SUMMARY
Introduction: Dementia constitutes an increasingly prevalent cognitive disorder with serious socioeconomic implications. Aims: In the present study, we aimed to evaluate the efficacy of aniracetam, either as monotherapy or combined with cholinesterase inhibitors (ChEIs), in terms of several neuropsychological parameters, in a considerable number of patients with dementia. Results: In our prospective, open‐label study, we enrolled a total of 276 patients (mean age 71 ± 8 years, 95 males) with cognitive disorders. Our study population comprised four groups: no treatment group (n = 75), aniracetam monotherapy group (n = 58), ChEIs monotherapy group (n = 68), and group of combined treatment (n = 68). Patients were examined with validated neuropsychological tests at baseline, 3, 6, and 12 months of treatment. In patients treated with aniracetam, all studied parameters were adequately maintained at 6 and 12 months, while emotional state was significantly improved at 3 months. In patients treated with ChEIs, we observed a significant cognitive deterioration at 12 months. The comparison between aniracetam and ChEIs in patients with relatively mild dementia (15 ≤ MMSE ≤ 25) revealed a significantly better cognitive performance with aniracetam at 6 months and improved functionality at 3 months. Comparing aniracetam monotherapy with combined treatment in the same population, aniracetam performed better in the cognitive scale at 6 months, and displayed a notable tendency for enhanced mood at 12 months and improved functionality at 6 months. Conclusions: Our findings indicate that aniracetam (a nootropic compound with glutamatergic activity and neuroprotective potential) is a promising option for patients with cognitive deficit of mild severity. It preserved all neuropsychological parameters for at least 12 months, and seemed to exert a favorable effect on emotional stability of demented patients.
Keywords: Alzheimer’s disease; Aniracetam; Cholinesterase inhibitors; Combined treatment; Dementia, Monotherapy; Neuropsychological parameters; Nootropics
Introduction
Dementia is widely recognized as a growing public health problem in all developed and developing countries [1, 2]. It usually affects older people and results in progressive cognitive decline, functional disability, depressive personality, and significantly disrupted quality of life for both patients and their caregivers [3, 4, 5, 6, 7]. Alzheimer's disease (AD) is the most common form of dementia, and constitutes a clinically, biochemically, pathologically, and genetically heterogeneous neurodegenerative disease with serious clinical and socioeconomic implications [2, 8].
The limited number of currently available agents against dementia and their moderate long‐term clinical efficacy emphasize the urgent need for new and more effective treatments, which should be ideally devoid of intrinsic toxicity, well tolerated by elderly patients and able to successfully counteract the progressive cognitive decline, through a positive effect on the underlying neurobiological mechanisms [9].
One of the major therapeutic strategies aimed to ameliorate the clinical manifestations of AD is to enhance cholinergic neurotransmission in memory‐associated regions of the brain by the use of cholinesterase inhibitors (ChEIs), which delay the breakdown of acetylcholine released into synaptic clefts [10]. Several studies have confirmed the positive effect of these drugs on parameters of cognition, activities of daily living (ADL), and emotional well‐being [10, 11], but there is a relative paucity of clinical data concerning long‐term favorable outcomes [11]. Furthermore, the issue of their cost effectiveness has evoked considerable debate in recent literature and has not been conclusively delineated yet [12]. On the other hand, nootropic agents are older and safe medications that have been clinically evaluated in the treatment of psychobehavioral symptoms of dementia, following stroke or AD [13]. They enhance primarily glutamatergic and secondarily cholinergic neurotransmission in the hippocampal and cortical region of the temporal lobe, and exert additional neuroprotective and synaptogenic effects [14]. One of these compounds, aniracetam, appears to be a particularly promising option for dementia‐related brain disorders, since accumulating experimental data suggest interaction with multiple neurotransmitter systems, antidepressant, and neurotrophic properties [14]. The excellent tolerability profile of aniracetam and the absence of remarkable drug interactions allow its widespread clinical use in patients with cognitive disorders, both as monotherapy and as part of combined treatment [15].
The present study reports the preliminary findings of the Hellenic AMNESIA Study (Aniracetam MoNotherapy compared with cholinEsterase inhibitors Study In Alzheimer’s). In this prospective study of 1‐year duration, we aimed to evaluate the efficacy of aniracetam, either as monotherapy or combined with ChEIs, in terms of cognitive, functional, and psychological parameters at 3, 6, and 12 months of treatment. In addition, we performed direct comparisons between the studied regimens in terms of clinical efficacy, and reviewed the relatively scarce clinical and experimental data regarding the role of aniracetam in targeting the multifactorial pathogenetic cascade of brain neurodegenerative diseases, such as AD.
Patients and Methods
Study Population
We evaluated a total of 276 patients diagnosed with cognitive disorders of variable severity, who were consecutively examined at the Memory and Dementia Outpatient Clinic of G. Papanikolaou General Hospital and other affiliated medical centers, and were enrolled in our study within 3 months. These patients were prospectively evaluated with four validated scales for neuropsychological assessment at 3, 6, and 12 months of follow‐up.
Study Design
This is a prospective, open‐label study, aimed to evaluate in a comparative way the clinical efficacy of three different treatment regimens in a considerable number of Greek patients suffering from several stages of cognitive impairment. Emphasis was placed on therapeutic effect, and not on the safety profile of the compared regimens.
All studied patients fulfilled the following inclusion criteria: minimum age of 50 years, diagnosis of cognitive impairment and classification according to the well‐established diagnostic criteria of the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV) of the American Psychiatric Association, the presence of a well‐trained and reliable caregiver, and finally recent neuroimaging evaluation obtained during the last 6 months. The major exclusion criteria included life‐threatening cardiovascular, pulmonary, gastrointestinal, hepatic, or endocrine disease, profound biochemical abnormalities, and severe manifested neuropsychiatric disorders other than dementia. The clinical diagnosis of possible or probable AD was established from clinical and neuropsychological examination, based on the diagnostic criteria of National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (impairment in at least two from the following cognitive domains: memory, language, perceptual skills, attention, constructive abilities, orientation, problem solving, and functional abilities) [16].
The study population was divided into four distinct groups, which were prospectively and comparatively investigated throughout the 1‐year study period. Although no computer‐generated randomization methodology was applied, the participating clinicians tried to divide consecutive patients into treatment groups in a roughly randomized manner (first patient one treatment, second patient different treatment, etc.). They tried to determine patients’ treatment without being influenced by the severity of their underlying cognitive impairment. The first group (n = 75) received no pharmacological treatment (no treatment group), but was assigned an individualized lifestyle and mental strengthening management plan consisting of healthy dietary choices, physical activity, and mental training exercises. These patients were either reluctant to receive any drug therapy or had discontinued treatment at least 2 years before their enrollment in the protocol. These patients had expressed their own will to remain untreated and served as a control arm in our study, in order to demonstrate the progressive neuropsychological deterioration expected and observed in such patients. The second group of patients (n = 68) was prescribed a regimen with ChEIs (ChEIs monotherapy group), including donepezil, rivastigmine, galantamine, memantine (NMDA antagonist), as well as combinations of memantine with either donepezil or rivastigmine or galantamine in similar proportions. The third group (n = 58) was assigned monotherapy with aniracetam, and the fourth group (n = 68) received a combination of aniracetam with a ChEI (combined treatment group). All pharmacologically treated patients were provided with the same recommendations for physical and mental exercises as the no‐treatment group. Dosing adjustments were based on the official prescribing information of aniracetam (1500 mg daily fixed dose) and ChEIs (greatly variable, depending on the specific agent), the clinical judgement of the therapist and the compliance, tolerance, and responsiveness of patients to assigned treatment. Both patients receiving aniracetam and ChEIs were treated with the optimal dosages of prescribed agents, after an appropriate dosing escalation, as clinically indicated. No patient of any study group was intentionally suboptimally treated. All patients treated with ChEIs reached optimal dosages, provided that no clinically relevant drug‐related side effects occurred.
The adherence to the study protocol was monitored by routine assessment of monotherapy or concomitant medication use. All patients who were initially assigned a specific treatment and were later on switched to another regimen because of poor efficacy, side effects, or patients’ own will were excluded from the statistical analysis of the study (n = 7). More analytically, two patients initially assigned to aniracetam monotherapy were switched to combined regimen because of insufficient response to aniracetam alone. For other two patients, the initial regimen of ChEIs monotherapy was later on reinforced by switch to combined regimen because of poor efficacy of ChEIs alone (inverse switch). For two other patients initially assigned the combined regimen, they were switched to aniracetam monotherapy because of side effects related to use of ChEIs. Finally, one more patient initially assigned the combined regimen ended up receiving only ChEIs for nonmedical reasons that were not clearly explained to the therapist. Patients who switched treatment happened to be well balanced in terms of their baseline characteristics (comparable demographics, type of cognitive impairment, severity of dementia), reasons for switch and initial regimen, so their removal from the analysis is expected to have only limited impact on the results of our study.
Parameters of Clinical Efficacy
The efficacy of studied regimens was assessed with four widely recognized neuropsychological tests, which were administered by a highly qualified clinical neuropsychologist (C.M.) with a great experience in the specific scales. The rater was blinded to the treatment followed by patients, maximizing objectivity and eliminating possible influential biases. More specifically, MMSE scale (Mini‐Mental State Examination) was used to assess patients’ cognitive performance. This scale grades patients’ orientation, judgement, visuospatial memory, recall ability, attention, concentration, language skills, and executive tasks. MMSE score ranges from 0 to 30, with higher values indicating a better cognitive state [17, 18]. FRSSD scale (Functional Rating Scale for Symptoms of Dementia) was used to assess the impact of dementia on patients’ daily activities and functional parameters, such as nutrition, dressing, personal hygiene, sleep integrity, urine incontinence, conversational skills, successful recall of names and events, spatial orientation, confusion, emotional balance, and social interactions. FRSSD score ranges from 0 to 42, with the higher values suggesting increasing functional incapacity. The cut‐off point of 5–6 has been used in order to distinguish patients with dementia from patients with MCI [19]. GDS scale (Geriatric Depression Scale) was used to assess the emotional state of participants. With 15 yes/no questions, this scale evaluates patients’ sadness, interest for life, pessimistic thoughts, ability to feel, and social withdrawal. GDS score ranges from 0 to 15, with higher values indicating more prominent depressive symptoms. The short version of GDS has been validated and standardized in the Greek population, and the cut‐off point for diagnosing clinically significant depression in demented patients has been determined to be 6–7 [20]. Finally, NPI questionnaire (Neuropsychiatric Inventory) was additionally administered in patients’ caregivers to provide a more thorough assessment of important psychopathological aspects of dementia such as apathy, anxiety, agitation, delusions, hallucinations, eating disorders, and sleeping difficulties [21].
Statistical Analysis
All numerical data were assessed for a normal distribution of their values with the nonparametric one‐sample Kolmogorov–Smirnov test. Normally distributed quantitative variables are expressed as mean values ± standard deviation, skewed data are presented by means of their median values and interquartile ranges, while categorical data are given as absolute and relative frequencies. Differences within the four studied groups between baseline performance and performance at 3, 6, and 12 months (prospective evaluation) were tested with Student's t‐test for paired samples (0–3 months, 0–6 months, 0–12 months) or Wilcoxon Signed Ranks test, depending on the number of cases and the normality of data distribution. Differences between treatment groups in terms of baseline characteristics and clinical efficacy parameters at 3, 6, and 12 months were evaluated with ANOVA (analysis of variance), and the Bonferroni rule was applied for post hoc comparisons in order to control for inflation of type I error. Categorical data were compared using the Chi‐Square test. P‐values (two‐tailed) were considered statistically significant at the level of 0.05. Analysis of the data was performed using the SPSS Statistical Package (version 16.0, SPSS, Chicago, IL, USA).
Results
Prospective Neuropsychological Evaluation of Study Groups at 3, 6, and 12 Months
The demographic and baseline clinical characteristics of the participants as a total cohort are summarized in Table 1, while Table 2 differentiates these characteristics according to treatment group. Our four study groups were practically comparable in terms of age, educational level, gender, and depressive symptoms (GDS scale), but aniracetam‐treated patients (group 2, n = 58) had a better baseline performance in MMSE and FRSSD scales, and were more commonly diagnosed with MCI compared to the other study groups.
Table 1.
Demographic and baseline clinical characteristics of the participants (N = 269 patients with cognitive disorders of variable severity)
| Age (in years) | 71 ± 8 |
| Male‐to‐female ratio | 95/174 |
| Years of educationa | 6 [4–6] |
| Mild cognitive impairment, n (%) | 33 (12.3) |
| Alzheimer's disease, n (%) | 201 (74.7) |
| Other forms of dementia,b n (%) | 35 (13) |
| MMSE score | 17.7 ± 6.6 |
| FRSSD score a | 8 [5–14] |
| GDS scorea | 3 [1–5.25] |
| NPI scorea | 8 [3–15] |
| ChEIs monotherapy, n (%) | 68 (25.3) |
| Aniracetam monotherapy, n (%) | 58 (21.6) |
| Combined treatment, n (%) | 68 (25.3) |
| No treatment, n (%) | 75 (27.8) |
Data are expressed as mean values ± standard deviation for normally distributed data.
aSkewed data such as years of education, FRSSD, GDS and NPI scores, are presented as median values plus the interquartile range, given into square brackets.
bOther forms of dementia include vascular, mixed and frontotemporal dementia diagnosed with DSM‐IV criteria of the American Psychiatric Association.
MMSE, Mini‐Mental State Examination; FRSSD, Functional Rating Scale for Symptoms of Dementia; GDS, Geriatric Depression Scale; NPI, Neuropsychiatric Inventory; ChEIs, Cholinesterase Inhibitors.
Table 2.
Demographic and clinical characteristics of the four study groups
| Baseline characteristics | ChEIs monotherapy | Aniracetam monotherapy | Combined treatment | No treatment | P significance |
|---|---|---|---|---|---|
| group 1 | group 2 | group 3 | group 4 | ||
| (n = 68) | (n = 58) | (n = 68) | (n = 75) | ||
| Age (years) | 71 ± 8 | 73 ± 8 | 74 ± 7 | 69 ± 8 | NS |
| Years of educationa | 6 [4–6] | 6 [5–12] | 6 [3–6] | 6 [5–6] | NS |
| MMSE score | 17.4 ± 5.1 | 23.5 ± 5 | 17.5 ± 5.8 | 13.7 ± 6.4 | * |
| FRSSD scorea | 11 [6–14] | 6 [3–8.8] | 9 [7–14] | 9 [5–19] | ** |
| GDS scorea | 2.5 [1–5] | 3 [1–5] | 2 [1–5.8] | 3.5 [1–7] | NS |
| NPI scorea | 8 [2.5–15.5] | 4 [0–10] | 9 [4–20] | Unavailable | *** |
| Male‐to‐female ratio (M/F) | 21/47 | 19/39 | 28/40 | 27/48 | NS |
| MCI, n (% within disease group) | 0 (0) | 26 (78.8) | 7 (21.2) | 0 (0) | ‡ |
| Alzheimer's disease, n (% within disease group) | 61 (30.3) | 22 (10.9) | 43 (21.4) | 75 (37.4) | ‡ |
| Other forms of dementia, n (% within disease group) | 7 (20) | 10 (28.6) | 18 (51.4) | 0 (0) | ‡ |
aSkewed data such as years of education, FRSSD, GDS, and NPI scores, are presented as median values plus the interquartile range, given into square brackets. For age and MMSE scores (normally distributed), data are presented as mean values ± SD.
*P≤ 0.001 for post hoc ANOVA comparisons (Bonferroni rule applied) between groups 1–2, 2–3, 1–4, 2–4, 3–4. Aniracetam‐treated patients (group 2) displayed the most favorable baseline cognitive profile (highest baseline MMSE score), while the no‐treatment group (group 4) displayed the worst. These differences were eliminated, when the analysis focused on patients with relatively mild‐to‐moderate cognitive impairment (15 ≤ MMSE ≤ 25).
**P= 0.02 for post hoc ANOVA comparison between groups 1 and 2, P= 0.007 for post hoc ANOVA comparison between groups 2 and 3. These differences were neutralized within the subpopulation of patients with baseline 15 ≤ MMSE ≤ 25.
***P= 0.02 for post hoc ANOVA comparison between groups 2 and 3. This difference was eliminated in patients with 15 ≤ MMSE ≤ 25.
‡P < 0.001 for prevalence of MCI between groups 2 and 1, 3, 4. The prevalence of MCI was significantly higher in patients treated with aniracetam compared to the other study groups. However, these patients (MMSE > 24) were not included in the final analysis focused on patients with 15 ≤ MMSE ≤ 25. As a result, the final study groups were well balanced in terms of the underlying diagnosis of cognitive disorder.
Figure 1 provides a schematic presentation of patients’ neuropsychological data at baseline, 3, 6, and 12 months of follow‐up, for every single treatment group (line graphs A–D).
Figure 1.
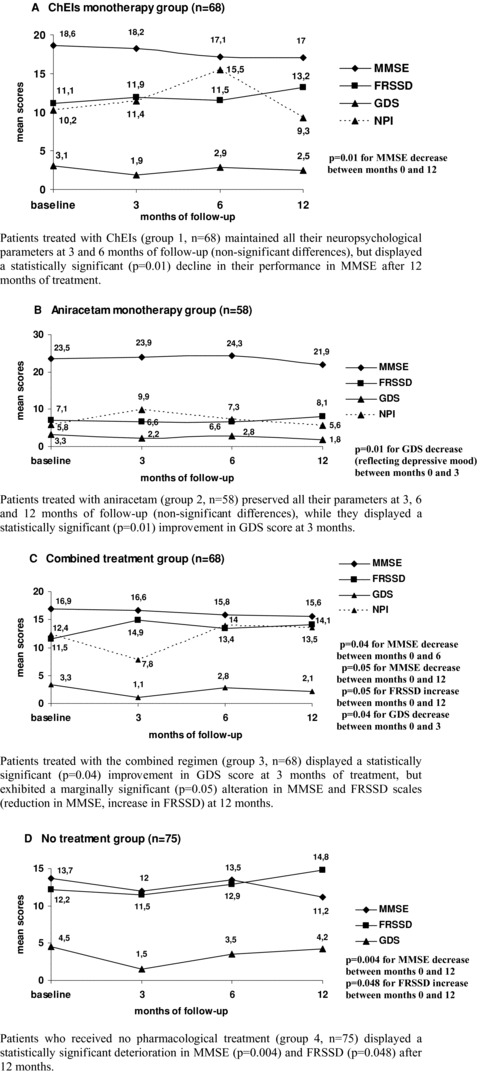
Prospective neuropsychological evaluation of all treatment groups across time. (A) ChEIs monotherapy group (n = 68): Patients treated with ChEIs (group 1, n = 68) maintained all their neuropsychological parameters at 3 and 6 months of follow‐up (nonsignificant differences), but displayed a statistically significant (P= 0.01) decline in their performance in MMSE after 12 months of treatment. (B) Aniracetam monotherapy group (n = 58): Patients treated with aniracetam (group 2, n = 58) preserved all their parameters at 3, 6, and 12 months of follow‐up (nonsignificant differences), while they displayed a statistically significant (P= 0.01) improvement in GDS score at 3 months. (C) Combined treatment group (n= 68): Patients treated with the combined regimen (group 3, n = 68) displayed a statistically significant (P= 0.04) improvement in GDS score at 3 months of treatment, but exhibited a marginally significant (P= 0.05) alteration in MMSE and FRSSD scales (reduction in MMSE, increase in FRSSD) at 12 months. (D) No treatment group (n= 75): Patients who received no pharmacological treatment (group 4, n = 75) displayed a statistically significant deterioration in MMSE (P= 0.004) and FRSSD (P= 0.048) after 12 months. MMSE, Mini‐Mental State Examination; FRSSD, Functional Rating Scale for Symptoms of Dementia; GDS, Geriatric Depression Scale; NPI, Neuropsychiatric Inventory. Note: An increase in FRSSD and GDS scores is interpreted as deterioration of patients’ functional and emotional (depression‐related) parameters, respectively.
The ChEIs monotherapy group (n = 68) remained stable in terms of cognitive, functional, and emotional parameters at 3 and 6 months of psychometric evaluation, but after 12 months of treatment, a statistically significant decline in their MMSE performance was interestingly noticed and reported (line graph A). More specifically, mean MMSE score in patients treated with ChEIs decreased from 18.6 ± 4.8 at baseline to 17 ± 6 at 12 months of treatment (P= 0.01), indicating a potentially deteriorating effect of ChEIs at 1 year of therapy, at least as reflected by MMSE scores.
In patients assigned to aniracetam monotherapy (n = 58), all examined parameters were adequately preserved after 6 and 12 months of treatment (P= nonsignificant for changes in MMSE, FRSSD, GDS, and NPI scores at 6 and 12 months), while emotional well‐being of aniracetam‐treated patients was substantially ameliorated at 3 months of monotherapy, as reflected by the significant decrease of GDS score from 3.3 to 2.2 (mean values) at 3 months of follow‐up (line graph B).
Patients who were prescribed combined treatment of both aniracetam and ChEIs (n = 68) exhibited a substantial improvement of their emotional state at 3 months of concurrent therapy, but there was a significant worsening of their cognitive performance (assessed by MMSE) at 6 months, as well as a marginally significant deterioration of MMSE and FRSSD parameters at 12 months (line graph C).
Last but not least, untreated patients (n = 75) demonstrated a considerable deterioration of their performance in MMSE and FRSSD scales, as shown by the statistically significant decrease in MMSE and increase in FRSSD at 12 months of follow‐up (line graph D), emphasizing the need for early therapeutic intervention (even as symptomatic management) in patients with dementia.
Since the initial cognitive state of participants assessed by their baseline MMSE score might have influenced the findings stated above, acting as a potential confounding factor, we secondarily studied patients with a baseline MMSE score between 15 and 25, representing relatively mild and moderate stages of dementia. Focusing our statistical analysis on this group of patients, who actually constituted the prevalent subpopulation in terms of severity staging (n = 151 patients with 15 ≤ MMSE ≤ 25 from a total of 276 enrolled patients), the following findings were observed: in patients treated with aniracetam, no significant mood enhancement could be confirmed at 3 months. However, there was a notable preservation of cognition, ADL, and neuropsychiatric symptoms in these patients, manifested by the nonsignificant changes in MMSE, FRSSD, GDS, and NPI scores at 3, 6, and 12 months. In the combined treatment group, the concurrent administration of a nootropic agent along with a ChEI was found to maintain patients’ performance in the examined scales at 3, 6, and 12 months, without exerting any adverse effects. Finally, patients who were prescribed only ChEIs demonstrated a significant deterioration of their MMSE performance after 12 months of treatment (decline of MMSE from 19.6 ± 3 to 18.4 ± 4.4, P= 0.02), similarly to the entire ChEIs monotherapy group, rendering this observation a rather consistent finding, which was practically independent from the severity of dementia.
Figure 2 is dedicated to the subgroup of 151 patients with relatively mild cognitive impairment and illustrates their prospective performance in MMSE, FRSSD, GDS, and NPI at 3, 6, and 12 months of follow‐up (line graphs A–D).
Figure 2.
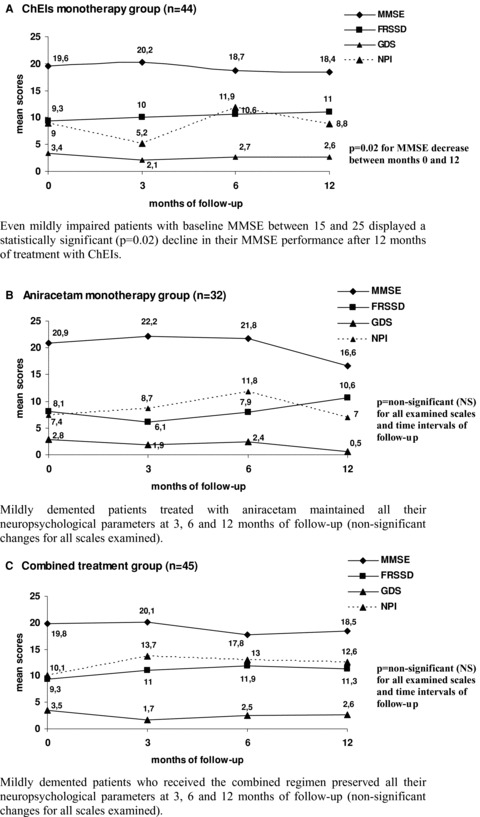
Follow‐up of 151 patients with cognitive disorders of relatively mild severity (MMSE: 15–25). (A) ChEIs monotherapy group (n = 44): Even mildly impaired patients with baseline MMSE between 15 and 25 displayed a statistically significant (P= 0.02) decline in their MMSE performance after 12 months of treatment with ChEIs. (B) Aniracetam monotherapy group (n = 32): Mildly demented patients treated with aniracetam maintained all their neuropsychological parameters at 3, 6, and 12 months of follow‐up (nonsignificant changes for all scales examined). (C) Combined treatment group (n= 45): Mildly demented patients who received the combined regimen preserved all their neuropsychological parameters at 3, 6, and 12 months of follow‐up (nonsignificant changes for all scales examined).
The more severely impaired patients of our cohort (MMSE ≤ 15) were 98. From these moderately to severely demented patients, 25 received ChEIs, only 4 received aniracetam, 21 received the combination, and 48 received no treatment. The number of patients treated with aniracetam was extremely small to allow any reliable statistical computations. Concerning the other treatment groups, we found no significant differences within and between treatment groups.
Comparative Evaluation of Efficacy between Studied Regimens
Aniracetam and ChEIs monotherapy groups were comparable regarding age, educational status, emotional state, and male‐to‐female ratio, but differed significantly in terms of baseline MMSE and FRSSD scores, with aniracetam‐treated patients presenting a more favorable cognitive and functional baseline profile (Table 2). Trying to counteract the confounding effect of different baseline status for the comparisons of efficacy at 3, 6, and 12 months of follow‐up, we focused our further analysis on the subpopulation with baseline MMSE between 15 and 25, namely patients with mild‐to‐moderate dementia that constituted the prevalent severity subgroup of our study population. This criterion was fulfilled by 151 patients, of whom 32 patients received aniracetam, 44 patients received ChEIs, 45 patients received the combination, and 30 patients received no drugs. With this adjustment, the two studied groups became fully comparable in terms of all potentially confounding parameters. Studying patients with 15 ≤ MMSE ≤ 25 facilitated direct and unbiased comparisons between aniracetam and ChEIs in terms of their short‐ and long‐term efficacy profile. These comparisons revealed a marginally significantly better performance in MMSE in the aniracetam group at 6 months of treatment (MMSE: 21.8 ± 3.6 vs. 18.7 ± 4.2, P= 0.05) and a significantly improved ADL at 3 months of treatment (FRSSD: 6.1 ± 4.6 vs. 10 ± 4.7, P= 0.04). For all the other scales and time intervals, no significant differences were reported.
When comparing aniracetam monotherapy with combined treatment (aniracetam and ChEIs), there was also a significant heterogeneity in terms of baseline performance in MMSE and FRSSD scales. We focused thus once again on patients with 15 ≤ MMSE ≤ 25, neutralized baseline differences and proceeded to direct comparisons between aniracetam monotherapy and combined treatment. Based on these comparisons between 32 patients treated with aniracetam and 45 patients treated with the combination, aniracetam performed significantly better in the cognitive scale at 6 months (P= 0.02), while patients treated with aniracetam displayed a notable tendency for enhanced mood at 12 months and improved ADL at 6 months (P= 0.08).
The final comparison was performed between the ChEIs monotherapy group (n = 68) and the combination group (n = 68). These two groups were significantly different in terms of baseline NPI scores, so we used once again the subgroup of mildly demented patients for the comparative evaluation of clinical efficacy between ChEIs and combined regimen. No statistically significant differences in terms of MMSE performance, ADL, and depressive mood at 3, 6, and 12 months could be observed, indicating comparable effectiveness of these two regimens in patients with relatively mild dementia.
It is noteworthy that in all comparisons described above, no significant gender‐related differentiation could be ascertained, indicating that the gender of patients with dementia had no clinically meaningful impact on their therapeutic responsiveness to assigned treatment of either type (nootropic or ChEI).
Figure 3 presents schematically a comparative evaluation of our three treatment groups (ChEIs, aniracetam, combination) in terms of patients’ neuropsychological parameters at 3, 6, and 12 months of follow‐up (bar charts A–D).
Figure 3.
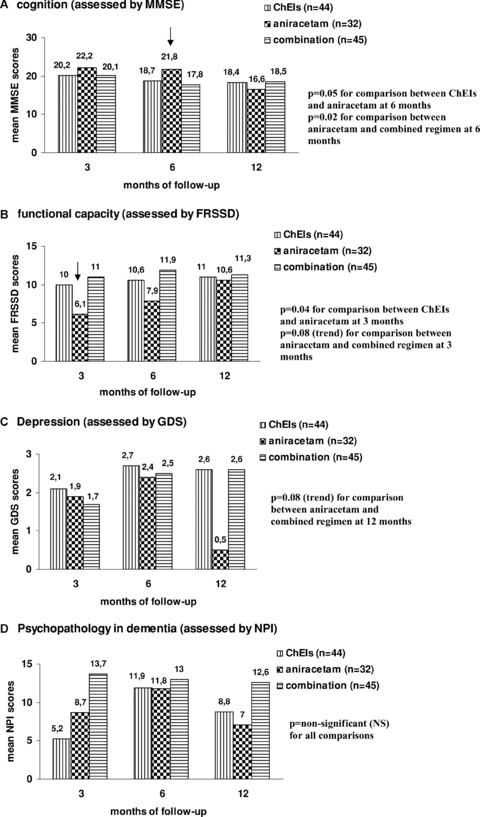
Comparative evaluation of performance in MMSE, FRSSD, GDS, and NPI among treatment groups at 3, 6, and 12 months of follow‐up. (Subgroup of patients with relatively mild cognitive disorders: N = 151) (A) Cognition (assessed by MMSE), (B) functional capacity (assessed by FRSSD), (C) Depression (assessed by GDS), and (D) Psychopathology in dementia (assessed by NPI). MMSE, Mini‐Mental State Examination; FRSSD, Functional Rating Scale for Symptoms of Dementia; GDS, Geriatric Depression Scale; NPI, Neuropsychiatric Inventory.
Summary of Treatment‐Related Side Effects
The study revealed no serious safety concerns related to any of the treatment regimens examined. Aniracetam was generally well tolerated and there was no discontinuation due to drug‐related adverse effects. The most commonly reported side effects of aniracetam were mild anxiety, irritability, and insomnia, which were however transient and self‐limiting and were no reason for treatment cessation. As far as ChEIs are concerned, the most commonly reported side effects included nausea, vomiting, and muscle cramps. Two patients receiving ChEIs discontinued treatment because of gastrointestinal disorders and symptomatic bradycardia, respectively. The patient with bradycardia was also treated with cardiovascular drugs.
Discussion
The present study evaluated prospectively a considerable number of community‐dwelling patients with cognitive decline, being allocated to three different treatment groups: aniracetam monotherapy, ChEIs monotherapy, and combined treatment with a ChEI and a nootropic compound. Direct comparisons have been also conducted among treatment groups in terms of patients’ short‐ and long‐term performance in simple and easily administered neuropsychological scales such as MMSE, FRSSD, GDS, and NPI. Given the currently limited therapeutic options for dementia, our findings substantiate the commonly accepted perception that treatment of cognitive disorders constitutes a challenging area of investigation, since data supporting the clinical superiority of a specific drug category are rather heterogeneous and contradictory.
In our cohort, patients treated with ChEIs displayed interestingly a significant deterioration in their MMSE performance after 12 months of treatment. One might assume that this unfavorable effect is possibly attributable to the increased severity of baseline cognitive impairment of these patients, since most ChEIs are clinically indicated for mild‐to‐moderate dementia (with the exception of donepezil and NMDA receptor antagonist memantine). However, when we focused on the subgroup of patients with 15 ≤ MMSE ≤ 25, namely patients with mild‐to‐moderate severity of cognitive impairment, we observed again that patients treated with ChEIs displayed a significantly worse 12‐month MMSE performance compared to baseline.
Since the introduction of the first ChEI (tacrine) in 1994, most clinicians and patients consider the cholinergic drugs (donepezil, rivastigmine, galantamine) to be the first‐line pharmacotherapy for mild‐to‐moderate AD. These drugs have slightly different pharmacological properties, but they all work by inhibiting the breakdown of intrasynaptic acetylocholine, a crucial neurotransmitter associated with memory, by blocking the enzymes acetylcholinesterase or butyrylocholinesterase into the synaptic clefts [10]. Despite the slight variations in the mode of action of the three ChEIs, there is no convincing evidence of any clinically meaningful differences between them with respect to efficacy [10, 22]. These drugs have proven their efficacy in modifying the clinical manifestations of mild‐to‐moderate AD, and have shown to significantly delay global cognitive impairment and loss of ADL associated with AD, for at least 6 months [10, 12, 22]. A large number of international well‐designed (multicenter, randomized, placebo‐controlled, double‐blind) studies have established the role of ChEIs as first‐line symptomatic treatment for patients with AD and other forms of dementia, such as vascular and mixed dementia. This is why our findings, regarding MMSE worsening in patients treated with ChEIs should be definitely viewed and interpreted with caution. A possible explanation for these findings might be the fact that we used a moderately sensitive and rather crude neuropsychological tool for cognitive assessment (MMSE), which might not reflect with absolute reliability the real cognitive status of examined patients. On the other hand, our department has a long‐standing considerable clinical experience with this scale and has consistently found it to correlate well with cognitive outcomes in patients with dementia, and most importantly with the progression of their cognitive performance across time. Nevertheless, there is still possibility that MMSE might not be able to adequately reflect all clinically relevant cognitive domains of our study population. The deteriorating effect of ChEIs observed in our study was based on a single cognitive scale of moderate sensitivity, and should not be interpreted as an overall worsening of all patients’ cognitive domains. Besides, some degree of further cognitive deterioration can be observed regardless of symptomatic treatment and it is therefore hard to establish a causal association. On the other hand, there are still some concerns regarding the use of ChEIs in everyday clinical practice, which are mainly related to their long‐term clinical efficacy [12], their cost‐effectiveness in terms of health economics [23], and their safety profile, especially in elderly demented patients with serious comorbidity and polypharmacy (fear for pharmacodynamic and pharmacokinetic interactions) [24]. The final conclusive answer as to whether ChEIs constitute a cost‐effective treatment for patients with AD (as indicated by several reviews and meta‐analyses) may be given only by additional large‐scale studies, examining both economic data and parameters of clinical efficacy.
As far as aniracetam is concerned, patients treated with this agent presented an adequate maintenance of their overall neuropsychological parameters at 6 and 12 months. They also presented a significantly improved emotional profile at 3 months (assessed by GDS), which was however not sustained at 6 and 12 months. A possible reason for this lack of significance at 12 months might be the relatively small number of patients treated with aniracetam, which is why further studies in larger numbers of demented patients are required. The fact that we found differences in GDS, but not in NPI, indicates that aniracetam might provide a mood‐enhancing benefit, while we found no evidence to support its role in alleviating other aspects of dementia psychopathology such as apathy, agitation, and anxiety. Another interesting finding of our study was the better 6‐month MMSE performance observed in aniracetam‐treated patients, compared with patients treated with ChEIs, within the subpopulation of patients with 15 ≤ MMSE ≤ 25. Contrary to these findings, in a previous study by Tsolaki et al., comparing nootropics with ChEIs, it was suggested that for patients with mild dementia, ChEIs performed better than nootropics in terms of cognitive performance [25]. However, in the same study, nootropics proved superior to ChEIs in moderate dementia at 12 months, while no significant differences were observed in the overall study population and in patients with severe dementia [25]. These results corroborate the findings of the present study, regarding comparable long‐term effects between aniracetam and ChEIs, especially for patients with mild‐to‐moderate cognitive impairment.
Aniracetam has been clinically evaluated for its cognition enhancing effects in patients with cognitive disorders with pretty encouraging results [9, 13, 26]. Results from trials in elderly patients with mild‐to‐moderate dementia of Alzheimer type suggest that aniracetam may be of clinical benefit, with further trials required, in order to confirm its efficacy profile, and to define more precisely those patients who are most likely to respond to treatment with nootropics [13]. Aniracetam at dose 1500 mg/day exhibits an excellent tolerability profile, and has proved to be more effective than placebo and clinically superior to piracetam [13]. Furthermore, based on preliminary evidence in the treatment of patients with dementia of cerebrovascular origin, aniracetam appears to be a promising therapeutic option in vascular dementia, as well [13, 26].
Its pleiotropic mechanism of action, promoting synaptogenesis, neuroprotection, and enhanced synaptic plasticity, might be in part responsible for both its cognitive and antidepressant actions. Aniracetam is a pyrrolidinone‐containing nootropic compound, which behaves as a dual allosteric positive modulator of AMPA‐sensitive and metabotropic glutamate receptors in a variety of systems, including intact brain tissue and cultured neurons [27]. The experimentally observed potentiation of glutamatergic activity by aniracetam provides the molecular explanation for the clinical efficacy of nootropic agents as cognition enhancers [27]. In addition to directly enhancing glutamatergic synaptic transmission, aniracetam activates the nicotinic receptors of acetylocholine in brain neurons, restoring partially the deficient cholinergic neurotransmission that constitutes the fundamental functional defect in AD [28, 29]. Experimental studies in rats have disclosed additional neurobiological actions of aniracetam, such as an indirect augmentation of dopaminergic neurotransmission through its cholinergic activity [30, 31], an increased expression of neurotrophic and neuroprotective factors such as BDNF (Brain‐Derived Neurotrophic Factor) and an enhanced synaptic transmission [32]. The experimental finding of increased BDNF levels after exposure to aniracetam might form the scientific basis for the significant antidepressant effect of aniracetam that was also manifested in the present study. At present, there are relatively scarce clinical data concerning the potential memory stabilizing effects of aniracetam in patients with dementia and MCI. On the other hand, recent discoveries in the behavioral pharmacology provide new indications for aniracetam in the treatment of various CNS disorders including impulsiveness, fear and anxiety, depression, posttraumatic stress disorder, sleep disorders, and cardiovagal abnormalities [14, 33]. Far more clinical trials are definitely needed in order to validate these new promising indications.
Concerning the combined treatment, we observed a notable decline in patients’ MMSE and FRSSD parameters at 6 and 12 months. However, when we restricted our analysis to patients with mild‐to‐moderate dementia, this striking difference disappeared, indicating that this paradoxical long‐term deterioration was possibly influenced by the poor initial cognitive state of patients. Interestingly, the combination of ChEIs with a nootropic agent appears to affect favorably long‐term outcomes of patients with relatively mild‐to‐moderate dementia, and this finding might have important clinical implications.
In the present study, we chose not to use a prespecified range of MMSE as an inclusion criterion, because we aimed to evaluate patients at several stages of cognitive impairment, being treated either with ChEIs, or with aniracetam, or with the combination. As a result, we included in our analysis both severely and mildly to moderately impaired patients. However, when it came to direct comparisons between treatment groups in terms of short‐ and long‐term clinical efficacy, we selected a representative subpopulation of our cohort, consisting of 151 demented patients with 15 ≤ MMSE ≤ 25, who were mainly consistent of mildly to moderately affected patients. Our findings, regarding the positive outcomes of aniracetam‐treated patients compared to ChEIs and combined treatment at 6 and 12 months, concern patients within the specific MMSE range, and cannot be extrapolated to more severely compromised patients. Furthermore, it cannot be precluded that the preservation of cognitive function observed with aniracetam might be partly mediated by its potential psychotropic or antidepressant effects.
Our study has several limitations inherent to its open‐label nature. First of all, it was not placebo‐controlled, introducing a bias related to the no‐treatment group. These patients had been never treated for dementia (unwilling to do so) or had decided themselves to discontinue treatment at least 2 years before enrollment. Since these patients had a mean MMSE score of 13.7, namely advanced cognitive impairment, it was considered relatively unethical to provide them with a placebo agent. On the other hand, it was useful to have them as a control arm in our study, in order to confirm that if patients with AD are left unassisted in the fight against dementia, they are highly likely to deteriorate in both cognitive and functional parameters within a year, emphasizing the need for early supportive treatment of any type. Furthermore, we had to exclude seven subjects from the analysis, who could not be properly classified into a specific treatment group, since they switched therapy at an intermediate time of follow‐up. Considering that these patients were well balanced in terms of their baseline characteristics (comparable demographics, type of cognitive impairment, severity of dementia), reasons for switch and initial regimen, their removal from the analysis was not expected to have considerable impact on the results of our study. In addition, we could have used a more comprehensive battery of neuropsychological tools for evaluation of patients. We selected instead four simple, easy to perform, and representative scales, driven by the peculiarities of the Greek demented population and the ability of our clinical neuropsychologists to administer the specific scales rapidly, and with significant accuracy and reproducibility. The relatively small number of patients assigned to every treatment group is also a further limitation. Despite the existing methodological limitations, we believe that our study reveals some trends that might have important clinical implications, if confirmed by further large‐scale studies. Despite the wealth of experimental evidence regarding the neurobiological actions of aniracetam, the clinical studies investigating its therapeutic potential are limited. The present study is trying to bridge—to some extent—the existing gap in scientific literature, and provide the stimulus for further researchers to test with prospective better designed studies the potential effects of aniracetam in large numbers of patients with dementia.
Given the open‐label nature of our study, caution is warranted for a careful interpretation of our findings and no safe conclusions can be drawn. However, based on our preliminary findings, we conclude that aniracetam, a nootropic agent with an AMPA‐potentiating and neuroprotective mechanism of action, should be tested in further clinical studies in order to prove, if it finally deserves any place in the therapeutic armamentarium against cognitive disorders. Its safety profile appears promising; its efficacy remains to be determined.
Conflict of Interest
The authors declare no financial conflicts of interest related to this article.
Authors’ Contribution
Chrysi C. Koliaki: Manuscript preparation, data analysis/interpretation, and literature review; Chaido Messini: Data collection and approval of article; Magda Tsolaki: Concept/design, data interpretation, data collection, and approval and critical review of the article.
Acknowledgments
We would like to thank wholeheartedly the caregivers of all demented patients who took part in our study, for ensuring patients’ compliance with the assigned treatment regimens. We are grateful to them for their constructive cooperation and their invaluable contribution to the completion of the study protocol.
Preliminary data from the Hellenic AMNESIA Study: Aniracetam MoNotherapy compared with cholinEsterase inhibitors Study In Alzheimer’s.
References
- 1. Alzheimer's Association .2009 Alzheimer's disease facts and figures. Alzheimers Dement 2009;5:234–270. [DOI] [PubMed] [Google Scholar]
- 2. Rice DP, Fillit HM, Max W, Knopman DS, Lloyd JR, Duttagupta S. Prevalence, costs, and treatment of Alzheimer's disease and related dementia: A managed care perspective. Am J Manag Care 2001;7:809–818. [PubMed] [Google Scholar]
- 3. Hurt CS, Banerjee S, Tunnard C, et al Insight, cognition and quality of life in Alzheimer's disease. J Neurol Neurosurg Psychiatry 2009;81:331–336. [DOI] [PubMed] [Google Scholar]
- 4. Banerjee S, Samsi K, Petrie CD, Alvir J, Treglia M, Schwam EM, del Valle M. What do we know about quality of life in dementia? A review of the emerging evidence on the predictive and explanatory value of disease specific measures of health related quality of life in people with dementia. Int J Geriatr Psychiatry 2009;24:15–24. [DOI] [PubMed] [Google Scholar]
- 5. Tsuno N, Homma A. What is the association between depression and Alzheimer's disease? Expert Rev Neurother 2009;9:1667–1676. [DOI] [PubMed] [Google Scholar]
- 6. Gillioz AS, Villars H, Voisin T, et al; for the REAL.FR Group . Spared and impaired abilities in community‐dwelling patients entering the severe stage of Alzheimer's disease. Dement Geriatr Cogn Disord 2009;28:427–432. [DOI] [PubMed] [Google Scholar]
- 7. Vellone E, Piras G, Talucci C, Cohen MZ. Quality of life for caregivers of people with Alzheimer's disease. J Adv Nurs 2008;61:222–231. [DOI] [PubMed] [Google Scholar]
- 8. Mesterton J, Wimo A, By A, Langworth S, Winblad B, Jönsson L. Cross sectional observational study on the societal costs of Alzheimer's disease. Curr Alzheimer Res 2010;7:358–367. [DOI] [PubMed] [Google Scholar]
- 9. Senin U, Abate G, Fieschi C, et al Aniracetam (Ro 13–5057) in the treatment of senile dementia of Alzheimer type (SDAT): Results of a placebo controlled multicentre clinical study. Eur Neuropsychopharmacol 1991;1:511–517. [DOI] [PubMed] [Google Scholar]
- 10. Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database Syst Rev 2006;1:CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takeda A, Loveman E, Clegg A, Kirby J, Picot J, Payne E, Green C. A systematic review of the clinical effectiveness of donepezil, rivastigmine and galantamine on cognition, quality of life and adverse events in Alzheimer's disease. Int J Geriatr Psychiatry 2006;21:17–28. [DOI] [PubMed] [Google Scholar]
- 12. Wolfson C, Oremus M, Shukla V, Momoli F, Demers L, Perrault A, Moride Y. Donepezil and rivastigmine in the treatment of Alzheimer's disease: A best‐evidence synthesis of the published data on their efficacy and cost‐effectiveness. Clin Ther 2002;24:862–886. [DOI] [PubMed] [Google Scholar]
- 13. Lee CR, Benfield P. Aniracetam . An overview of its pharmacodynamic and pharmacokinetic properties, and a review of its therapeutic potential in senile cognitive disorders. Drugs Aging 1994;4:257–273. [DOI] [PubMed] [Google Scholar]
- 14. Nakamura K. Aniracetam: Its novel therapeutic potential in cerebral dysfunctional disorders based on recent pharmacological discoveries. CNS Drug Rev 2002;8:70–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Endo H, Tajima T, Yamada H, et al Pharmacokinetic study of aniracetam in elderly patients with cerebrovascular disease. Behav Brain Res 1997;83:243–244. [DOI] [PubMed] [Google Scholar]
- 16. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 17. Folstein MF, Folstein SE, McHugh PR: ‘Mini‐ Mental State’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 18. Fountoulakis K, Tsolaki M, Chantzi H, Kazis A. Mini Mental State Examination. A validation study in elderly demented patients in Greece. Enkephalos (Greece) 1994;31:93–102. [Google Scholar]
- 19. Hutton TJ: Alzheimer's disease In: Rakel RE, editor. Conn's current therapy. Philadelphia : WB Saunders, 1990; 780 p. [Google Scholar]
- 20. Fountoulakis KN, Tsolaki M, Iacovides A, Yesarage J, O’Hara R, Kazis A, Ierodiaconou C. The validation of the short form of the Geriatric Depression Scale (GDS) in Greece. Aging (Milano) 1999;11:367–372. [DOI] [PubMed] [Google Scholar]
- 21. Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308–2314. [DOI] [PubMed] [Google Scholar]
- 22. López‐Pousa S, Turon‐Estrada A, Garre‐Olmo J, et al Differential efficacy of treatment with acetylcholinesterase inhibitors in patients with mild and moderate Alzheimer's disease over a 6‐month period. Dement Geriatr Cogn Disord 2005;19:189–195. [DOI] [PubMed] [Google Scholar]
- 23. Loveman E, Green C, Kirby J, Takeda A, Picot J, Payne E, Clegg A. The clinical and cost‐effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer's disease. Health Technol Assess 2006;10:1–160. [DOI] [PubMed] [Google Scholar]
- 24. Bordier P, Garrigue S, Lanusse S, Margaine J, Robert F, Gencel L, Lafitte A. Cardiovascular effects and risk of syncope related to donepezil in patients with Alzheimer's disease. CNS Drugs 2006;20:411–417. [DOI] [PubMed] [Google Scholar]
- 25. Tsolaki M, Pantazi T, Kazis A. Efficacy of acetylcholinesterase inhibitors versus nootropics in Alzheimer's disease: A retrospective, longitudinal study. J Int Med Res 2001;29:28–36. [DOI] [PubMed] [Google Scholar]
- 26. Canonico V, Forgione L, Paoletti C, et al Efficacy and tolerance of aniracetam in elderly patients with primary or secondary mental deterioration. Riv Neurol 1991;61:92–96. [PubMed] [Google Scholar]
- 27. Nicoletti F, Casabona G, Genazzani AA, Copani A, Aleppo G, Canonico PL, Scapagnini U. Excitatory amino acids and neuronal plasticity: Modulation of AMPA receptors as a novel substrate for the action of nootropic drugs. Funct Neurol 1992;7:413–422. [PubMed] [Google Scholar]
- 28. Nakamura K, Shirane M. Activation of the reticulothalamic cholinergic pathway by the major metabolites of aniracetam. Eur J Pharmacol 1999;380:81–89. [DOI] [PubMed] [Google Scholar]
- 29. Shirane M, Nakamura K. Group II metabotropic glutamate receptors are a common target of N‐anisoyl‐GABA and 1S,3R‐ACPD in enhancing ACh release in the prefrontal cortex of freely moving SHRSP. Neuropharmacology 2000;39:866–872. [DOI] [PubMed] [Google Scholar]
- 30. Nakamura K, Shirane M, Koshikawa N. Site‐specific activation of dopamine and serotonin transmission by aniracetam in the mesocorticolimbic pathway of rats. Brain Res 2001;897:82–92. [DOI] [PubMed] [Google Scholar]
- 31. Shirane M, Nakamura K. Aniracetam enhances cortical dopamine and serotonin release via cholinergic and glutamatergic mechanisms in SHRSP. Brain Res 2001;916:211–221. [DOI] [PubMed] [Google Scholar]
- 32. O’Neill MJ, Bleakman D, Zimmerman DM, Nisenbaum ES. AMPA receptor potentiators for the treatment of CNS disorders. Curr Drug Targets CNS Neurol Disord 2004;3:181–194. [DOI] [PubMed] [Google Scholar]
- 33. Kihara M, Nishikawa S, Nakasaka Y, Tanaka H, Takahashi M. Autonomic consequences of brainstem infarction. Auton Neurosci 2001;86:202–207. [DOI] [PubMed] [Google Scholar]


