SE and analyzed using ANOVA using SPSS 11.0 (SPSS Inc., Chicago, IL, USA). Post hoc comparisons of means were made using Scheffe's or Tukey's method where appropriate.
Results
Effects of Radiation on Apoptosis and Necrosis of C17.2 Cells
When C17.2 cells were treated with an increasing dose of irradiation, the MTS assay revealed a dose‐dependent reduction of cell viability 24 h postirradiation (Figure 1). The Hoechst 33342 discrimination staining was run in parallel to the MTS assay and replicated results of the dose‐dependent toxic effects of irradiation in C17.2 cells.
Figure 1.
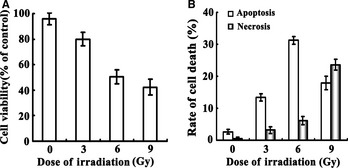
Radiation‐induced neural toxicity on C17.2 NSCs. Panel A, C17.2 NSCs received different doses of irradiation treatment, and the cell viability was analyzed by the MTS assay. The effect of irradiation on C17.2 NSCs shows a dose‐dependent decrease in cell viability. Panel B, Data from flow cytometric analysis shown that when cells were exposed to the lower dose of irradiation (≤6 Gy), apoptosis was the leading cause of cell death. As the dose of irradiation increased to 9 Gy, necrosis began to play a prominent role in cell viability. NSC, neural stem cell.
Data of flow cytometric analysis showed that the mean percentage of apoptotic cells present in our control culture was (2.61 ± 0.71)%. In culture treated with 3, 6, and 9 Gy irradiation, the means were (13.3 ± 1.14)%, (31.2 ± 1.23)%, and (17.9 ± 2.1)%, respectively, thus, progressively increasing with dosage until decreasing at the 9 Gy. Additionally, the percentage of cells in culture with necrosis increased in proportion to the radiation dose received (3.21 ± 1.06)% in the 3 Gy group, (6.13 ± 1.33)% in the 6 Gy group, and (23.6 ± 1.68)% in the 9 Gy group. From the data presented above, we observed that apoptosis was the most prominent in the 6 Gy irradiation group. Apoptosis then begins to decline at the higher dose (9 Gy) group (Figure 1). Therefore, the 6 Gy group was chosen to carry out the following experiment to further investigate probable mechanisms for radiation‐induced cell apoptosis.
Effect of bFGF on Radiation‐Induced Apoptosis and the Expression of FGFR1 in C17.2 NSCs
The 6 Gy irradiation group was used to observe the protective effect of different concentrations of bFGF on the apoptosis of C17.2 NSCs. Our results showed that with the increased concentrations of bFGF, apoptosis of C17.2 NSCs decreased. The mean percentage of apoptotic C17.2 NSCs decreased slightly to (23.3 ± 1.81)% when treated with the lowest concentration of bFGF (25 ng/mL) and decreased further to (10.1 ± 2.23)% and (7.83 ± 1.75)% when treated with 50 and 100 ng/mL bFGF, respectively (Figure 2).
Figure 2.
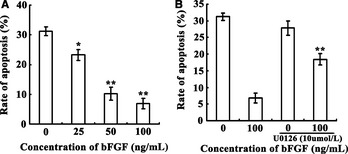
Effect of bFGF on radiation‐induced apoptosis. Panel A, with increasing concentrations of bFGF, apoptosis of C17.2 cells decreased. The most protective concentration of bFGF for C17.2 NSCs against apoptosis was 100 ng/mL. Panel B, C17.2 NSCs pretreated with 10 μM of U0126 1 h before receiving 6 Gy irradiation could prevent the protective effect from the 100 ng/mL bFGF treatment on cell apoptosis. *P < 0.05, **P < 0.01 compared to control group. bFGF, basic fibroblast growth factor; NSC, neural stem cell.
Immunocytochemistry (ICC) staining of FGFR1 revealed that when treated with bFGF, expression of FGFR1 increased significantly. In accordance with ICC, Western blots revealed that treatment with bFGF could clearly elevate the expression of FGFR1. U0126 treatment had no effect on the expression of FGFR1 that was induced by bFGF (Figure 3).
Figure 3.
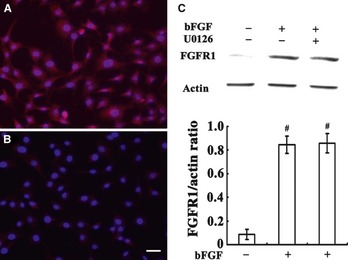
Effect of bFGF on the expression of FGFR1 in C17.2 NSCs. Panel A, B, ICC staining of FGFR1 in C17.2 NSCs. Panel B, increased expression of FGFR1 after bFGF treatment. Panel C, Western blots for FGFR1 expression showing the average densitometric values after β‐actin normalization represents the protein levels for each data point (n = 3). #P < 0.001 compared to untreated group. Scale bar, 30 μm. bFGF, basic fibroblast growth factor; ICC, immunocytochemistry; NSC, neural stem cell.
Effects of Pretreatment with U0126 on Radiation‐Induced Apoptosis in C17.2 NSCs
U0126, a MEK 1/2 Inhibitor, has been shown to be a highly selective inhibitor of MEK 1 and MEK 2. C17.2 NSCs were pretreated with 10 μM of U0126 1 h prior to receiving the 6 Gy irradiation treatment, with or without 100 ng/mL bFGF added 5 min postirradiation. Data from flow cytometric analysis showed that U0126 pretreatment could prevent the protective effect of the 100 ng/mL of bFGF on cells apoptosis partly; the percentage of apoptosis increased from (7.83 ± 1.75)% to (18.4 ± 1.71)% (Figure 2). In addition, Western blots revealed that U0126 treatment reduced the expression of p‐ERK1/2 compared to the control group (Figure 4).
Figure 4.
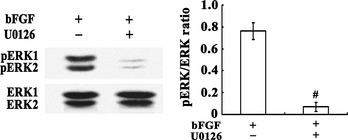
Effects of U0126 on the expression of ERK1/2 in C17.2 NSCs. Western blots for ERK1/2 showed that U0126 reduced the phosphorylation of ERK1/2. The average densitometric values after β‐actin normalization represents the protein levels for each data point (n = 3). #P < 0.001 compared to U0126 untreated group. NSC, neural stem cell.
Discussion
Previous studies showed 19, 20 that cells with a shorter cell division period and average lifetime (such as endothelial cells, astrocytes and oligodentrocytes) were more sensitive to radiation and therefore more likely to develop radiation‐induced neural injury. Neurons, in most cases, do not undergo cell division and as a result were considered to be exempt from the effects of irradiation 21. NSCs, which have the capacity for self‐renewal, proliferation, and pluripotentiality, were considered to be more sensitive to radiation than neuroglia cells and neurons 7. A recent study indicated that inhibition of neurogenesis has been an underlying mechanism for radiation‐induced CNS damage. When exposed to a single dose of 5 Gy, NSCs were clearly affected, while no injury effects were observed on neuroglia cells and neurons 22. Scientists have speculated that radiation‐induced attenuation of differentiation and the persistent changes in protein expression, rather than a cytotoxic mechanism, are responsible for the induced injury of NSCs that leads to radiation‐associated neural injury 15. However, until now, very little work has been performed to establish a radiation‐induced neural injury model in vitro, using a NSC line and the detailed mechanisms remain to be elucidated.
As a result, it is very important to establish an in vitro radiation model for the relevant mechanistic studies underlying radiation‐induced neural injury. In this study, using the C17.2 NSCs, we have established the first novel radiation model and used it to study the characteristic effects of radiation treatment. Our results have revealed that a single exposure at 3 Gy is sufficient to induce the C17.2 NSCs death. In addition, we showed dose‐dependent cell death as the dose of irradiation was increased. Furthermore, when the C17.2 NSCs were exposed to the lower dose of irradiation, apoptosis was the leading type of cell death. However, as the dose of irradiation increased, necrosis plays a prominent role as well.
As one of the potential neurotrophic factors whose role has yet to be clearly identified, the major biological effect of bFGF on neurons and astrocytes is its induction effect on proliferation 10. Additionally, bFGF has been shown to be effective in the regeneration of peripheral neural injuries. Presently, bFGF has widely been studied in degenerative diseases of the CNS and diseases related to the light‐induced injury of the retina 23, 24. However, its effects on radiation‐associated neural injury were still unclear. In 1997, it was initially reported that bFGF could protect astrocytes from x‐ray‐induced injury 25. Furthermore, in 2002, research from Nieder indicated that bFGF had some protective effects on radiation‐induced injury to the spinal cord 26. However, the effects of bFGF on radiation‐induced injury of NSCs were still unclear. To explore the possible protective effect and underlying mechanism of bFGF on radiation‐induced injury of NSCs, different concentrations of bFGF were administered to the medium of C17.2 NSCs 5 min postirradiation. The results showed that after 24 h, cellular apoptosis was inhibited with increasing bFGF concentration in a dose‐dependent manner, and the maximum concentration of bFGF for C17.2 NSCs that was protective against apoptosis was 100 ng/mL. Moreover, our study showed that externally generated bFGF could induce an increased expression of FGFR1 in C17.2 NSCs. It has been shown that FGFR1 plays a critical role in cellular proliferation and differentiation during animal development and adult homeostasis. In NSCs, bFGF could work concurrently with the FGFR1 to activate multiple signal pathways that increase proliferation, survival, and differentiation, both in vivo and in vitro 27.
The extracellular signal‐regulated kinase (ERK) signaling pathway is a major determinant for the control of several diverse cellular processes such as proliferation, survival, differentiation, and motility and played a crucial role in mediating the neurotrophic effect of bFGF 28. It has been shown that recombinant human bFGF can induce phosphorylation of ERK1/2 in a concentration‐ and time‐dependent manner in cultured rat cerebral cortical neurons 29. Furthermore, Yang et al. 30 demonstrated that bFGF consistently activated ERK activity in bone marrow stromal cells, resulting in neuronal differentiation. These findings suggest an important role for ERK in the neuronal cell cycles. In this investigation, we studied whether this signal transduction pathway was involved in the protective mechanism of bFGF in radiation‐induced neural injury. After adding bFGF to the medium of C17.2 NSCs, the expression of ERK1/2 and p‐ERK1/2 was increased. When U0126, the specific inhibitor of MEK1/2, was administered to the medium, the expression of FGFR1 and ERK1/2 was unchanged, but the phosphorylation of p‐ERK1/2 decreased. These results revealed that the protective effect of bFGF against radiation‐induced apoptosis in C17.2 NSCs may act through the ERK1/2 pathway and can be prevented with treatment of U0126. These results were similar to Gu's research on endothelial cells 31. Additionally, our data showed that blockage of the ERK1/2 signaling pathway could only partially prevent the protective effect of bFGF in NSCs after irradiation. This suggests that other proteins may also be involved in the bFGF‐mediated protection against radiation, and further research is required.
In conclusion, our study successfully developed a novel cellular model for research of radiation‐induced apoptosis using C17.2 NSCs. This model can be used in vitro for defining the mechanisms of radiation‐induced injuries in the nervous system. Furthermore, we found that bFGF could protect neurons from radiation‐induced injury in vitro through the ERK1/2 signaling pathway. This finding is helpful for future researchers who may be searching for effective therapeutic strategies for neural irradiation injury.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This study was supported by a grant given to Jun Liu from the National Natural Science Foundation of China (No. 30970966).
The first two authors contributed equally to this work.
References
- 1. Tsao MN, Lloyd NS, Wong RK, Rakovitch E, Chow E, Laperriere N. Radiotherapeutic management of brain metastases: a systematic review and meta‐analysis. Cancer Treat Rev 2005;31: 256–273. [DOI] [PubMed] [Google Scholar]
- 2. Kantor G, Laprie A, Huchet A, Loiseau H, Dejean C, Mazeron JJ. Radiation therapy for glial tumors: technical aspects and clinical indications. Cancer Radiother 2008;12: 687–694. [DOI] [PubMed] [Google Scholar]
- 3. Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation‐induced encephalopathy. J Clin Oncol 1994;12: 627–642. [DOI] [PubMed] [Google Scholar]
- 4. Schultheiss TE, Kun LE, Ang KK, Stephens LC. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys 1995;31: 1093–1112. [DOI] [PubMed] [Google Scholar]
- 5. Liu Y, Xiao S, Liu J, Zhou H, Liu Z, Xin Y, Suo WZ. An experimental study of acute radiation‐induced cognitive dysfunction in a young rat model. AJNR Am J Neuroradiol 2010;31: 383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Welzel G, Fleckenstein K, Mai SK, Hermann B, Kraus‐Tiefenbacher U, Wenz F. Acute neurocognitive impairment during cranial radiation therapy in patients with intracranial tumors. Strahlenther Onkol 2008;184: 647–654. [DOI] [PubMed] [Google Scholar]
- 7. Hellström NA, Björk‐Eriksson T, Blomgren K, Kuhn HG. Differential recovery of neural stem cells in the subventricular zone and dentate gyrus after ionizing radiation. Stem Cells 2009;27: 634–641. [DOI] [PubMed] [Google Scholar]
- 8. Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X‐irradiation. Cancer Res 2003;63: 4021–4027. [PubMed] [Google Scholar]
- 9. Haynes LW. Fibroblast (heparin‐binding) growing factors in neuronal development and repair. Mol Neurobiol 1988;2: 263–289. [DOI] [PubMed] [Google Scholar]
- 10. Abe K, Saito H. Effects of basic fibroblast growth factor on central nervous system functions. Pharmacol Res 2001;43: 307–312. [DOI] [PubMed] [Google Scholar]
- 11. Maric D, Maric I, Chang YH, Barker JL. Prospective cell sorting of embryonic rat neural stem cells and neuronal and glial progenitors reveals selective effects of basic fibroblast growth factor and epidermal growth factor on self‐renewal and differentiation. J Neurosci 2003;23: 240–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jordan PM, Ojeda LD, Thonhoff JR, Gao J, Boehning D, Yu Y, Wu P. Generation of spinal motor neurons from human fetal brain‐derived neural stem cells: role of basic fibroblast growth factor. J Neurosci Res 2009;87: 318–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thula TT, Schultz G, Tran‐Son‐Tay R, Batich C. Effects of EGF and bFGF on irradiated parotid glands. Ann Biomed Eng 2005;33: 685–695. [DOI] [PubMed] [Google Scholar]
- 14. Qiu W, Leibowitz B, Zhang L, Yu J. Growth factors protect intestinal stem cells from radiation‐induced apoptosis by suppressing PUMA through the PI3K/AKT/p53 axis. Oncogene 2010;29: 1622–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bajinskis A, Lindegren H, Johansson L, Harms‐Ringdahl M, Forsby A. Low‐dose/dose‐rate γ radiation depresses neural differentiation and alters protein expression profiles in neuroblastoma SH‐SY5Y cells and C17.2 neural stem cells. Radiat Res 2011;175: 185–192. [DOI] [PubMed] [Google Scholar]
- 16. Zhao Z, Lu R, Zhang B, et al. Differentiation of HT22 neurons induces expression of NMDA receptor that mediates homocysteine cytotoxicity. Neurol Res 2012;34: 38–43. [DOI] [PubMed] [Google Scholar]
- 17. Liu J, Li L, Suo WZ. HT22 hippocampal neuronal cell line possesses functional cholinergic properties. Life Sci 2009;84: 267–271. [DOI] [PubMed] [Google Scholar]
- 18. Liu J, Rasul I, Sun Y, Wu G, Li L, Premont RT, Suo WZ. GRK5 deficiency leads to reduced hippocampal acetylcholine level via impaired presynaptic M2/M4 autoreceptor desensitization. J Biol Chem 2009;284: 19564–19571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pena LA, Fuks Z, Kolesnick RN. Radiation‐induced apoptosis of endothelial cells in the murine central nervous system: protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res 2000;60: 321–327. [PubMed] [Google Scholar]
- 20. Li YQ, Jay V, Wong CS. Oligodendrocytes in the adult rat spinal cord undergo radiation‐induced apoptosis. Cancer Res 1996;56: 5417–5422. [PubMed] [Google Scholar]
- 21. Romero AA, Gross SR, Cheng KY, Goldsmith NK, Geller HM. An age‐related increase in resistance to DNA damage‐induced apoptotic cell death is associated with development of DNA repair mechanisms. J Neurochem 2003;84: 1275–1287. [DOI] [PubMed] [Google Scholar]
- 22. Lu F, Wong CS. A clonogenic survival assay of neural stem cells in rat spinal cord after exposure to ionizing radiation. Radiat Res 2005;163: 63–71. [DOI] [PubMed] [Google Scholar]
- 23. Grothe C, Timmer M. The physiological and pharmacological role of basic fibroblast growth factor in the dopaminergic nigrostriatal system. Brain Res Rev 2007;54: 80–91. [DOI] [PubMed] [Google Scholar]
- 24. Rosenthal R, Malek G, Salomon N, et al. The fibroblast growth factor receptors, FGFR‐1 and FGFR‐2, mediate two independent signalling pathways in human retinal pigment epithelial cells. Biochem Biophys Res Commun 2005;337: 241–247. [DOI] [PubMed] [Google Scholar]
- 25. Noel F, Ijichi A, Chen JJ, Gumin GJ, Tofilon PJ. X‐ray‐mediated reduction in basic fibroblast growth factor expression in primary rat astrocyte cultures. Radiat Res 1997;147: 484–489. [PubMed] [Google Scholar]
- 26. Nieder C, Price RE, Rivera B, Andratschke N, Ang KK. Experimental data for administration of insulin‐like growth factor 1 (IGF‐1) and basic fibroblast growth factor (bFGF) for prevention of radiation myelopathy. Strahlenther Onkol 2002;178: 147–152. [DOI] [PubMed] [Google Scholar]
- 27. Galvez‐Contreras AY, Gonzalez‐Castaneda RE, Luquin S, Gonzalez‐Perez O. Role of fibroblast growth factor receptors in astrocytic stem cells. Curr Signal Transduct Ther 2012;7(1): 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xiao Z, Kong Y, Yang S, Li M, Wen J, Li L. Upregulation of Flk‐1 by bFGF via the ERK pathway is essential for VEGF‐mediated promotion of neural stem cell proliferation. Cell Res 2007;17: 73–79. [DOI] [PubMed] [Google Scholar]
- 29. Abe K, Saito H. Neurotrophic effect of basic fibroblast growth factor is mediated by the p42/p44 mitogen‐activated protein kinase cascade in cultured rat cortical neurons. Brain Res Dev Brain Res 2000;122: 81–85. [DOI] [PubMed] [Google Scholar]
- 30. Yang H, Xia Y, Lu SQ, Soong TW, Feng ZW. Basic fibroblast growth factor‐induced neuronal differentiation of mouse bone marrow stromal cells requires FGFR‐1, MAPK/ERK, and transcription factor AP‐1. J Biol Chem 2008;283: 5287–5295. [DOI] [PubMed] [Google Scholar]
- 31. Gu QY, Wang DW, Wang LS. MEK inhibitor U0126 may partly block the inhibitory effect of bFGF on radiation‐induced apoptosis in endothelial cells. J Radiat Res Radiat Process 2004;1: 39–42. [Google Scholar]


