SUMMARY
Aims: To investigate the incidence of depression at different time points within the first year after stroke in mainland China and to identify risk factors related to a poor 1‐year prognosis in stroke patients. Methods: Subjects with acute cerebrovascular diseases were recruited and enrolled from 56 hospitals in mainland China between April 2008 and April 2010. Demographic data, previous disease history, and clinical data were collected. Four follow‐up visits were occurred within the first year after stroke. The modified Rankin Scale ≥2 represents an unfavorable prognosis. Depression was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition and was divided into persistent, recurrent and transient types. Results: The 1‐year cumulative incidence of depression in stroke patients was 41.8%. Logistic regression analysis showed that the 1‐year prognosis level was associated with age, disability before onset, neurological functional deficit level at admission, and a range of depression types. The odds ratio for persistent depression is the highest (OR = 7.615, P < 0.0001, 95% confidence interval 5.011–11.572). Conclusions: In our study, depression occurred in >40% of patients within the first year after stroke. Persistent depression is the first independent determinant of prognosis during the first year after stroke.
Keywords: Cohort studies, Depression, Incidence, Prognosis, Prospective studies, Risk factors, Stroke
Introduction
Stroke is a prevalent disease endangering human health. Poststroke depression (PSD) is the most frequent emotional obstacle after stroke. Many studies have shown that PSD not only affects recovery of language and somatic function, but also lowers quality of life and increases disability rate and mortality [1, 2]. Different international studies have reported different levels of PSD incidence at different times postevent [3, 4, 5, 6, 7, 8]. A report by Bour et al. stated that 1 month after stroke, the prevalence of PSD was 18.8%. Thirty percent of patients who were depressed in the first 3 months did not reach cut‐off levels on depression screening instruments at subsequent assessments. In 44% of these 30% patients who were depressed in the first 3 months symptoms recurred. Persistent patients were more disabled and suffered more often from major depression [8].
There are many studies on PSD among the Chinese population [9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19]. Most published studies in mainland China were cross‐sectional study and few were prospective. Most data were obtained from a single center or a limited number of hospitals. Meanwhile, the study designs showed large diversities in terms of case sources, inclusion criteria, stroke types, diagnostic criteria for PSD, time point for observing the stroke, follow‐up frequency and intervals, and statistical analysis methodologies [17, 18, 19]. Therefore, large‐scale multicenter prospective studies with uniform diagnostic criteria and observation period are urgently needed. Meanwhile, there is a need for more attention to be paid to the course of depression poststroke to improve our understanding of its impact on patients, as well as the impact of different types of depression and the ways in which these affect the function of stroke patients among mainland Chinese.
The Prospective Cohort study on Incidence and Outcome of Patients with Poststroke Depression in China (PRIOD), sponsored by the Ministry of Health of China, is the first large‐scale national multicenter prospective cohort study on PSD in mainland China, number ISRCTN62169508, at http://www.controlledtrials.com/isrctn.
Subjects and Methods
The present study aimed to perform a subanalysis of the findings of PRIOD to gain a better understanding of the incidence of depression within the first year after stroke, and to identify any correlations between the course of depression and prognosis of stroke patients among mainland Chinese.
Screening of Subjects
Between April 2008 and April 2010, stroke patients admitted to the Neurology Departments of 56 centers nationwide were screened and recruited.
The inclusion criteria were as follows: (1) fulfillment of the definition of stroke by meeting WHO criteria; namely, sudden focal or comprehensive neurological deficit of cerebrovascular cause that persists beyond 24 h, excluding cerebral dysfunction caused by other nonvascular factors (stroke patients included in our study had cerebral infarction and brain parenchyma hemorrhage, which were established by computed tomography or magnetic resonance imaging); (2) onset of stroke within 14 days of consultation; (3) age ≥18 years; (4) willingness to cooperate with examination and follow‐up; and (5) written informed consent provided by the patient or their legal guardian.
The following exclusion criteria were applied: (1) previous dementia or other known neurological diseases that could affect cognitive function; (2) history of alcohol or drug abuse; and (3) communication obstacles, including obvious aphasia that impeded the patient from finishing psychological assessment.
Methods
Patients’ clinical baseline data, including demographic data (age, gender, residence status, education background, marital status, personal characteristics, whether they had medical insurance, left‐handedness or right‐handedness), past medical conditions, past depression or psychiatric disease, stroke risk factors (e.g., hypertension, diabetes, smoking or alcohol addiction), modified Rankin scale (mRS) score before stroke, stroke types, lesion location, and National Institutes of Health Stroke Scale (NIHSS) score on admission, were collected. Other clinical data, including NIHSS score within 14 ± 2 days after onset, cognitive function within 14 ± 2 days after onset (assessed by the Mini Mental State Examination; MMSE), and other systemic complications (e.g., atrial vibration, urinary infection, lung infection, deep vein thrombus, and cardiac infarction), were also collected. Diagnosis of depression was performed by well‐trained physicians according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM‐IV) [20]. The Hamilton Rating Scale for Depression‐17 (HRSD‐17) scale was used to rate patients’ depression status [21]. The study used the Chinese version of the HRSD. The mRS was used to determine functional status. Previous studies have linked unfavorable prognosis to mRS scores ≥2 [22, 23]. Therefore, in the present study, we considered mRS <2 to represent functional independence and a favorable prognosis, and mRS ≥2 to represent an unfavorable prognosis.
Follow‐up assessments included diagnosis of depression according to the DSM‐IV, assessment of the HRSD‐17 at day 14 ± 2, 3 and 6 months, and day 360 ± 7 after onset, mRS score at day 360 ± 7 after onset, and mortality within 1 year after onset.
Patients were formally assessed at five time points (T1 = admission; T2 = 14 days poststroke; T3 = 3 months poststroke; T4 = 6 months poststroke; and T5 = 12 months poststroke). Baseline assessments were conducted by experienced allied health clinicians. The assessments were made only after patients gave written informed consent.
Clinical physiologists responsible for follow‐up at each center received strict and systematic training on various scales. HRSD‐17 and MMSE scores were assessed by qualified psychiatric or psychological physicians. The study was approved by the medical ethics committee of Beijing Tiantan Hospital affiliated to Capital Medical University, and was conducted in compliance with the Declaration of Helsinki Guidelines for Protection of Human Subjects.
The Course of Depression
Transient depression was defined as depression at only one time point or at two or three consecutive follow‐up points after diagnosis of depression, and no later recurrence of depression symptoms. Persistent depression was defined as depression occurring persistently after the onset of depression symptoms. Recurrent depression was defined as depression at four follow‐up visits at nonconsecutive time points [8].
Different Assessment Scales
Stroke severity: stroke severity was assessed using the NIHSS [24].
Cognition: the MMSE was used for assessments of cognitive function [25].
Statistical Analysis
SAS 9.2 (SAS Institute Inc., Cary, NC, USA) was used for statistical analysis. Continuous data are expressed as medians (interquartile range). Discrete data are expressed as frequencies and percentiles. The χ2 test or Fisher's test was used for analysis. Group differences between continuous data and one‐way orderly data were analyzed by the Wilcoxon rank‐sum test. A multivariate logistic regression model was adopted for multivariate analysis. Comprehensive data on depressed and nondepressed participants were analyzed to determine the relationships between PSD and poststroke function (mRS score at 12 months); individuals lacking mRS scores at 12 months were excluded. To further understand the course of depression in stroke patients, an analysis of the incidence and course of depression at different follow‐up stages was performed (n = 1687). For all analyses, a two‐tailed probability value of P < 0.05 was considered statistically significant.
Results
A total of 2828 patients were recruited (mean age, 61.5 ± 11.7 years). Among them, there were 2324 patients with cerebral infarction, 446 patients with hemorrhage, 56 patients with subarachnoid hemorrhage, and two patients with unclear diagnoses. In total, the 2770 patients with cerebral infarction and hemorrhage (male, n = 1822; female, n = 948) who were eligible for inclusion were enrolled in the study. Patients with subarachnoid hemorrhage or unclear diagnosis were excluded from further analysis. Among 70 patients who died, 59 died of cerebral infarction and 11 died of hemorrhage. Fifteen patients died within 3 months after stroke, a further 18 died within 6 months, and a further 37 died within 12 months. The mortality rate at 12 months was 2.50%. Patients were lost to follow‐up: 21 were lost at 14 days, 452 lost at 3 months, 675 lost at 6 months, and 194 patients were lost at 1 year. The proportion lost to follow‐up at 1 year was 7.00%.
Comparison of Data between Patients with and without Follow‐Up Data for All Time Points
There were 1687 patients with follow‐up data for all time points and 1083 patients without follow‐up data for all time points. There was no significant difference in demographic characteristics, including gender (P= 0.1720), age (P= 0.9651), marital status (P= 0.2045), literacy level (P= 0.2978), personal characteristics (P= 0.2357), course of smoking (P= 0.064), drinking (P= 0.423) psychiatric disease (P= 0.9816), family course of psychiatric disease (P= 0.3554), focus sides (P= 0.2969), residence status (P= 0.0832), 14‐day MMSE score (P= 0.1850), whether depression presented within the first 14 days (P= 0.0512), and left‐ and right‐handedness (P= 0.1952), between the patients with and without follow‐up data for all time points. There were significant differences in stroke types (P= 0.0066), admission NIHSS scores (P= 0.0004), 14‐day NIHSS scores (P < 0.0001), and receiving health education (P= 0.0011), indicating more severe neurological deficits in patients who did not finish 1‐year follow‐up, with greater numbers having cerebral hemorrhage and fewer having received health education.
Incidence and Characteristics of PSD
Among patients with follow‐up data at all time points (n = 1687), there were 705 with depressive symptoms and 982 without symptoms of depression. The PSD cumulative incidence within 1 year was 41.8%. There were 1441 patients with cerebral infarction and 246 patients with hemorrhage; these were divided into different groups according to depression time points and stroke types. Among patients with cerebral infarction, 858 (59.5%) did not have depression, 408 (28.3%) had transient depression, 113 (7.84%) had persistent depression, and 62 (4.31%) had recurrent depression. Among patients with hemorrhage, 124 (50.4%) did not have depression, 75 (30.5%) had transient depression, 32 (13.0%) had persistent depression, and 15 (6.1%) had recurrent depression. The incidence of depression in patients with hemorrhage was higher than that in patients with cerebral ischemia, with a significant difference (49.6% vs. 40.5%, χ 2= 7.2090, P= 0.0073). When adjusted on the basis of NIHSS scores at admission, there was no significant difference (χ 2= 3.8394, P= 0.0501).
Table 1 shows the incidence of newly occurring depression at each time point within 1 year after stroke. The highest incidence occurred in the acute poststroke period and at 3 months after stroke (28.4% and 11.3%, respectively). PSD incidence showed a gradually decreasing tendency; reduced to 5.18% at 1 year, from 28.4% in the acute period. Among 705 patients with depression, the morbidities of transient depression, persistent depression and recurrent depression at 1 year follow‐up were 68.5%, 20.6%, and 10.9%, respectively.
Correlative Factor Analysis of 1‐year Prognosis of Stroke Patients
The favorable prognosis was defined as mRS 0–1 and unfavorable prognosis was defined as mRS 2–5. Among 1687 patients with stroke, 1203 patients had favorable prognosis and 484 patients had unfavorable prognosis. Among 1203 patients with favorable prognosis, a total of 398 patients (33.1%) manifested depressive symptoms, while among 484 patients with unfavorable prognosis, 307 patients (63.4%) manifested depressive symptoms. The difference between the groups was significant (χ2 = 448.26, P < 0.0001).
Correlative factor analysis of the 1‐year prognosis of stroke patients (n = 1687) with complete follow‐up data was performed. The analysis indicated that poor prognosis was associated with female gender, age, high literacy, left‐handedness, diabetes, disability before onset, hemorrhagic stroke, severe neurological injury on admission and at day 14 after onset, low recognition, complications, and whether depression occurred within 1 year. Depression was divided into transient, persistent and recurrent types according to its occurrence at different time points. Logistic regression analysis was performed for factors with P < 0.05, adjusted for age, gender, and other confounding factors. The results indicated that the 1‐year physical disability level was associated with age, disability before onset, neurological injury level on admission, and all depression types (Figure 1). Persistent depression had the highest odds ratio, (OR: 7.615, 95% confidence interval [CI] 5.011–11.572) indicating a greater risk of unfavorable prognosis and more severe negative effects. The OR for recurrent depression was 4.701 (95% CI 2.721–8.122). The OR for transient depression was 2.309 (95% CI 1.756–3.307) (Figure 1).
Figure 1.
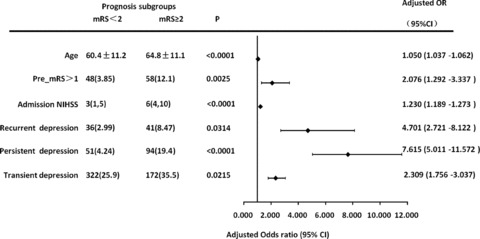
Association between poststroke depression and prognosis. Related risk factors and univariate probability values for comparisons between prognosis subgroups are reported. Odds ratios for poststroke depression adjusted by age, sex, education, handedness, diabetes, stroke type, and mini‐mental state examination (MMSE) score at 14 days after stroke are plotted. NIHSS indicates National Institutes of Health Stroke Scale; mRS indicates modified Rankin scale.
Among the 1687 stroke patients with follow‐up data in our study, 437 were admitted to stroke units (25.9%). Among these, 318 patients had good prognoses (72.8%), and 119 had poor prognoses (27.3%). A total of 1250 patients were admitted to regular units, among which 885 had good prognoses (70.8%) and 365 had poor prognoses (29.2%). Statistical analysis revealed that being admitted to a stroke unit was not associated with the 1‐year functional prognosis of stoke patients (P= 0.4335).
Discussion
Stroke is a critical challenge for public health. Approximately one‐third of all stroke patients will experience PSD. There are many studies on PSD among the Chinese population. The Prince of Wales Hospital in Hong Kong took the lead in the study focusing on the frequency, clinical manifestations, and radiological predictors of depression after stroke in Hong Kong area. It was a single‐center follow‐up study, with Structured Clinical Interview for DSM‐IV as the main scale [9, 10, 11, 12]. Quite a few cross‐sectional or retrospective studies on PSD have also been published by authors from Taiwan, China. In their studies, the diagnosis of depression was mainly based on Geriatric Depression Scale‐short form, HRSD, and Centre for Epidemiologic Studies Depression Scale, or based on the doctors’ judgment. The subjects included community‐dwelling chronic stroke patients, inpatients with acute stroke, and outpatients with stroke. The reported incidence of depression varied from 34.3–62.2%[13, 14, 15, 16]. Fu et al. surveyed the prevalence of depressive symptoms among Chinese stroke inpatients. A total of 384 eligible stroke subjects with a mean disease course of 1.3 ± 1.4 years, were consecutively enrolled from the departments of neurology in 10 tertiary hospitals in Beijing, Shanghai, Guangzhou, and Chengdu. With a HRSD score of ≥17 as the cut‐off value for the diagnosing depression, the prevalence of depression among stroke patients was found to be 9.9%[17]. In 2009, Zhang et al., for the first time in China, diagnosed PSD by using the World Health Organization Composite International Diagnostic Interview, yielding a 3‐month accumulative prevalence of 27.3% from a single center [18].
To the best of our knowledge, our study is the first large scale national multicenter prospective cohort study on PSD and its prognosis in mainland China. In our study, the cumulative incidence of PSD within the 3 months was 36.4%, higher than the 27.3% incidence reported by Zhang et al. [18]. This discrepancy might be related to differences in assessment scales and the populations studied.
Meanwhile, very few of previous studies have performed dynamic follow‐ups to observe PSD incidence at different time points. In our study, the 1 year cumulative incidence of PSD in stroke patients was 41.8%, consistent with levels reported in international studies [8, 26]. The 1 year cumulative incidence of PSD reported by Aben et al. and Bour et al. was 38.7%[26] and 36.2%, respectively [8]. Bour et al. observed PSD incidence for 1 year at 3‐month intervals, and found that PSD incidence changed dynamically. However, they did not observe PSD incidence during the acute period after stroke [8]. Our study examined patients at 14 days, 3 months, 6 months and 12 months, including the acute, subacute and chronic periods, providing insight into the incidence and characteristics of depression during the first year after stroke.
Longitudinal studies based on individual data revealed that although the prevalence of depression remained consistent over time, individuals were depressed at different time points [27]. We also observed newly occurring depression at every time point within the first year after stroke, in particular during the acute period and at 3 months after stroke, with morbidities of 28.4% and 11.3%, respectively. The incidence in the acute period was the highest. This gradually decreased with time to 5.18% at 1 year.
In our study, depression was divided into persistent, recurrent, and transient types. Our study found that the majority of patients with PSD had transient depression. Over time, depression symptoms dissipated in greater than 60% of stroke patients. We observed persistent depression in 20.6% of stroke patients, with rates of 20.2% in the acute period and 22.2% at 3 months after stroke.
PSD is associated with worsened functional outcome and deterioration of quality of life [28, 29]. However, in terms of the relationships between PSD and functional outcomes, the results of previous studies are inconsistent. Many studies have shown that compared with patients without depression, the functional outcomes and rehabilitation status of patients with PSD and stroke survivors with symptoms of depression are poor [1, 2, 28, 30, 31]. We also found that depression was related to functional rehabilitation of stroke patients, and that 63.4% of patients with poor functional outcome manifested symptoms of depression (P < 0.0001).
However, some researchers believe that PSD is irrelevant to prognosis. Cassidy and colleagues studied 50 patients who received rehabilitation treatment 3–12 months after stroke and described two significant results. First, depression was unrelated to the extent of functional disability after stroke. Second, there was no significant relationship between depression and early functional outcome after rehabilitation. Patients with major depressive disorders were administered psychotropic medication in this study, leading to inaccuracy of results [32]. De Weerd et al. found that depression (P = 0.057) was irrelevant to functional dependence [33]. Their study only observed 57 patients, so the small sample may limit extrapolation of these results.
Our study indicated a definite relationship between the course of PSD and functional prognosis. Logistic regression analysis showed that persistent depression was strongly associated with poor functional prognosis (OR = 7.615, 95% CI 5.011–11.572). Compared with the other two types of depression, persistent depression was linked with the highest risk of poor prognosis.
Chemerinsk et al. found that alleviation of depression in the months after stroke and early effective treatment of depression had a positive effect on rehabilitation outcomes in stroke patients, compared with persistent PSD [34]. Some studies have shown that depression itself does not cause poor outcomes directly. However, depression hinders patients’ enthusiasm for participating in rehabilitation exercises, and hence tends to be associated with poor outcomes [35].
In our study, 10.9% patients had recurrent depression within the 1 year follow‐up period. Among patients experiencing depression in the acute period, 20.2% had persistent depression and 13.6% had recurrent depression. Statistical analysis showed that recurrent depression was associated with 1 year functional prognosis (OR = 4.701, 95% CI 2.721–8.122). In addition, 5.18% patients had newly occurring depression at 1 year after onset of stroke.
Therefore, clinicians treating stroke patients should pay attention to the risks associated with PSD. Even patients in whom the symptoms of depression are alleviated also need periodic assessments of psychological status. In the acute period and following the subacute period, as well as in the long‐term chronic stage, early identification of recurrent depression might improve functional prognosis through systematic and dynamic screening of negative emotions in stroke patients.
In accordance with other study results, our study found that the 1 year functional disability level was associated with older age, disability before onset, and neurological function levels at admission [28].
Moreover, in randomized trials, acute stroke units are associated with improved patient outcomes [36]. Stroke units are now recommended as the minimum standard of care for all patients admitted to hospital with a diagnosis of stroke [37]. Nevertheless, our study found that being admitted to a stroke unit was irrelevant to 1 year functional prognosis (P= 0.4335). This was possibly due to the heterogeneity, as well as different grades of involved hospitals, inconsistent levels of stroke units in hospitals of different grades, and existing heterogeneity within stroke units. Hence, the outcome that stroke unit is irrelevant to prognosis of stroke function is confounded by differences in such factors.
Our study, in comparing PSD and non‐PSD patients, found there was no statistically significant difference with regard to the incidence of hemorrhagic stroke and ischemic stroke (P= 0.0501 adjusted by NIHSS scores). This conclusion is consistent with most other study results [18, 38]. Some studies have considered that the incidence of depression is associated with multiple lacunar infarction [39]. Our study merely divided stroke into ischemic stroke and hemorrhagic stroke. Further subtype categorization of ischemic stroke and analysis of the correlation between different ischemic stroke subtypes and incidence of depression were not performed. Therefore, our study could only show that incidence of depression is irrelevant to ischemic and hemorrhagic stroke, but could not further illuminate the association with subtypes of ischemic stroke.
There was no significant difference in demographic factors and incidence of depression in the acute period between patients with complete follow‐up data sets and those with incomplete follow‐up data sets. However, there was a significant difference in stroke type (P = 0.0066), admission NIHSS (P = 0.0004), 14‐day NIHSS score (P < 0.0001), and whether health education had been received (P = 0.0011), indicating the presence of severe neurological functional deficits in patients who did not complete follow up, and those who did not receive health education. The compliance of patients was associated with the degree of severity of neurological functional deficit and whether they had received health education during hospitalization. Education and counseling interventions have led to improved functional and social patient outcomes. This approach helps patients and their spouses make optimal adjustments to living with stroke [40].
Limitations
Many of our results are consistent with those of other studies both in China and abroad. Nevertheless, our study also has some limitations. First, our study is based on observation of hospitalized patients, and excluded patients in the community. Second, being limited by the assessment scales used, patients with severe aphasia, cognition impairment and consciousness impediment were excluded; thus, the enrolled subjects had relatively moderate deficits in neurological function and lower mortality. Third, in terms of the correlative analysis of prognosis, our study did not perform a classification analysis of depression severity, and did not analyze the relationship between a patient's functional status and whether antidepression therapy was given to treat complications.
Conclusion and Future Directions
The present multicenter large‐scale sampling study performed in mainland China revealed that depression occurred in >40% of patients within the first year after stroke. All stroke patients should undergo periodic screening and assessment for depression. Depression can be recurrent, and requires periodic assessment of psychological status to ensure early identification. The present work suggests that persistent depression is the first independent determinant of prognosis during the first year after stroke in mainland Chinese patients.
It is important to continue to address the management of psychological status after stroke, and especially to monitor for symptoms of depression, not just in the acute and immediate subacute time frames, but also in the longer term chronic period.
Author Contributions
Yong‐Jun Wang, MD: research design and concept development, critical revisions, and approval of submitted versions; Chun‐Xue Wang, MD: draft revisions, literature review, drafting the article, and review of submitted versions; Ning Zhang, MD: literature review, data collection, data analysis, drafting the article, and review of submitted versions; An‐Xin Wang, B.S. Med: statistical consultation and data analysis; Ying Bai, MM: data collection; Yong Zhou, MD: research design; Yi‐Long Wang, MD: research design; Tong Zhang, MD: data collection, drafting and approval of submitted versions; Juan Zhou, MM: data collection; Xing‐Quan Zhao, MD: study conception; Xin Yu, MD: research design and concept development; Xin‐Yu Sun, MD: research design and study conception; Zhao‐Rui Liu, MD: statistical consultation.
Disclosure
This study was funded jointly by the Beijing Science and Technology Committee (grant no. 7102050), the National Science Foundation (grant no. 81071115), the Young Scientists Fund of the Beijing Health Bureau (grant No. 2009–009), and the National 11th Five‐year Scientific and Technological Brainstorm Project (grant No. 2006BA101A11). This study was also supported by Pfizer Pharmaceutical Company.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgment
We thank all participating hospitals, colleagues, and imaging technicians. The authors are grateful to all of the colleagues, nurses, and patients involved in the study. The authors thank De‐Jun Liang, Li‐Ping Liu, Xian‐Wei Wang, Rong Cai and Gai‐Fen Liu for their assistance and kind support.
References
- 1. Hadidi N, Treat‐Jacobson DJ, Lindquist R. Poststroke depression and functional outcome: A critical review of literature. Heart Lung. 2009;38:151–162. [DOI] [PubMed] [Google Scholar]
- 2. Haas DC, Davidson KW, Schwartz DJ, et al Depressive symptoms are independently predictive of carotid atherosclerosis. Am J Cardiol 2005;95:547–550. [DOI] [PubMed] [Google Scholar]
- 3. Aström M, Adolfsson R, Asplund K. Major depression in stroke patients. A 3‐year longitudinal study. Stroke 1993;24:976–982. [DOI] [PubMed] [Google Scholar]
- 4. Kouwenhoven SE, Kirkevold M, Engedal K, Kim HS. Depression in acute stroke: Prevalence, dominant symptoms and associated factors. A systematic literature review. Disabil Rehabil 2011;33:539–556. [DOI] [PubMed] [Google Scholar]
- 5. Kauhanen M, Korpelainen JT, Hiltunen P, et al Poststroke depression correlates with cognitive impairment and neurological deficits. Stroke 1999;30:1875–1880. [DOI] [PubMed] [Google Scholar]
- 6. Aben I, Verhey F, Strik J, Lousberg R, Lodder J, Honig A. A comparative study into the one year cumulative incidence of depression after stroke and myocardial infarction. J Neurol Neurosurg Psychiatry 2003;74:581–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry 2002;52:253–264. [DOI] [PubMed] [Google Scholar]
- 8. Bour A, Rasquin S, Aben I, Boreas A, Limburg M, Verhey F. A one‐year follow‐up study into the course of depression after stroke. J Nutr Health Aging 2010;14:488–493. [DOI] [PubMed] [Google Scholar]
- 9. Chen YK, Wong KS, Mok V, Ungvari GS, Tang WK. Health‐related quality of life in patients with poststroke emotional incontinence. Arch Phys Med Rehabil 2011;92:1659–1662. [DOI] [PubMed] [Google Scholar]
- 10. Tang WK, Chan SS, Chiu HF, et al Poststroke depression in Chinese patients: Frequency, psychosocial, clinical, and radiological determinants. J Geriatr Psychiatry Neurol 2005;18:45–51. [DOI] [PubMed] [Google Scholar]
- 11. Tang WK, Chen YK, Lu JY, et al White matter hyperintensities in post‐stroke depression: A case control study. J Neurol Neurosurg Psychiatry 2010;81:1312–1315. [DOI] [PubMed] [Google Scholar]
- 12. Tang WK, Lu JY, Chen YK, et al Association of frontal subcortical circuits infarcts in poststroke depression: A magnetic resonance imaging study of 591 Chinese patients with ischemic stroke. J Geriatr Psychiatry Neurol 2011;24:44–49. [DOI] [PubMed] [Google Scholar]
- 13. Fuh JL, Liu HC, Wang SJ, Liu CY, Wang PN. Poststroke depression among the Chinese elderly in a rural community. Stroke 1997;28:1126–1129. [DOI] [PubMed] [Google Scholar]
- 14. Hsieh LP, Kao HJ. Depressive symptoms following ischemic stroke: A study of 207 patients. Acta Neurol Taiwan 2005;14:187–190. [PubMed] [Google Scholar]
- 15. Huang CY, Hsu MC, Hsu SP, Cheng PC, Lin SF, Chuang CH. Mediating roles of social support on poststroke depression and quality of life in patients with ischemic stroke. J Clin Nurs 2010;19:2752–2762. [DOI] [PubMed] [Google Scholar]
- 16. Hung JW, Tsay TH, Chang HW, Leong CP, Lau YC. Incidence and risk factors of medical complications during inpatient stroke rehabilitation. Chang Gung Med J 2005;28:31–38. [PubMed] [Google Scholar]
- 17. Fu CW, Xu B, Zhan SY, Luan RS, Chen WQ. A cross‐sectional study on the prevalence of depressive and/or anxiety symptoms in neurological patients from four cities in China. Zhonghua Liu Xing Bing Xue Za Zhi 2006;27:803–807. [PubMed] [Google Scholar]
- 18. Zhang T, Wang C, Liu L, et al A prospective cohort study of the incidence and determinants of post‐stroke depression among the mainland Chinese patients. Neurol Res 2010;32:347–3452. [DOI] [PubMed] [Google Scholar]
- 19. Zhang T, Meng JM, Xiang MJ. A prospective depression after cerebral strokes. Chin J Psychiatry 1996;29:73–76. [Google Scholar]
- 20. Association Psychological Association . Diagnostic and statistical manual of mental disorders 4th ed. Association Psychological Association, Washington , DC , 1994. [Google Scholar]
- 21. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Uyttenboogaart M, Stewart RE, Vroomen PC, De Keyser J, Luijckx GJ. Optimizing cutoff scores for the Barthel index and the modified Rankin scale for defining outcome in acute stroke trials. Stroke 2005;36:1984–1987. [DOI] [PubMed] [Google Scholar]
- 23. Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke 1999;30:1538–1541. [DOI] [PubMed] [Google Scholar]
- 24. Lyden P, Brott T, Tilley B, et al Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke 1994;25:2220–2226. [DOI] [PubMed] [Google Scholar]
- 25. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 26. Aben I, Lodder J, Honig A, Lousberg R, Boreas A, Verhey F. Focal or generalized vascular brain damage and vulnerability to depression after stroke: A 1‐year prospective follow‐up study. Int Psychogeriatr 2006;18:19–35. [DOI] [PubMed] [Google Scholar]
- 27. Herrmann N, Black SE, Lawrence J, Szekely C, Szalai JP. The Sunnybrook Stroke study: A prospective study of depressive symptoms and functional outcome. Stroke 1998;29:618–624. [DOI] [PubMed] [Google Scholar]
- 28. Wolfe CD, Crichton SL, Heuschmann PU, et al Estimates of outcomes up to ten years after stroke: Analysis from the prospective South London stroke register. PLoS Med doi: 10.1371/j.1001033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Robinson RG, Spalletta G. Poststroke depression: A review. Can J Psychiatry 2010;55:341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van de Weg FB, Kuik DJ, Lankhorst GJ. Post‐stroke depression and functional outcome: A cohort study investigating the influence of depression on functional recovery from stroke. Clin Rehabil 1999;13:268–272. [DOI] [PubMed] [Google Scholar]
- 31. Pohjasvaara T, Vataja R, Leppävuori A, Kaste M, Erkinjuntti T. Depression is an independent predictor of poor long‐term functional outcome post‐stroke. Eur J Neurol 2001;8:315–319. [DOI] [PubMed] [Google Scholar]
- 32. Cassidy EM, O’Connor R, O’Keane V. Prevalence of post‐stroke depression in an Irish sample and its relationship with disability and outcome following inpatient rehabilitation. Disabil Rehabil 2004;26:71–77. [DOI] [PubMed] [Google Scholar]
- 33. de Weerd L, Rutgers WA, Groenier KH, van der Meer K. Perceived wellbeing of patients one year post stroke in general practice–recommendations for quality aftercare. BMC Neurology. doi: 10.1186/1471-2377-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chemerinski E, Robinson RG, Kosier JT. Improved recovery in activities of daily living associated with remission of poststroke depression. Stroke. 2001;32:113–117. [DOI] [PubMed] [Google Scholar]
- 35. Moretti R, Bernobich E, Esposito F, et al Depression in vascular pathologies: The neurologist's point of view. Vasc Health Risk Manag 2011;7:433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lindsay Govan, Christopher J. Weir and Peter Langhorne. Collaboration organised inpatient (stroke unit) care for stroke. Stroke 2008;39:2402–2403. [Google Scholar]
- 37. Adams HP Jr, del Zoppo G, Alberts MJ, et al Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 2007;38:1655–1711. [DOI] [PubMed] [Google Scholar]
- 38. Toso V, Gandolfo C, Paolucci S, Provinciali L, Torta R, Grassivaro N. Post‐stroke depression: Research methodology of a large multicentre observational study (DESTRO). Neurol Sci 2004;25:138–144. [DOI] [PubMed] [Google Scholar]
- 39. Clark MS, Rubenach S, Winsor A. A randomized controlled trial of an education and counselling intervention for families after stroke. Clin Rehabil 2003;17:703–712. [DOI] [PubMed] [Google Scholar]
- 40. Hackett ML, Anderson CS. Predictors of depression after stroke: A systematic review of observational studies. Stroke 2005;36:2296–2301. [DOI] [PubMed] [Google Scholar]


