SUMMARY
Background: The current prognostic models for mortality and functional outcome after intracerebral hemorrhage (ICH) are not simple enough. To predict the outcome of ICH, a new simple model, ICH index (ICHI), was established and evaluated in this study. Methods: Medical records of all cases with ICH in our hospital from January 2008 to August 2009 were reviewed. Multiple linear regression analyses were used to assess the contributions of independent variables to hospital mortality after ICH. Results: Age, serum glucose, white blood cell counts (WBC), and Glasgow Coma Scale (GCS) score were found to be greatly associated with mortality. A formula of ICH index [ICHI = age (years)/10 + glucose (mmol/L) + WBC (109/L) – GCS score] was established. Furthermore, the receiver operating characteristic (ROC) analyses were performed to estimate the predictive value of the ICHI. The model showed an area under the ROC curve (AURC) of 0.923 (95% CI: 0.883–0.963, P < 0.001). The best cut‐off value of ICHI for mortality was 18, which gave sensitivity, specificity, and Youden's index of 0.65, 0.95, and 0.60, respectively. The hospital mortality was extremely increased when 18 < ICHI < 28 (mortality 72.0%) and when ICHI ≥28 (mortality 100%), in contrast with overall mortality (21.6%). Conclusion: The ICHI can be a simple predictive model and complementary to other prognostic models.
Keywords: Hospital mortality, ICH, ICH index, Predictive model, Outcome
Introduction
Intracerebral hemorrhage (ICH), which is a common neurological emergency, constitutes 10–15% of all strokes and has a high risk of morbidity and mortality than cerebral infarction or subarachnoid hemorrhage (SAH) [1, 2]. ICH kills about half of those affected within 1 month and leaves most survivors disabled [3]. Surgical treatments for ICH using a variety of methods have yielded either negative or inconclusive results [4, 5, 6, 7, 8]. Likewise, no medical treatment has been shown conclusively to benefit patients with ICH [9, 10, 11, 12].
Despite the lack of efficacious treatments for ICH, an early and accurate predictive model for outcome in patients after ICH can help clinicians make a better decision and improve the efficiency of the treatment. The model needs to be not only accurate and professional, but also simple and easily obtained. Currently, a number of prognostic models for mortality and functional outcome after ICH have been established [13, 14, 15, 16, 17, 18, 19]. The models usually include criteria related to neurological condition, various other clinical and laboratory parameters, and neuroimaging findings. The usefulness of the models is limited because of complexity. The ICH score, which is well accepted, is a simple grading scale for ICH. However the ICH volume is still needed to be estimated with the use of the “ABC/2” method. In some developing and underdeveloped area, it is impossible that there are neuroimaging equipments (like CT) and clinicians specifically trained in neurology in hospitals with poor medical condition. The purpose of this study is to develop a much simpler but reliable model to predict the hospital death in patients with ICH. And the model includes criteria from neurological condition and laboratory parameters that can be obtained in the first time, and can be rapidly and accurately assessed even by nonspecifically trained doctor.
Materials and Methods
A retrospective review of medical records of patients with ICH treated in the First Affiliated Hospital of Chongqing Medical University, Chongqing from January 2008 to August 2009 was undertaken. A total of 227 subjects (49 subjects died in hospital) aged 14 to 95 years were recruited in this study.
All variables used for the model were extracted from data available at the time of initial ICH evaluation. The Glasgow Coma Scale (GCS) score at initial presentation was used. The systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) were recorded when the first blood pressure was measured after hospital arrival. Other recorded characteristics including sex, age, smoking status, drinking status, and medical history were obtained after hospital arrival. The first time hematological and biochemical variables after hospital arrival were also recorded. Outcome was assessed as mortality in hospital after ICH. Patients who were alive at hospital discharge were assumed to have been alive in hospital after ICH.
Comparison for baseline characteristics between two sets of patients was performed with ANOVA for continuous parameters and χ2‐test for categorical parameters. Continuous data were expressed as mean ± standard deviation (SD). Multiple linear regression analyses were used to assess the contribution of baseline characteristics to mortality in hospital after ICH. Furthermore, the receiver operating characteristic (ROC) curve was plotted and the area under the ROC curve (AURC) was used to evaluate the predictive value of the model. Cut points of value were chosen to produce a simple and reliable model. Statistical analyses were performed with SPSS software version 15.0 (SPSS Inc., Chicago, IL, USA).
Results
A total of 249 patients who went to our hospital with ICH between January 2008 and August 2009 were selected for this study. In total, 227 patients, with available and complete information, formed the cohort for data analysis. The overall hospital mortality was 21.6% (n = 49), with no sex‐difference (21.5% in male, and 21.7% in female, P= 0.978). Significant differences were found from the dead patients to the alive in hospital in the following variables: age (63.96 ± 16.75 years, 58.30 ± 15.60 years, P= 0.028), GCS score (8.55 ± 4.29, 13.35 ± 2.39, P < 0.001), white blood cell counts (WBC = 14.23 ± 5.48 109/L, 8.34 ± 3.21 109/L, P < 0.001), SBP (175.61 ± 41.57 mmHg, 157.18 ± 30.00 mmHg, P= 0.001), DBP (100.35 ± 24.10 mmHg, 93.31 ± 16.95 mmHg, P= 0.021), HR (87.22 ± 21.49 beat/min, 79.33 ± 15.39 beat/min, P= 0.004), and levels of Serum Glucose (8.73 ± 3.05 mmol/L, 6.29 ± 1.48 mmol/L, P < 0.001), total protein (TP) (73.09 ± 7.75 g/L, 69.29 ± 7.29 g/L, P= 0.002), albumin (ALB) (44.30 ± 7.70 g/L, 41.54 ± 5.33 g/L, P= 0.005), and high‐density‐lipoprotein cholesterol (HDL‐C; 1.52 ± 0.38 mmol/L, 1.30 ± 0.40 mmol/L, P= 0.020). The comparison for baseline characteristics between two sets of patients was shown in Table 1.
Table 1.
Characteristics of study subjects dead or alive in hospital
| Characteristics | Patients dead in hospital mean ± SD (n = 49) | Patients alive in hospital mean ± SD (n = 178) | P |
|---|---|---|---|
| Male% (n) | 63.3 (31) | 63.5 (113) | 0.978 |
| Age (years) | 64 ± 17 | 58 ± 16 | 0.028 |
| GCS score | 9 ± 4 | 13 ± 2 | <0.001 |
| SBP (mmHg) | 176 ± 42 | 157± 30 | 0.001 |
| DBP (mmHg) | 100 ± 24 | 93 ± 17 | 0.021 |
| HR (beats/min) | 87 ± 21 | 79 ± 15 | 0.004 |
| Glu (mmol/L) | 8.7 ± 3.1 | 6.3 ± 1.5 | <0.001 |
| TP (mmol/L) | 73 ± 8 | 69 ± 7 | 0.002 |
| ALB (mmol/L) | 44 ± 8 | 42 ± 5 | 0.005 |
| Na (mmol/L) | 140 ± 6 | 139 ± 5 | 0.385 |
| K (mmol/L) | 3.96 ± 0.54 | 3.88 ± 0.55 | 0.401 |
| Urea (mmol/L) | 6.7 ± 4.1 | 6.0 ± 2.7 | 0.165 |
| Scr (mmol/L) | 107 ± 99 | 85 ± 91 | 0.145 |
| LDL‐C (mmol/L) | 2.68 ± 0.78 | 2.74 ± 0.85 | 0.761 |
| HDL‐C (mmol/L) | 1.52 ± 0.38 | 1.30 ± 0.40 | 0.020 |
| TC (mmol/L) | 4.87 ± 0.81 | 4.72 ± 0.97 | 0.510 |
| TG (mmol/L) | 1.14 ± 0.85 | 1.39 ± 0.76 | 0.179 |
| WBC (109/L) | 14.2 ± 5.5 | 8.34± 3.2 | <0.001 |
| RBC (1012/L) | 4.5 ± 0.8 | 4.4 ± 0.6 | 0.488 |
| HGB (g/L) | 138 ± 23 | 133 ± 21 | 0.221 |
| PLT (109/L) | 168 ± 119 | 174 ± 68 | 0.682 |
GCS, Glasgow coma scale; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; Glu, serum glucose; TP, total protein; ALB, albumin; Na, serum sodium; K, potassium; Urea, urea nitrogen; Scr, serum creatinine; LDL‐C, low‐density lipoprotein cholesterol; HDL‐C, high‐density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides; WBC, white blood cell count; RBC, red blood cell count; HGB, hemoglobin; PLT, platelets.
Furthermore, multiple linear regression analyses were used to access the contribution of variables (age, GCS scores, SBP, DBP, HR, serum glucose, TP, ALB, HDL‐C, and WBC) to hospital mortality after ICH as a whole. Finally, we found that age, GCS scores, serum glucose, and WBC significantly affected the hospital mortality (P= 0.015, 0.001, 0.010, and <0.001, respectively; Table 2).
Table 2.
Multiple linear regression analyses to assess the contribution of variables to mortality
| Variable | Unstandardized coefficients, B | t | P |
|---|---|---|---|
| Age (year) | 0.004 | 2.474 | 0.015 |
| GCS score | −0.031 | 3.287 | 0.001 |
| WBC (109/L) | 0.026 | 3.778 | <0.001 |
| Glu (mmol/L) | 0.033 | 2.608 | 0.010 |
| SBP (mmHg) | −0.000 | −0.061 | 0.951 |
| DBP (mmHg) | 0.003 | 1.571 | 0.118 |
| HR (beats/min) | −0.001 | −0.605 | 0.546 |
| TP (mmol/L) | 0.001 | 0.208 | 0.835 |
| ALB (mmol/L) | −0.001 | −0.156 | 0.876 |
| HDL‐C (mmol/L) | 0.019 | 0.331 | 0.741 |
GCS, Glasgow coma scale; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; Glu, serum glucose; TP, total protein; ALB, albumin; HDL‐C, high‐density lipoprotein cholesterol; WBC, white blood cell count.
The ICH index (ICHI) to predict the hospital mortality after ICH was established. It was calculated by the value of age, serum glucose, WBC, and GCS score. The ICHI = age (years)/10 + serum glucose (mmol/L) + WBC (109/L) – GCS score. For example, a 58‐year‐old ICH patient arrived hospital with a level of Serum Glucose 10.2 mmol/L, count of WBC 11.2 × 109/L, and GCS score 14. The ICHI was 13 (ICHI = 58/10 + 10.2 + 11.2 – 14 = 13.2 ≈ 13).
To evaluate the predictive value of the ICHI, ROC analyses were performed (Figure 1). The model for prediction of hospital mortality after ICH showed an AURC of 0.923 (95% CI: 0.883–0.963, P < 0.001). Figure 2 shows the hospital mortality in different range of ICHI. Patients with ICHI ≤ 9 hardly died in hospital (mortality 1.52%), whereas all patients with ICHI ≥ 28 died in hospital. When ICHI was between 19 and 27, the mortality was extremely higher than the overall mortality (72.0% vs. 21.6%). Slight difference of mortality in patients with ICHI from 10 to 18 was found in contract with overall mortality (28.8% vs. 21.6%). Thus, it suggested that the best cut‐off value of ICHI for mortality was 18, which gave sensitivity, specificity, and Youden's index of 0.65, 0.95, and 0.60, respectively. The hospital mortality after ICH was extremely increased when the ICHI was more than 18.
Figure 1.
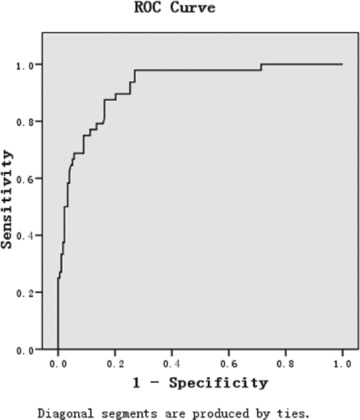
ROC curves of ICH index for the prediction of hospital mortality after ICH.
Figure 2.
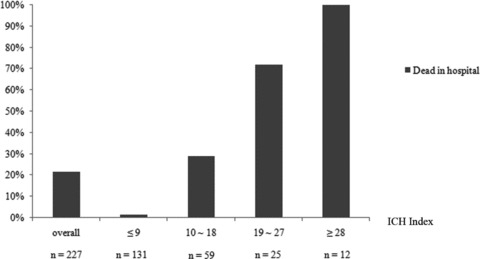
The ICH index and hospital mortality. Hospital mortality increases as the ICH index increases.
Discussion
Clinical grading scales play a key role in the assessment and management of patients with acute neurological disorders. The practical applicability of every clinical grading scale depends on its simplicity and accuracy of outcome prediction.
The Hemphill's ICH score [20] is a clinical grading scale composed of factors including a basic neurological examination (GCS score), a baseline patient characteristic (age), and initial neuroimaging (ICH volume, IVH, infratentorial/supratentorial origin). And ICH hematoma volume was measured on the initial head CT scan with the use of the ABC/2 method [21]. The presence or absence of IVH was also noted on initial head CT. The total ICH score is the sum of the points of the various characteristics, and every point was given different weight according to its influence on outcomes. Compared with ICH score, ICH‐GS [22] is a reference scoring system. And although the independent predictors of ICH‐GS are the same as ICH score, different cutoffs and scoring advances sensitivity and the prognostic power in predicting in‐hospital and 30‐day mortality. Different from ICH score and ICH‐GS, our prediction model includes age, GCS score, serum glucose, and WBC, which reduces our reliance on neuroimaging equipment. When patients were accepted in community hospitals or hospitals which dose not have neuroimaging equipment, this prediction can be used by clinicians to assess prognosis more accurately on the basis of data generally available at the time of initial diagnosis and better enable them to advise patients and their families. Compared to several predictive models for ICH, which have been previously developed and validated [13, 14, 15, 16, 17, 18, 19], the present model is much simple. In a well‐accepted model, the ICH score, the head CT are essentially needed [15]. In this study, a more convenient model (ICHI) to predict the hospital mortality after ICH was evaluated. The variables used in this predictive model can be easily obtained, and only four variables (age, GCS score, serum glucose, WBC) were needed. Neuroimaging findings, which are essential for the prevalent predictive models, are not included in this model. The model will be more useful in undeveloped or developing countries where medical conditions are poor. Furthermore, clinicians have no need to estimate the ICH volume and location. It can be rapidly and accurately assessed at the very early time of presentation without any neuroimaging findings, especially by personnel not specifically trained in neurology.
Serum glucose and WBC, which have been found to be independent predictors for ICH outcome, are designed in our model. To the best of our knowledge, it was the first time serum glucose, WBC, GCS score, and age are considered as a whole in predicting the ICH outcome. Thus ICHI will be complementary to other prognostic models. The GCS score, which is a reproducible and reliable neurological assessment tool [23], has been used in many predictive models [13, 16, 18, 24,]. Age has been found to be an independent predictor of ICH outcome [24]. In the ICH score, only very old age (≥80 years) is associated with 30‐day mortality [15]. WBC is consistently associated with outcome of hemorrhagic and ischemic stroke [25, 26, 27, 28]. Recently, it has been proved that hyperglycemia may worsen the prognosis of ICH [29, 30]. The formula of ICHI was established according to the contribution of these four variables accessed by multiple linear regression analyses.
This study has several limitations. First, hospital mortality may not be as good to assess the outcome of ICH as 30‐day mortality. Some patients, who were alive in hospital, might die soon after discharged. The hospital mortality (21.6%) of ICH in our study was smaller than 30‐day mortality in other studies (45%) [15]. Second, the data of ICH volume and location in this study were not completed, so it is impossible to compare the performance of the ICH score and the present predictive model. Third, primary data collection is incomplete. Because of some factors are not readily assessable on initial ICH presentation, we did not collect the data about the medical history and premorbid drug therapy status of ICH patients such as statin and antiplatelet therapy (APT). And a research [31] reveals that there is association of previous statin use and decreased mortality after ICH, and a systematic review [32] concludes that APT use at the time of ICH compared to no APT use was independently associated with increased mortality but not with poor functional outcome. APT and statin prior to ICH was common and some medical history such as hypertension is associated with the death and functional outcome of ICH, while these data are not included in this study, as the reference point, should be taken into account for the outcome of ICH. Last but not least, the cases are patients with ICH. There are some ICH analogous diseases, such as traumatic brain injury, SAH, or ischemic stroke. However, due to the pathogeny, mechanisms, clinical feature and prognosis of ICH are different from traumatic brain injury, SAH and ischemic stroke, we can not confirm that the ICHI have predictive values in outcome of hemorrhagic, ischemic stroke, traumatic brain injury and SAH. Therefore, we should do further studies so as to validate the predictive value of the model in patients ICH, and to evaluate the predictive value in patients with SAH, or cerebral infarction.
Prospective
Several prognostic models for ICH have proven to be reliable and accurate in predicting 30‐day mortality and ICH‐GS has demonstrated to performed equally well in predicting good functional outcome at 30‐day follow up [20, 22, 31]. To improve applicable range of ICHI, long‐term functional outcome should be observed and predicted. We are collecting more detailed data and a prospective observational cohort study will be carried out to testify the practical applicability of ICHI and improve it.
Conflict of Interest
There was no support from any organization for the submitted work; no financial relationships with any organization that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: The study was approved by Medical ethic committee in the Second Hospital of Shanxi Medical University and The First Affiliated Hospital of Chongqing Medical University. The study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
We thank the staff for their support and assistance.
The first two authors contributed equally to this study.
References
- 1. Broderick JP, Brott TG, Tomsick T, Miller R, Huster G. Intracerebral hemorrhage more than twice as common as subarachnoid hemorrhage. J Neurosurg 1993;78:188–191. [DOI] [PubMed] [Google Scholar]
- 2. Caplan LR. Intracerebral haemorrhage. Lancet 1992;14;656–658. [DOI] [PubMed] [Google Scholar]
- 3. Dennis MS. Outcome after brain haemorrhage. Cerebrovasc Dis 2003;16:9–13. [DOI] [PubMed] [Google Scholar]
- 4. Auer LM, Deinsberger W, Niederkorn K, et al J Neurosurg 1989;70:530–535. [DOI] [PubMed] [Google Scholar]
- 5. Batjer HH, Reisch JS, Allen BC, Plaizier LJ, Su CJ. Failure of surgery to improve outcome in hypertensive putaminal hemorrhage: A prospective randomized trial. Arch Neurol 1990;47:1103–1106. [DOI] [PubMed] [Google Scholar]
- 6. Juarez VJ, Lyons M. Interrater reliability of the Glasgow Coma Scale. J Neurosci Nurs 1995;27:283–286. [DOI] [PubMed] [Google Scholar]
- 7. Kimura K, Iguchi Y, Inoue T, Shibazaki K, Matsumoto N, Kobayashi K, Yamashita S. Hyperglycemia independently increases the risk of early death in acute spontaneous intracerebral hemorrhage. J Neurol Sci 2007;255:90–94. [DOI] [PubMed] [Google Scholar]
- 8. Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit Care Med 1999; 27:617–621. [DOI] [PubMed] [Google Scholar]
- 9. Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001;32:891–897. [DOI] [PubMed] [Google Scholar]
- 10. Lisk DR, Pasteur W, Rhoades H, Putnam RD, Grotta JC. Early presentation of hemispheric intracerebral hemorrhage: Prediction of outcome and guidelines for treatment allocation. Neurology 1994;44:133–139. [DOI] [PubMed] [Google Scholar]
- 11. Poungvarin N, Bhoopat W, Viriyavejakul A, Rodprasert P, Buranasiri P, Sukondhabhant S, Hensley MJ, Strom BL. Effects of dexamethasone in primary supratentorial intracerebral hemorrhage. N Engl J Med 1987;316:1229–1233. [DOI] [PubMed] [Google Scholar]
- 12. Tuhrim S, Dambrosia JM, Price TR, Mohr JP, Wolf PA, Hier DB, Kase CS. Intracerebral hemorrhage: External validation and extension of a model for prediction of 30‐day survival. Ann Neurol 1991;29:658–663. [DOI] [PubMed] [Google Scholar]
- 13. Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy‐to‐use predictor of 30‐day mortality. Stroke 1993;24:987–993. [DOI] [PubMed] [Google Scholar]
- 14. Godoy DA, Piñero G, Di Napoli M. Predicting mortality in spontaneous intracerebral hemorrhage: Can modification to original score improve the prediction? Stroke 2006;37:1038–1044. [DOI] [PubMed] [Google Scholar]
- 15. Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001;32:891–897. [DOI] [PubMed] [Google Scholar]
- 16. Juvela S, Heiskanen O, Poranen A, Valtonen S, Kuurne T, Kaste M, Troupp H. The treatment of spontaneous intracerebral hemorrhage: A prospective randomized trial of surgical and conservative treatment. J Neurosurg 1989;70:755–758. [DOI] [PubMed] [Google Scholar]
- 17. Morgenstern LB, Frankowski RF, Shedden P, Pasteur W, Grotta JC. Surgical treatment for intracerebral hemorrhage (STICH): A single‐center, randomized clinical trial. Neurology 1998;51:1359–1363. [DOI] [PubMed] [Google Scholar]
- 18. Qureshi AI, Bliwise DL, Bliwise NG, Akbar MS, Uzen G, Frankel MR. Rate of 24‐hour blood pressure decline and mortality after spontaneous intracerebral hemorrhage: A retrospective analysis with a random effects regression model. Crit Care Med 1999;27:480–485. [DOI] [PubMed] [Google Scholar]
- 19. Tellez H, Bauer RB. Dexamethasone as treatment in cerebrovascular disease. I. A controlled study in intracerebral hemorrhage. Stroke 1973;4:541–546. [DOI] [PubMed] [Google Scholar]
- 20. Claude Hemphill J III, Bonovich David C, Besmertis L, et al The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001;32:891–897. [DOI] [PubMed] [Google Scholar]
- 21. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, Khoury J. The ABCs of measuring intracerebral hemorrhage volumes. Stroke 1996;27:1304–1305. [DOI] [PubMed] [Google Scholar]
- 22. Ruiz‐Sandoval Jose L, Chiquete E, Romero‐Vargas S, et al Grading scale for prediction of outcome in primary intracerebral hemorrhages. Stroke 2007;38:1641–1644. [DOI] [PubMed] [Google Scholar]
- 23. Holling M, Jeibmann A, Gerss J, Fischer BR, Wassmann H, Paulus W, Hasselblatt M, Albert FK. Prognostic value of histopathological findings in aneurysmal subarachnoid hemorrhage. J Neurosurg 2009;110:487–491. [DOI] [PubMed] [Google Scholar]
- 24. Italian acute stroke study group haemodilution in acute stroke: Results of the Italian haemodilution trial. Lancet 1988;1:318–321. [PubMed] [Google Scholar]
- 25. Grau AJ, Boddy AW, Dukovic DA, Buggle F, Lichy C, Brandt T, Hacke W; CAPRIE Investigators . Leukocyte count as an independent predictor of recurrent ischemic events. Stroke 2004;35:1147–1152. [DOI] [PubMed] [Google Scholar]
- 26. Efstathiou SP, Tsioulos DI, Zacharos ID, et al A new classification tool for clinical differentiation between haemorrhagic and ischaemic stroke. J Intern Med 2002;252:121–129. [DOI] [PubMed] [Google Scholar]
- 27. Elkind MS, Sciacca RR, Boden‐Albala B, Rundek T, Paik MC, Sacco RL. Relative elevation in baseline leukocyte count predicts first cerebral infarction. Neurology 2005;64:2121–2125. [DOI] [PubMed] [Google Scholar]
- 28. Juvela S. Risk factors for impaired outcome after spontaneous intracerebral hemorrhage. Arch Neurol 1995;52:1193–1200. [DOI] [PubMed] [Google Scholar]
- 29. Juarez VJ, Lyons M. Interrater reliability of the Glasgow Coma Scale. J Neurosci Nurs 1995;27:283–286. [DOI] [PubMed] [Google Scholar]
- 30. Godoy DA, Piñero GR, Svampa S, Papa F, Di Napoli M. Hyperglycemia and short‐term outcome in patients with spontaneous intracerebral hemorrhage. Neurocrit Care 2008;9:217–229. [DOI] [PubMed] [Google Scholar]
- 31. Cheung RT, Zou LY. Use of the original, modified, or new intracerebral hemorrhage score to predict mortality and morbidity after intracerebral hemorrhage. Stroke 2003;34:1717–1722. [DOI] [PubMed] [Google Scholar]
- 32. Thompson BB, Béjot Y, Caso V, et al Prior antiplatelet therapy and outcome following intracerebral hemorrhage. Neurology 2010;75:1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]


