Introduction
Hypothermia has been reported in many clinical states and sometimes it is life threatening. However, there are rare cases reported about hypothermia related with Parkinson's disease (PD) 1, 2, 3. We now present a patient with PD, who developed an episode of severe hypothermia. The unique changes both in electrocardiogram (ECG) and in electroencephalogram (EEG) during hypothermia were presented and discussed.
Case Report
An 89‐year‐old woman was presented to our hospital with conscious impairment for 1 day. She was diagnosed with Parkinson's disease about 10 years ago and has been taking carbidopa‐levodopa at 500–750 mg/day since then. Her symptoms, such as slowness of movement did not significantly deteriorate in nearly 10 years, with Hoehn‐Yahr Stage II. During last 2 years, her slow movement worsened, but she did not add the dose of carbidopa‐levodopa. In recent 2 months, she had prominent deterioration of her motor slowness (Hoehn‐Yahr Stage IV). Her caregiver and son lived with her in the house with air‐conditioner, keeping room temperature around 20°C. One day before the admission, she was found by her caregiver in a new onset state of mutism in the afternoon, with her eyes opening spontaneously. She sat in a warm room and showed mere response to outside stimuli and no voluntary movement or facial expression, accompanied with urinary and fecal incontinence. But no static tremor or shivering was noticed. No obvious daytime sleepiness or disturbed nocturnal sleep was observed. Her son and caregiver stated that she was carefully looked after and slept with electric blanket covered. They denied any exposure to cold environment before and after sudden onset of mutism and they did not measure her body temperature.
On examination, her aural canal temperature was 30.9°C, blood pressure 112/67 mmHg, heart rate 50 beats/min, respiratory rate 20 /min, with an oxygen saturation of 96.7% while breathing room air. Glasgow coma scale was 8. She was noted to have obvious conjunctival edema. On auscultation and percussion, the lungs were clear and no cardiac murmur was present. Abdomen was flat and bowel sound was audible. No abnormal mass was palpable. Neurological examination revealed generalized muscular rigidity with masked face and neck stiffness. She was in a stupor and exhibited minor myoclonia in bilateral upper extremities which could be intensified by passive extension of the fingers. Deep tendon reflexes were brisk and bilateral, Babinski sign was negative.
Results of routine laboratory tests, including complete blood count, electrolytes, fasting serum glucose, hepatic and renal function, C‐reactive protein, Troponin‐I, and antinuclear antibody, were all normal. Arterial blood gas showed metabolic acidosis with pH of 7.297, pCO2 49.5 mmHg, pO2 99.5 mmHg, BE 3.1 mM, SB 21.7 mM, AG 15.9 mM. Her thyroid function showed free T4 of 13.23 pM, free T3 of 2.62 pM, and thyroid stimulating hormone of 2.18 mIU/L. Her thyroid peroxidase antibody (TPO‐Ab) and thyroglobulin antibody (TG‐Ab) were normal. Serum adrenocorticotropic hormone (ACTH) was also normal, 22.52 pg/mL in 8:00 a.m. and 27.13 pg/mL in 4:00 p.m. Brain CT scan and magnetic resonance images were generally normal.
Her first electrocardiography (ECG) taken immediately after admission (30.9°C, Figure 1A) demonstrated bradycardia and Osborn waves. The next day after admission (35.4°C, Figure 2), 16‐channel electroencephalography (EEG) with scalp electrodes placed according to international 10–20 system was firstly recorded. At that time, she was still in a state of stupor. EEG demonstrated no alpha rhythm, but widespread theta waves of 80 μV (4–6c/s) and delta waves of 120 μV (1.5–3c/s) throughout both hemispheres. The frequent triphasic discharges of up to 100 μV with sharp contour were conspicuous. These waves mainly occurred synchronously in left hemisphere channels, with intervals of 1.5–3 s.
Figure 1.
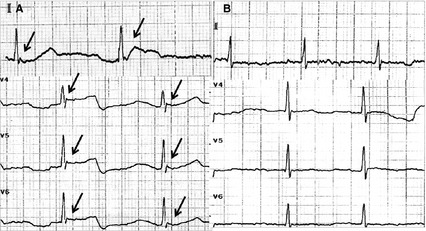
A 12‐lead electrocardiogram (ECG) was taken immediately after admission (when aural canal temperature was 30.9°C) and showed the obvious Osborn wave on lead II and lead V4–V6 (black arrow in A). The repeated ECG taken after the patient's body temperature returned to normal (aural canal temperature was 37.0°C) revealed no Osborn waves (B).
Figure 2.
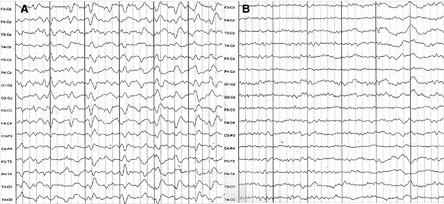
The first and second electroencephalogram (EEG) were taken immediately after admission (A, aural canal temperature 35.4°C) and several days later (B, 37.0°C). The time interval between two solid vertical lines is 3 s.
After several days' rewarming by warm environment in hospital and blanket, her body temperature was gradually getting back to normal (37.0°C) on the 4th day in hospital. She became alert and talked by simple phrases. Conjunctival edema and myoclonus also disappeared. The second ECG (Figure 1B) revealed no Osborn waves. The repeated EEG (37°C, Figure 2) demonstrated alpha rhythm, instead of triphasic waves, with theta waves of 50 μV (4–6c/s) and delta waves of 50 μV (2–3c/s) widespread throughout both hemispheres. Her caregiver was asked to check her body temperature regularly after she was discharged.
Discussion
In the current report, we describe one patient with PD who developed severe hypothermia, during which both EEG and ECG demonstrated striking changes. After the body temperature returned to normal, her consciousness recovered and the abnormalities of ECG and EEG were also reversible.
The remarkably reversible triphasic waves in EEG are of interest. The coexistence of myoclonus and triphasic waves was reported in patients with Creutzfeldt‐Jakob disease and serotonin syndrome 4. But these reasons were ruled out in the current case because of the reversible characteristic and no history of taking serotonin reuptake inhibitor. Previous report also showed periodic triphasic activity following levodopa treatment 5. This chance also seems to be small in our case because she kept taking carbidopa‐levodopa in the hospital. Myoclonus was reported to develop during brain hypothermia and disappeared after rewarming 6. The illustrated hypothermia and concomitant reversible triphasic waves in PD were also reported in two cases 1. We thus speculate that the reversible triphasic waves and myoclonus in the present case were related to accidental hypothermia. The metabolic derangements, such as metabolic acidosis and low ATP level during hypothermia, may explain the underlying mechanisms.
Osborn waves on ECG, also known as J waves, was reported when the body temperature drops and resolute gradually with rewarming. It is found that the amplitude of Osborn wave was inversely correlated with body temperature, thus the amplitude may be used as an indicator and monitored during the rewarming process. Osborn wave is best observed in the inferior and lateral precordial leads and occur due to transient electrophysiological changes during cooling. During lower temperature, heterogeneous distribution of a transient action potential with notch in epicardium but not in endocardium across the ventricular wall leads to the electrocardiographic Osborn wave. Specific Osborn wave was reported in one case of accidental hypothermia in PD 3.
We suggest that hypothermia is related to PD, not just an accidental event. The common causes of hypothermia, such as endocrinologic (hypopituitarism, hypoadrenalism, hypothyroidism, hypoglycemia), environmental and dermatologic diseases have been ruled out in this case. Interestingly, the absence of shivering during hypothermia leads to the suspicion of the thermoregulation dysfunction or even the pathology of hypothalamus. Animal experiment has shown the change of body temperature and thermal preference owing to the lesion of median preoptic area of hypothalamus 7. In patients with PD, the histopathological and functional changes in hypothalamic nuclei have been reported. PET study in PD showed the declines of 18F‐dopa uptake in hypothalamus 8. Notably, the selective loss of hypothalamic hypocretin cell and the deficiency of cerebrospinal fluid hypocretin (orexin)‐1 have been shown in patients with PD associated with disturbances of metabolism and thermoregulation 9. Hypocretin was thought to promote heat production 10. However, we did not notice the disturbance of sleepiness in this case, which was reported to be related with low levels of hypocretin. The relationship between hypocretin and hypothermia needs to be further clarified.
We suggest that PD could be a predisposing cause of severe hypothermia, and the reversible EEG and ECG changes may be characteristic in hypothermia. Although it is rare to see a patient with PD who presented symptoms caused by hypothermia, the recognition of clinical entity of hypothermia and the related EEG and ECG changes is important to prevent misdiagnosis. Future study may be needed to find the possible mechanism, such as the reasons of transient dysfunction in diffuse cerebral areas and hypocretin in hypothermia in PD.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
Dr. Lou is supported by grants from the National Natural Science Foundation of China (81070915 and 81171095), the Science and Technology Department of Zhejiang Province (2008C14078), Health Bureau of Zhejiang Province (WKJ2010‐2‐010) and Zhejiang Provincial Natural Science Foundation of China (LR12H09001).
References
- 1. Gubbay SS, Barwick DD. Two cases of accidental hypothermia in Parkinson's disease with unusual.E.G. findings. J Neurol Neurosurg Psychiatry 1966;29: 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hama K, Miwa H, Kondo T. Life‐threatening hypothermia in Parkinson's disease. Mov Disord 2009;24: 945–946. [DOI] [PubMed] [Google Scholar]
- 3. Sehara Y. Hypothermia with Osborn waves in Parkinson's disease. Intern Med 2009;48: 615–618. [DOI] [PubMed] [Google Scholar]
- 4. Dike GL. Triphasic waves in serotonin syndrome. J Neurol Neurosurg Psychiatry 1997;62: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Neufeld MY. Periodic triphasic waves in levodopa‐induced encephalopathy. Neurology 1992;42: 444–446. [DOI] [PubMed] [Google Scholar]
- 6. Bouwman NA, Verhagen WI, Meulstee J. EEG abnormalities in poikilothermia suggesting Creutzfeldt‐Jakob disease. Clin EEG Neurosci 2009;40: 196–199. [DOI] [PubMed] [Google Scholar]
- 7. Ray B, Mallick HN, Kumar VM. Changes in sleep‐wakefulness in the medial preoptic area lesioned rats: role of thermal preference. Behav Brain Res 2005;158: 43–52. [DOI] [PubMed] [Google Scholar]
- 8. Pavese N, Rivero‐Bosch M, Lewis SJ, Whone AL, Brooks DJ. Progression of monoaminergic dysfunction in Parkinson's disease: a longitudinal 18F‐dopa PET study. Neuroimage 2011;56: 1463–1468. [DOI] [PubMed] [Google Scholar]
- 9. Drouot X, Moutereau S, Nguyen JP, et al. Low levels of ventricular CSF orexin/hypocretin in advanced PD. Neurology 2003;61: 540–543. [DOI] [PubMed] [Google Scholar]
- 10. Kanosue K, Maruyama M, Nagashima K. The central organization of the thermoregulatory system. Tokyo: Springer‐Verlag, 2001. [Google Scholar]


