Summary
Aims
To explore the role and underlying mechanism of miR‐124 in stroke.
Methods
miR‐124 expression was determined by real‐time PCR. The effect of miR‐124 on infarct area was assessed in middle cerebral artery occlusion (MCAO) mice. The influence of miR‐124 on oxygen and glucose deprivation (OGD) induced neuron apoptosis and death was examined by immunofluorescence. The effect of miR‐124 on apoptosis‐related proteins was determined by Western blot.
Results
The level of miR‐124 is significantly increased in ischemic penumbra as compared with that in nonischemic area of MACO mice. Brain tissue of stroke‐prone spontaneously hypertensive rats (SHR‐SP) also showed higher level of miR‐124 as compared with that of spontaneously hypertensive rats (SHR). Consistently, OGD treatment obviously increased miR‐124 level in primary neurons. In vivo, miR‐124 overexpression significantly decreased, while miR‐124 knockdown significantly increased, the infarct area of MCAO mice. In vitro, gain or loss of miR‐124 function resulted in reduced or increased neuron apoptosis and death induced by OGD, and increased or reduced antiapoptosis protein, Bcl‐2 and Bcl‐xl, respectively.
Conclusions
miR‐124 plays a neurons‐protective role via apoptosis‐inhibiting pathway in ischemic stroke.
Keywords: Apoptosis, MicorRNA‐124, Neuron, Stroke
Introduction
Stroke represents a major public health problem. It is one of the leading causes of death and adult disability worldwide. One in six people worldwide will have at least one stroke in their lifetime. Ischemic stroke accounts for 85% of all strokes 1, 2, 3, 4. Extensive research has demonstrated that cerebral ischemia could trigger a cascade of pathological events, and eventually lead to irreversible apoptotic and necrotic neuronal death in the ischemic regions 5, 6, 7. Although sustained effort has been made in exploring the mechanisms of ischemic cerebral stroke, further studies about the delicate regulation of stroke‐induced neuronal death and neurological dysfunction are still required.
Noncoding (nc) RNAs represent approximately 98% of the transcriptional output in the eukaryotic genome and are currently considered as the master controllers of the transcription and translation that decides the organ‐ and cell‐specific protein repertoire 8. miRNAs (18–25 nucleotides) are the most studied of all classes of ncRNAs. miRNAs modulate protein expression by binding to complementary or partially complementary target mRNAs, leading to their degradation or translation inhibition 9. It has been found that >20% of the miRNAs alter in the ischemic brain, indicating that miRNAs have emerged as key mediators in both pathogenic and pathological aspects of ischemic stroke biology10, 11, 12, 13. Identification of these miRNAs could provide novel therapeutic targets for ischemic stroke.
miR‐124, almost exclusively expressed in the central nerve system and neuronal cells, are 100 times higher than in other organs 14. It was perfectly conserved at the nucleotide level from worms to humans and expressed throughout the embryonic and adult CNS 15. miR‐124 has been found to regulate neuronal differentiation during CNS development and adult neurogenesis 16, 17, 18. Recently, Weng et al. found that plasma concentration of miR‐124 was significantly elevated at 6 h and remained elevated at 48 h after middle cerebral artery occlusion (MCAO) in the rat, suggesting that plasma miR‐124 may act as a candidate biomarker for early detection of cerebral infarction 19, 20. We speculate that plasma miR‐124 is released from the infarcted brain tissue, and miR‐124 may play an important role in cerebral ischemic stroke.
In this study, we found miR‐124 level was significantly elevated, in ischemic penumbra as compared with that in nonischemic area in MACO mice, in brain tissue of SHR‐SP as compared with that of SHR, and in primary neurons after OGD treatment. In vivo and in vitro, studies demonstrated that miR‐124 protect against cerebral infarction in MACO mice and apoptosis in primary OGD‐triggered neurons. This role is possibly mediated by the upregulation of miR‐124 on antiapoptosis protein, Bcl‐2, and Bcl‐xl. The study provides new insights into the understanding of cerebral protection and suggests miR‐124 may act as a neuroprotective agent for stroke therapeutics.
Materials and Methods
Reagents
The miR‐124 agomir, antagomir, and their negative control were from RiboBio (Guangzhou, China). The antisense miR‐124 vivo‐morpholino (5′‐GGCATTCACCGCGTGCCTTAATTGT‐3′) and the control vivo‐morpholino (5′‐GGCAATGACCCCGTCCCTTAATTCT‐3′) were from Gene Tools (Philomath, OR, USA). Anti‐GAPDH antibodies were from Protein Tech (Chicago, IL, USA). Anticaspase 8 antibody, anti‐cleaved caspase 8 antibody, anti‐Bad antibody, anti‐Bax antibody, anti‐Bcl‐xl antibody, and anti‐Bcl‐2 antibody were from Cell Signaling (Danvers, MA, USA).
Animals
C57BL/6 mice (18–22 g) were purchased from Sino‐British SIPPR/BK Laboratory Animals (Shanghai, China). Male SHR‐SP and SHR rats were provided by the animal center of Second Military Medical University. Animals were housed in controlled conditions (temperature 23 ± 2°C, humidity 60 ± 10%, lighting 8 a.m. to 8 p.m., free access to water, and a standard rodent diet). All animal experiments were performed in accordance with the National Institute of Health's “Guide for the Care and Use of Laboratory Animals”, with the approval of the Scientific Investigation Board of Second Military Medical University, China.
Middle Cerebral Artery Occlusion (MCAO)
C57BL/6 mice underwent MCAO by electrocoagulation as described previously 21. Briefly, mice were anesthetized with 15% chloral hydrate. After a 1‐cm skin incision between right eye and ear, a burr hole was drilled through the temporal skull. The dura mater was removed, and the middle cerebral artery was permanently occluded using a bipolar electrocoagulation forceps. Twenty‐four hours after MCAO, C57BL mice were analgesia sacrificed to get the brains. Serial coronal sections (1 mm apart) were stained with 2,3,5‐triphenyltetrazolium chloride monohydrate (TTC; Sigma‐Al‐drich, St. Louis, MO, USA) for assessing infarct area. Sections were digitized, and the area of infarct and noninfarct tissues was calculated by the software ImageJ (NIH, Bethesda, MD, USA) 22.
Primary Neuron Culture
Primary cultured neurons from cortices derived from embryonic day 16–18 C57BL/6 mice were prepared according to previously established protocols 23. Briefly, neurons were isolated from cerebral cortices of fetus C57BL/6. The cerebral cortices of fetus mice were dissected out under microscope. The meninges were removed followed by digestion of the cortices with 0.25% trypsin (GIBCO, Grand Island, NY, USA). Cells were cultured on poly‐D‐lysine‐coated plates at a density of 1 × 106 cells/ml in DMEM medium (GBICO) with 10% fetal bovine serum (FBS; GBICO). After 4–6 h, the cultures were replenished with Neurobasal medium (GIBCO) supplemented with 2% B27, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.5 mM glutamine (GIBCO), in incubator at 37°C with 5% CO2. The medium was changed every 3 days. All experiments were performed 1 week after isolation.
Apoptosis Detection
Cell apoptosis and death were examined by Annexin V‐FITC Apoptosis Detection Kit (Keygen Biotech, Nanjing, China). Late apoptotic or necrotic cells were labeled red with the PI staining. Images were acquired under a fluorescent microscope (IX‐7; Olympus, Tokyo, Japan) with 12.8 M pixel recording digital color‐cooled camera (DP72; Olympus). Death and apoptosis rate was calculated as the PI‐positive cells divided by the total number of cells counted 23.
Oxygen and Glucose Deprivation (OGD)
Cultured neurons underwent ischemia‐like conditions via exposure to OGD by transfer to glucose‐free DMEM medium in a box gassed with 94% N2, 5% CO2, and 1% O2 at 37°C for 6 or 12 h. Control neuron cultures were grown in DMEM containing glucose (25 mM) and incubated under normal culture conditions for the same period 22.
RNA Quantification
Total RNA, containing miRNAs, was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following manufacturer's instructions. For miR‐124 analysis, Taqman MicroRNA Assays (ABI, Carlsbad, CA, USA) were used. The relative expression level of miR‐124 was normalized to that of internal control sno234 using a 2‐ΔΔCt cycle threshold method.
Immunoblotting
Western blot analysis was performed as described in a previously report 22 with some modifications. Briefly, neurons were lysed with M‐PER protein extraction reagent (Pierce, Rockford, IL, USA) supplemented with the protease inhibitor mixture (CalBiochem, San Diego, CA, USA). After centrifugation, proteins in the supernatant were separated through SDS‐PAGE and transferred onto nitrocellulose membranes. Immunoblottings were performed as described previously. The images were captured and analyzed by the Odyssey infrared fluorescence imaging system (Li‐Cor Bioscience, Lincoln, NE, USA). The data were analyzed using ImageJ software (NIH).
Statistics
Data are represented as mean ± SD. Statistical significance was determined using a Student's t‐test. For measurement data, whether the variances were standard normal distributions was judged by the Bartlett test. When the data were standard normal distributions, the F‐test (ANOVA, two‐sided) was performed to evaluate the variances. P‐values of <0.05 were deemed to be significantly different.
Results
MiR‐124 Expression is Upregulated in Brain Tissue of Experimental Stroke Mice, SHR‐SP Rat, and OGD‐treated Neurons
To confirm miR‐124 alteration, experimental model of stroke was used. Twenty‐four hours later after MCAO in mice, the miR‐124 level of brain tissue was measured by real‐time PCR. As shown in Figure 1A, the level of miR‐124 in the ischemic penumbra was nearly three times higher than that in nonischemic area.
Figure 1.
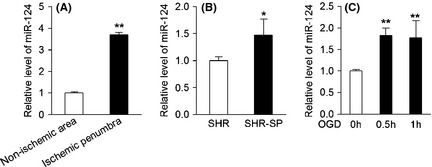
MiR‐124 expression is upregulated in the brain tissue of experimental stroke mice, SHR‐SP rat, and OGD‐treated neurons. (A) Real‐time PCR detection of miR‐124 in ischemic penumbra and nonischemic area of C57BL/6 mice 24 h after MCAO (n = 6, **P < 0.01). (B) Real‐time PCR detection of miR‐124 in the brain tissue of male SHR or SHR‐SP aged 5 months (n = 4, *P < 0.05). (C) Real‐time PCR detection of miR‐124 in primary neurons with or without OGD exposure (n = 3, **P < 0.01). Data are represented as mean ± SD.
Stroke‐prone spontaneously hypertensive rats is another commonly used animal model of stroke. These rats develop a stroke at a mean age of 10 (males) to 14 months (females) in our laboratory 24. A comparison of SHR‐SP with SHR would provide objective assessment for the pathogenesis of stroke, with ruling out the effect of hypertension. As shown in Figure 2B, the miR‐124 level in brain tissue of SHR‐SP rat was significantly increased by approximately 50% as compared with that of SHR rat.
Figure 2.
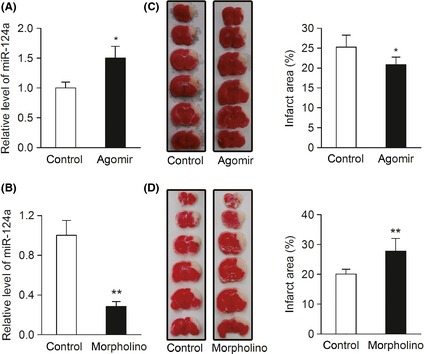
MiR‐124 protects against acute ischemic cerebral injury in MCAO mice. (A, B) Real‐time PCR detection of miR‐124 in the brain tissues of C57BL/6 mice 3 days after tail‐vein injection with miR‐124 agomir, vivo‐morpholino, and their matched controls. (n = 3, *P < 0.05, **P < 0.01). (C) C57BL/6 mice were injected via tail‐vein with 100 nmol/kg of miR‐124a agomir or negative control for 3 days, and then underwent MCAO by electrocoagulation. 24 h after MCAO operation, infarct area was evaluated by TTC staining. (n = 7, *P < 0.05). Left, representative images of TTC‐stained brain sections; right, quantitative analysis of infarct area. (D) C57BL/6 mice were injected via tail‐vein with 2 mg/kg of antisense miR‐124 vivo‐morpholino or control vivo‐morpholino for 3 days, and then treated as described in (C). (n = 8, **P < 0.01). Data are represented as mean ± SD.
We have also used a cell culture model of stroke, oxygen, and glucose deprivation 25, 26, to validate the specific change of miR‐124 under ischemia condition. As expected, primary neurons showed elevated miR‐124 expression at 0.5 and 1 h after OGD (Figure 1C).
MiR‐124 Protects Against Acute Ischemic Cerebral Injury in MCAO Mice
To evaluate the role of miR‐124 in vivo, we examined the effect of miR‐124 on infarct area of MACO mice. Intravenous injection of miR‐124 agomir or vivo‐morpholino was performed to overexpression or knockdown miR‐124 in brain (Figure 2A, B). Quantitative assessment of TTC‐stained sections showed that overexpression of miR‐124 decreased the infarct area 24 hs after MCAO operation as compared with that in control group (Figure 2C), and knockdown of miR‐124 significantly increased the infarct area (Figure 2D). Together, these data indicate that miR‐124 provided protective effect in stroke.
MiR‐124 Inhibits OGD‐induced Neuronal Apoptosis and Death
Neuronal cell apoptosis and death are prominent features observed after stroke 27, 28. To explore the neuroprotective effect of miR‐124 in vitro, the effect of miR‐124 on cell death and late apoptosis (labelled red with PI staining) in OGD‐treated neurons was observed. As shown in Figure 3A, D, OGD treatment significantly increased PI‐staining positive cells. miR‐124 agomir (overexpression of miR‐124, Figure 2E) significantly reduced cell death and late apoptosis as compared with that in control group (Figure 3B,D), and its antagomir (knockdown of miR‐124, Figure 2E) significantly increased cell death and late apoptosis (Figure 3C,D).
Figure 3.
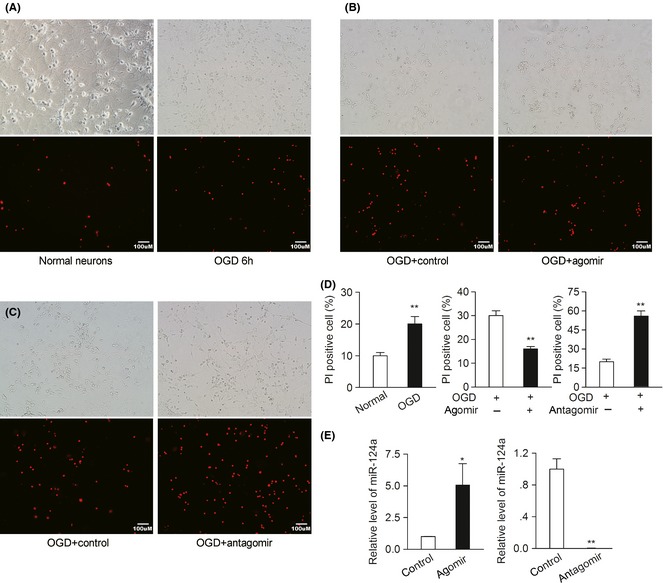
MiR‐124 inhibits OGD‐induced neuronal apoptosis and death. (A) Cell apoptosis and cell death detection in primary cultured neurons before and after OGD for 6 h. (B) Cell apoptosis and cell death detection 6 h after OGD in primary cultured neurons preincubated with agomir control and miR‐124a agomir for 48 h. (C) Cell apoptosis and cell death detection 6 h after OGD in primary cultured neurons preincubated with antagomir control or miR‐124a antagomir for 48 h. (D) Quantitative analysis of PI‐staining positive neurons. Death and apoptosis rate was calculated as the PI‐positive cells divided by the total number of cells counted. (n = 3, **P < 0.01). (E) Real‐time PCR detection of miR‐124 in primary neurons transfected with miR‐124 agomir or antagomir for 48 h. (n = 3, *P < 0.05, **P < 0.01). Data are represented as mean ± SD.
MiR‐124 Upregulates the Expression of Antiapoptosis Protein under OGD condition
As miR‐124 was found to protect against neurons apoptosis, the effect of miR‐124 on the expression of apoptosis‐related proteins under OGD condition was observed to clarify the possible neuroprotective mechanism. As shown in Figure 4A, miR‐124 agomir significantly increased, while miR‐124 antagomir markedly decreased, the expression of antiapoptosis proteins Bcl‐2 and Bcl‐xl. However, other apoptosis‐related proteins, such as caspase 8, cleaved caspase 8, Bax and Bad, did not change under the same conditions (Figure 4B–D).
Figure 4.
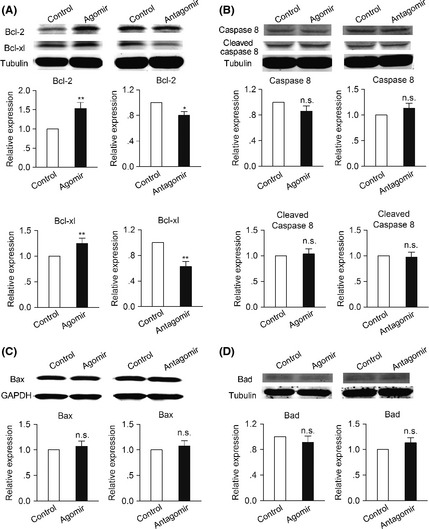
MiR‐124 upregulates the expression of antiapoptosis protein under OGD condition. Western blotting detection of (A) Bcl‐2, Bcl‐xl, (B) caspase 8, cleaved caspase 8, (C) Bax, and (D) Bad 6 h after OGD in primary neurons preincubated with miR‐124 agomir or antagomir and their corresponding control for 48 h. (n = 3, *P < 0.05, **P < 0.01). Data are represented as mean ± SD.
Discussion
Cerebral ischemia triggers a cascade of pathological events, including excitotoxicity within minutes, a robust inflammatory response within hours, and apoptosis within hours and days of stroke onset, ultimately cause irreversible neuronal injury in stroke‐affected brain tissue. Increasing evidence indicates neuronal cell death is a prominent feature observed after stroke. It has revealed that apoptosis plays a critical role in neuronal death in the ischemic penumbra after focal cerebral ischemia 6, 27, 28. Identification of the miRNAs that are involved in regulation of stroke‐related neurons death and apoptosis networks could provide insight into new therapeutic avenues.
Recently, miRNA expression profiling has been examined in stroke in vivo and in vitro. Many individual stroke‐responsive miRNAs were shown to play a role in mediating the secondary brain damage and functional outcome after experimental stroke. miR‐145 is upregulated in rat brain following focal ischemia. Antagomir of miR‐145 could significantly decreased infarction 29. MiR‐424 levels were decreased in the plasma of patients with acute ischemic stroke, as well as in mouse plasma and ipsilateral brain tissue after ischemia. Overexpression of miR‐424 lessened the ischemic brain injury 30. miR‐181 increases in the core, but decreases in the penumbra in response to stroke. Increased miR‐181 exacerbated injury both in vitro and in the mouse stroke model 31.
In this study, we found miR‐124, a CNS‐specific miRNA, was significantly elevated in response to ischemic stress in vivo and in vitro, which may be a protective response. Further data demonstrated that miR‐124 could provide protection against ischemic injury via inhibiting neurons apoptosis in experimental stroke. In accordance with our findings, some other miRNAs that regulate apoptosis have been clarified. For example, induction of miR‐497 following focal ischemia promotes ischemic neuronal death by negatively regulating antiapoptosis proteins, bcl‐2, and bcl‐w 32. miR‐21 increases neuronal survival after stroke by targeting and blocking translation of the gene that encodes Fas ligand (a receptor that signals to induce apoptosis) 33.
The Bcl‐2 family proteins have either pro‐ or antiapoptotic functional capabilities. They have been traditionally classified under three subsets. One class inhibits apoptosis (Bcl‐2, Bcl‐xl, Bcl‐w, Mcl1, and A1), whereas a second class promotes apoptosis (Bax, Bak, and Bok). The third specific class, known as BH3‐only proteins (Bad, Bim, Bid, DP5, Bik, Bmf, Noxa, and Puma), has a conserved BH3 domain 34. Caspase 8 (Casp8), a member of a mammalian caspase family, has been demonstrated to play a key role in mediating Fas‐induced apoptosis 35. Cleaved caspase 8 is an active fragment of caspase 8 when cleaved at Asp387. The Bcl‐2 family proteins may play a critical role in ischemic neuronal death 36. Bcl‐2 and Bcl‐xl are key regulators in attenuating stroke‐induced apoptotic cell death 37, 38. Bid could promote cell death after ischemia 39. Also, ischemia increased caspase 8 activities, and caspases may act as treatment targets in stroke and neurodegenerative diseases 35, 40.
Our studies found that miR‐124 could increase antiapoptosis proteins, Bcl‐2 and Bcl‐xl, which may be the molecular mechanism of miR‐124 protection against neuronal apoptosis. Many targets of miR‐124 have been identified, such as protein jagged‑1 (Jag‐1, also known as Notch‐ligand receptor), SOX‐9 (an adult neurogenesis transcription factor), DLX2 (a transcription factor involved in neuronal subtype specification), and so on 16, 41. How miR‐124 regulates Bcl‐2 and Bcl‐xl proteins needs further investigations.
In conclusion, our data have unveiled that miR‐124 protects neurons against apoptosis and brain injury in experimental stroke. miR‐124, as a novel antiapoptosis regulator, may develop into a neuroprotective agents for stroke therapeutics. Still, the role of miRNAs in stroke studies remains largely unknown. The elucidation of miRNA mechanisms involved in neuronal death and underlying pathogenesis of cerebral ischemia should be further widely explored.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by grants from the 973 National Key Basic Research Program of China (2009CB521901, to Ding‐Feng Su) and National Natural Science Foundation of China (No. 30973525, No. 81273606 to Xia Liu).
The first two authors contributed equally to this work.
References
- 1. Beal CC. Gender and stroke symptoms: a review of the current literature. J Neurosci Nurs 2010;42:80–87. [PubMed] [Google Scholar]
- 2. Bushnell CD. Stroke and the female brain. Nat Clin Pract Neurol 2008;4:22–33. [DOI] [PubMed] [Google Scholar]
- 3. Williams GR. Incidence and characteristics of total stroke in the United States. BMC Neurol 2001;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen PH, Gao S, Wang YJ, Xu AD, Li YS, Wang D. Classifying Ischemic Stroke, from TOAST to CISS. CNS Neurosci Ther 2012;18:452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 1999;22:391–397. [DOI] [PubMed] [Google Scholar]
- 6. An YT, Zhu P, Zhong Y, Sheng YC, Zhao Z, Min Y, et al. A neuroprotective mechanism of YGY‐E in cerebral ischemic injury in rats. CNS Neurosci Ther 2012;18:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ma XJ, Cheng JW, Zhang J, Liu AJ, Liu W, Guo W, et al. E‐selectin deficiency attenuates brain ischemia in mice. CNS Neurosci Ther 2012;18:903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jacquier A. The complex eukaryotic transcriptome: unexpected pervasive transcription and novel small RNAs. Nat Rev Genet 2009;10:833–844. [DOI] [PubMed] [Google Scholar]
- 9. Fabian MR, Sonenberg N. The mechanics of miRNA‐mediated gene silencing: a look under the hood of miRISC. Nat Struct Mol Biol 2012;19:586–593. [DOI] [PubMed] [Google Scholar]
- 10. Wu P, Zuo X, Ji A. Stroke‐induced microRNAs: the potential therapeutic role for stroke. Exp Ther Med 2012;3:571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhalala OG, Srikanth M, Kessler JA. The emerging roles of microRNAs in CNS injuries. Nat Rev Neurol 2013;9:328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vemuganti R. The microRNAs and stroke: no need to be coded to be counted. Transl Stroke Res 2010;1:158–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zheng HW, Wang YL, Lin JX, Li N, Zhao XQ, Liu GF, et al. Circulating MicroRNAs as potential risk biomarkers for hematoma enlargement after intracerebral hemorrhage. CNS Neurosci Ther 2012;18:1003–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mishima T, Mizuguchi Y, Kawahigashi Y, Takizawa T. RT‐PCR‐based analysis of microRNA (miR‐1 and ‐124) expression in mouse CNS. Brain Res 2007;1131:37–43. [DOI] [PubMed] [Google Scholar]
- 15. Lagos‐Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue‐specific microRNAs from mouse. Curr Biol 2002;12:735–739. [DOI] [PubMed] [Google Scholar]
- 16. Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR‐124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci 2009;12:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu JY, Chung KH, Deo M, Thompson RC, Turner DL. MicroRNA miR‐124 regulates neurite outgrowth during neuronal differentiation. Exp Cell Res 2008;314:2618–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The MicroRNA miR‐124 promotes neuronal differentiation by triggering brain‐specific alternative pre‐mRNA splicing. Mol Cell 2007;27:435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laterza OF, Lim L, Garrett‐Engele PW, Vlasakova K, Muniappa N, Tanaka WK, et al. Plasma MicroRNAs as sensitive and specific biomarkers of tissue injury. Clin Chem 2009;55:1977–1983. [DOI] [PubMed] [Google Scholar]
- 20. Weng H, Shen C, Hirokawa G, Ji X, Takahashi R, Shimada K, et al. Plasma miR‐124 as a biomarker for cerebral infarction. Biomed Res 2011;32:135–141. [DOI] [PubMed] [Google Scholar]
- 21. Zhang XH, Lei H, Liu AJ, Zou YX, Shen FM, Su DF. Increased oxidative stress is responsible for severer cerebral infarction in stroke‐prone spontaneously hypertensive rats. CNS Neurosci Ther 2011;17:590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zou YX, Zhang XH, Su FY, Liu X. Importance of riboflavin kinase in the pathogenesis of stroke. CNS Neurosci Ther 2012;18:834–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu AJ, Zang P, Guo JM, Wang W, Dong WZ, Guo W, et al. Involvement of acetylcholine‐alpha7nAChR in the protective effects of arterial baroreflex against ischemic stroke. CNS Neurosci Ther 2012;18:918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu AJ, Ma XJ, Shen FM, Liu JG, Chen H, Su DF. Arterial baroreflex: a novel target for preventing stroke in rat hypertension. Stroke 2007;38:1916–1923. [DOI] [PubMed] [Google Scholar]
- 25. Shi R, Weng J, Zhao L, Li XM, Gao TM, Kong J. Excessive autophagy contributes to neuron death in cerebral ischemia. CNS Neurosci Ther 2012;18:250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang HF, Guo F, Cao YZ, Shi W, Xia Q. Neuroprotection by manganese superoxide dismutase (MnSOD) mimics: antioxidant effect and oxidative stress regulation in acute experimental stroke. CNS Neurosci Ther 2012;18:811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Love S. Apoptosis and brain ischaemia. Prog Neuropsychopharmacol Biol Psychiatry 2003;27:267–282. [DOI] [PubMed] [Google Scholar]
- 28. Yuan J. Neuroprotective strategies targeting apoptotic and necrotic cell death for stroke. Apoptosis 2009;14:469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dharap A, Bowen K, Place R, Li LC, Vemuganti R. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab 2009;29:675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhao H, Wang J, Gao L, Wang R, Liu X, Gao Z, et al. MiRNA‐424 protects against permanent focal cerebral ischemia injury in mice involving suppressing microglia activation. Stroke 2013;44:1706–1713. [DOI] [PubMed] [Google Scholar]
- 31. Ouyang YB, Lu Y, Yue S, Xu LJ, Xiong XX, White RE, et al. miR‐181 regulates GRP78 and influences outcome from cerebral ischemia in vitro and in vivo . Neurobiol Dis 2012;45:555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yin KJ, Deng Z, Huang H, Hamblin M, Xie C, Zhang J, et al. miR‐497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol Dis 2010;38:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Buller B, Liu X, Wang X, Zhang RL, Zhang L, Hozeska‐Solgot A, et al. MicroRNA‐21 protects neurons from ischemic death. FEBS J 2010;277:4299–4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Youle RJ, Strasser A. The BCL‐2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 2008;9:47–59. [DOI] [PubMed] [Google Scholar]
- 35. Velier JJ, Ellison JA, Kikly KK, Spera PA, Barone FC, Feuerstein GZ. Caspase‐8 and caspase‐3 are expressed by different populations of cortical neurons undergoing delayed cell death after focal stroke in the rat. J Neurosci 1999;19:5932–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Graham SH, Chen J, Clark RS. Bcl‐2 family gene products in cerebral ischemia and traumatic brain injury. J Neurotrauma 2000;17:831–841. [DOI] [PubMed] [Google Scholar]
- 37. Wiessner C, Allegrini PR, Rupalla K, Sauer D, Oltersdorf T, McGregor AL, et al. Neuron‐specific transgene expression of Bcl‐XL but not Bcl‐2 genes reduced lesion size after permanent middle cerebral artery occlusion in mice. Neurosci Lett 1999;268:119–122. [DOI] [PubMed] [Google Scholar]
- 38. Martinou JC, Dubois‐Dauphin M, Staple JK, Rodriguez I, Frankowski H, Missotten M, et al. Overexpression of BCL‐2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron 1994;13:1017–1030. [DOI] [PubMed] [Google Scholar]
- 39. Plesnila N, Zinkel S, Le DA, Amin‐Hanjani S, Wu Y, Qiu J, et al. BID mediates neuronal cell death after oxygen/ glucose deprivation and focal cerebral ischemia. Proc Natl Acad Sci U S A 2001;98:15318–15323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schulz JB, Weller M, Moskowitz MA. Caspases as treatment targets in stroke and neurodegenerative diseases. Ann Neurol 1999;45:421–429. [DOI] [PubMed] [Google Scholar]
- 41. Liu XS, Chopp M, Zhang RL, Tao T, Wang XL, Kassis H, et al. MicroRNA profiling in subventricular zone after stroke: MiR‐124a regulates proliferation of neural progenitor cells through Notch signaling pathway. Plos One 2011;6:e23461. [DOI] [PMC free article] [PubMed] [Google Scholar]


