Supplemental Digital Content is available in the text.
Keywords: animals; downregulation; glutamine; mice; myocytes, smooth muscle; neoplasms; transcription factors
Abstract
Rationale:
TEAD (TEA domain transcription factor) 1—a major effector of the Hippo signaling pathway—acts as an oncoprotein in a variety of tumors. However, the function of TEAD1 in vascular smooth muscle cells (VSMCs) remains unclear.
Objective:
To assess the role of TEAD1 in vascular injury–induced smooth muscle proliferation and delineate the mechanisms underlying its action.
Methods and Results:
We found that TEAD1 expression is enhanced in mouse femoral artery after wire injury and correlates with the activation of mTORC1 (mechanistic target of rapamycin complex 1) signaling in vivo. Using an inducible smooth muscle–specific Tead1 KO (knockout) mouse model, we found that specific deletion of Tead1 in adult VSMCs is sufficient to attenuate arterial injury–induced neointima formation due to inhibition of mTORC1 activation and VSMC proliferation. Furthermore, we found that TEAD1 plays a unique role in VSMCs, where it not only downregulates VSMC differentiation markers but also activates mTORC1 signaling, leading to enhanced VSMC proliferation. Using whole-transcriptome sequencing analysis, we identified Slc1a5 (solute carrier family 1 member 5)—a key glutamine transporter—as a novel TEAD1 target gene. SLC1A5 overexpression mimicked TEAD1 in promoting mTORC1 activation and VSMC proliferation. Moreover, depletion of SLC1A5 by silencing RNA or blocking SLC1A5-mediated glutamine uptake attenuated TEAD1-dependent mTORC1 activation and VSMC proliferation.
Conclusions:
Our study unravels a novel mechanism by which TEAD1 promotes VSMC proliferation via transcriptional induction of SLC1A5, thereby activating mTORC1 signaling and promoting neointima formation.
Vascular smooth muscle cells (VSMCs) are the major cell type in blood vessels. Unlike skeletal or cardiac myocytes that are terminally differentiated, VSMCs retain a remarkable plasticity. In response to environmental cues such as vascular injury, VSMCs may modulate from a quiescent status to a hyperproliferative phenotype that contributes to vascular remodeling and neointima formation.1 Abnormal proliferation of VSMCs is key to a number of occlusive vascular diseases in humans, such as atherosclerosis, intimal hyperplasia associated with restenosis, and vein graft stenosis.1 Although substantial progress has been made with unraveling the origin of intimal VSMCs in arterial injury and atherosclerosis,2–4 the mechanisms involved in triggering VSMC proliferation are far from being completely understood.
Editorial, see p 1282
In This Issue, see p 1277
Meet the First Author, see p 1278
As major downstream nuclear effectors of Hippo signaling, TEAD (TEA domain transcription factor) proteins are a family of 4 transcription factors (TEAD1–4) that bind to a consensus DNA sequence 5′-CATTCC-3′, named the muscle-specific cytidine-adenosine-thymidine (MCAT) element.5 Through interaction with a variety of cofactors, such as YAP (yes-associated protein) and TAZ (transcriptional coactivator with PDZ-binding motif), TEADs bind to MCAT-containing genes that regulate cell growth, thereby leading to oncogenic transformation.6–8 Furthermore, the expression of TEAD proteins is upregulated in many cancer types and correlates with poor survival in patients with cancer.7 In addition to their recognized roles in cancer, TEADs have been shown to regulate the expression of multiple smooth muscle (SM)–specific and cardiac muscle–specific genes during cardiovascular development.9–11 In SM, while TEADs are required for the initial transcriptional activation of key SM differentiation genes such as Myocd (Myocardin) and Acta2 (SMA [SM α-actin]) during embryonic development, we previously demonstrated that in adult SM, TEAD1 represses SM-specific gene expression by disrupting the interaction of myocardin with the key transcription factor SRF (serum response factor).9,10,12 However, whether TEAD1 regulates VSMC proliferation and neointima formation in vivo remains unknown.
mTORC1 (mechanistic target of rapamycin complex 1) is a master growth regulator that can be activated by various signals, such as growth factors and amino acids. The downstream effects of activated mTORC1 signaling and subsequent cell growth are mediated through the phosphorylation of p70S6K (p70 ribosomal protein S6 kinase-1), S6 (ribosomal protein S6), and 4EBP1 (eukaryotic initiation factor-4E–binding protein-1), ultimately leading to enhanced cell growth and proliferation by promoting protein synthesis and triggering proteasome-mediated degradation of the key cell cycle inhibitor p27kip1.13–15 The key role of mTORC1 in regulating VSMC proliferation and neointima formation is supported by the fact that mTORC1 inhibitors (eg, rapamycin) are commonly used in the clinical setting to limit intimal hyperplasia associated with restenosis after angioplasty.16,17 However, how mTORC1 is activated in VSMCs after vascular injury is largely unknown. Recent studies have demonstrated that SLC1A5 (solute carrier family 1 member 5)—a high-affinity L-glutamine transporter that is highly expressed in several cancer types18–20—promotes the uptake of L-glutamine, leading to the activation of mTORC1 signaling and thus promotes cancer cell proliferation.20,21 However, to date, the role of SLC1A5 in VSMC proliferation and the upstream regulatory mechanism of SLC1A5 expression in VSMCs have not been examined.
In this study, we aimed to identify the role and the downstream actions of upregulated expression of TEAD1 in VSMC proliferation and neointima formation. We found that TEAD1 expression is enhanced in mouse femoral artery after wire injury and correlates with the activation of mTORC1 signaling in vivo. Because Tead1 global KO (knockout) mice are embryonic lethal,22,23 we recently generated Tead1F (flox) mice24 to circumvent this embryonic lethality and to allow us to study TEAD1 function specifically in postnatal VSMCs. Using an inducible SM-specific Tead1 KO mouse model, we found that specific deletion of Tead1 in adult VSMCs is sufficient to attenuate arterial injury–induced neointima formation by inhibiting mTORC1 activation and attenuating VSMC proliferation. Using whole-transcriptome sequencing analysis, we identified Slc1a5 as a novel TEAD1 transcriptional target that mediates, at least in part, TEAD1-induced mTORC1 signaling activation and VSMC proliferation. Our study suggests that TEAD1-SLC1A5-glutamine uptake is a promising therapeutic axis to ameliorate occlusive vascular disease.
Methods
The authors declare that all supporting data are available within the article and its Online Data Supplement. The RNA-sequencing (seq) data generated in this study have been deposited in the Sequences Read Archive at the NCBI (National Center for Biotechnology Information) under accession number PRJNA492803.
Tead1 global heterozygote mice were generated as we recently reported.24 SM-specific Tead1iKO (inducible KO) mice were generated by crossing female Tead1F mice24 with male mice expressing tamoxifen-inducible Cre driven by the SM-specific gene Myh11 (SM myosin heavy chain [SM MHC]) Myh11-CreERT2+) Only male mice were used in this study because SM MHC-CreERT2 transgene is only localized in the Y chromosome.25 At 10 weeks of age, male mice (Myh11-CreERT2+/Tead1F/F) were randomly assigned to 2 groups. These 2 groups of mice were then intraperitoneally injected with vehicle sunflower oil (served as control) or tamoxifen (1 mg per mouse, iKO), respectively, once a day for 10 days with 2 days’ break after the first 5 injections. Two weeks after the last injection, wire injury was performed in the left femoral artery (LFA) as described previously.26,27 The right femoral artery in the same mouse served as sham control. All mice used in this study were maintained on a C57BL/6 background. The use of experimental mouse for arterial injury procedures and BSL-2 (biosafety level 2) viral work was approved by the Institutional Animal Care and Use Committee and Biosafety Committees at Augusta University. A detailed, expanded Methods section is included in the Online Data Supplement.
Results
TEAD1 Expression Is Induced After Arterial Injury and Positively Correlates With mTORC1 Activation in VSMCs In Vivo
Before investigating TEAD1 function in the vasculature in vivo, we examined the relative mRNA abundance of all 4 TEAD family genes in cultured vascular cells, including VSMCs, endothelial cells (ECs), and macrophages. Quantitative polymerase chain reaction data revealed that in comparison to other TEAD genes, TEAD1 is the most abundant member of the TEAD family in human coronary artery SM cells (HCASMCs; Online Figure IA through IC). Furthermore, Western blotting data revealed that TEAD1 protein is readily detectable in VSMCs, across all the examined species (Online Figure ID). Next, we examined the expression of TEAD1 after arterial injury, where VSMCs actively proliferate in vivo, thereby contributing to neointima formation.1 We found that, compared with control right femoral arteries, the expression levels of TEAD1 and YAP—a key TEAD transcriptional cofactor2—are enhanced in the LFAs after wire injury, a mouse arterial injury model that mimics postangioplasty restenosis in humans (Figure 1A and 1B).27 Furthermore, TEAD1 was highly expressed in both the neointimal area and the expanding adventitial area after arterial injury (Figure 1C and 1D). Notably, the enhanced expression of TEAD1 not only correlated with upregulation of the proliferative markers pH3 (phospho-histone H3) and PCNA (proliferating cell nuclear antigen) and downregulation of contractile SM markers (SM MHC [myosin heavy chain], MLCK [myosin light-chain kinase], calponin, and SM22α [smooth muscle protein 22-alpha]; Figure 1A and 1B) but also correlated with enhanced phosphorylation of S6 and 4EBP1 (functional readouts of activated mTORC128) in VSMCs (Figure 1A, 1B, 1E, and 1F). These data demonstrate that TEAD1 induction, in response to vascular injury, correlates with SM proliferation and mTORC1 activation in vivo.
Figure 1.
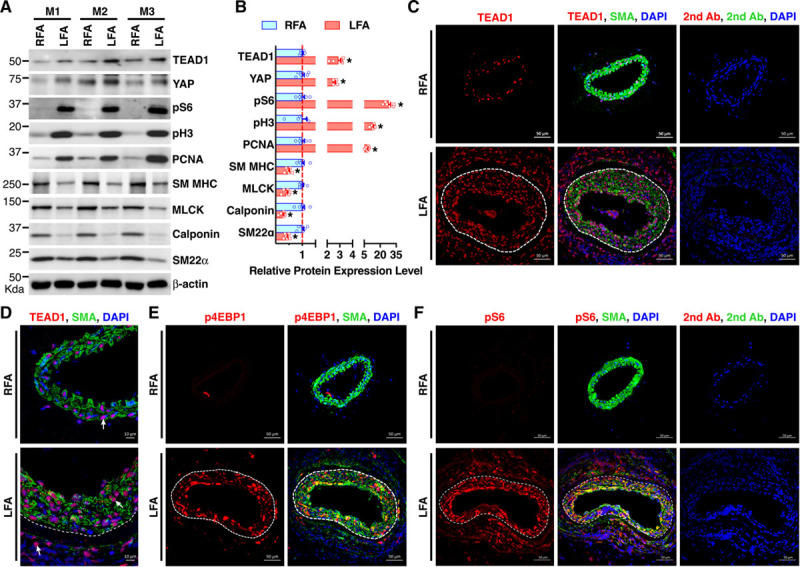
TEAD (TEA domain transcription factor) 1 expression is induced after arterial injury and positively correlates with mTORC1 (mechanistic target of rapamycin complex 1) activation in vascular smooth muscle cells in vivo. A, Control right femoral arteries (RFAs) or wire-injured left femoral arteries (LFAs) were harvested from adult male C57BL/6 mice (M) 7 d post-injury and analyzed by Western blot. B, Densitometric analysis of protein expression in A normalized to the loading control (β-actin) and expressed relative to signals from the uninjured control RFA (set to 1, red line). C and D, Control RFAs or wire-injured LFAs were harvested from adult male C57BL/6 mice 14 d post-injury and analyzed by immunofluorescence (IF) staining for TEAD1 (red) or SMA (smooth muscle α-actin; green). Nuclei were counterstained with DAPI (4’,6-diamidino-2-phenylindole; blue). Representative TEAD1 staining is shown by arrows (D). E and F, IF was performed similar to C except for using antibodies for activated mTOCR1 markers (E) p4EBP1 (phospho-eukaryotic initiation factor-4E–binding protein-1) or (F) pS6 (phospho-ribosomal protein S6), respectively. White dashed line denotes internal elastic lamina. The sections stained with fluorophore-labeled secondary antibodies (2nd Ab) and DAPI served as negative control. MLCK indicates myosin light-chain kinase; SM MHC, smooth muscle myosin heavy chain; and SM22α, smooth muscle protein 22-alpha. *P<0.05.
SM-Specific Deletion of Tead1 Attenuates Neointima Formation and Decreases VSMC Proliferation In Vivo
To determine the functional role of TEAD1 in vivo, we first performed wire injury in LFA in Tead1 global HET (heterozygous) mice because Tead1 global KO mouse is embryonic lethal.22,24 Characterization of the Tead1 HET mice demonstrated that TEAD1 protein expression in HET mice is reduced to ≈50% in both aortic and heart tissues compared with WT (wild type) control mice. This decreased TEAD1 expression did not affect SM or cardiac contractile protein expression or gross morphology of intact right femoral artery (Online Figure IIA through IID). However, compared with littermate WT control mice, Tead1 HET mice exhibited markedly reduced intimal area and intima-to-media ratio in injured LFA by ≈58% and ≈61%, respectively, without affecting the relative medial area in LFA at day 14 post-wire injury (Online Figure IIE through IIH). These results demonstrate that heterozygous deletion of Tead1 attenuates neointima formation after arterial injury.
To study TEAD1 function specifically in postnatal VSMCs in vivo, we generated SM-specific Tead1iKO mice. Compared with control mice, iKO mice have markedly reduced TEAD1 mRNA and protein expression specifically in the medial VSMCs (Online Figure IIIA through IIIF). Similar to the global Tead1HET mice, deletion of Tead1 in adult VSMCs did not alter SM contractile gene expression, overall vessel morphology, or VSMC numbers (Online Figure IIIC, IIIE, and IIIG), indicating that TEAD1 is not required for the maintenance of quiescent VSMCs in adult mice. To test whether SM-specific deletion of Tead1 attenuates neointima formation, we performed LFA wire injury in iKO mice versus control mice. We found that SM-specific Tead1 KO markedly reduced the intimal area and the intima-to-media ratio in injured LFA by ≈70% and ≈72%, respectively, without affecting the relative medial area at day 14 post-injury (Figure 2A through 2D). Furthermore, SM-specific Tead1 iKO significantly decreased the total number of EdU (5-ethynyl-2′-deoxyuridine)- and Ki67-positive VSMCs in the neointimal area (Figure 2E through 2H). These data suggest that SM-specific deletion of Tead1 attenuates neointima formation because of impaired VSMC proliferation.
Figure 2.
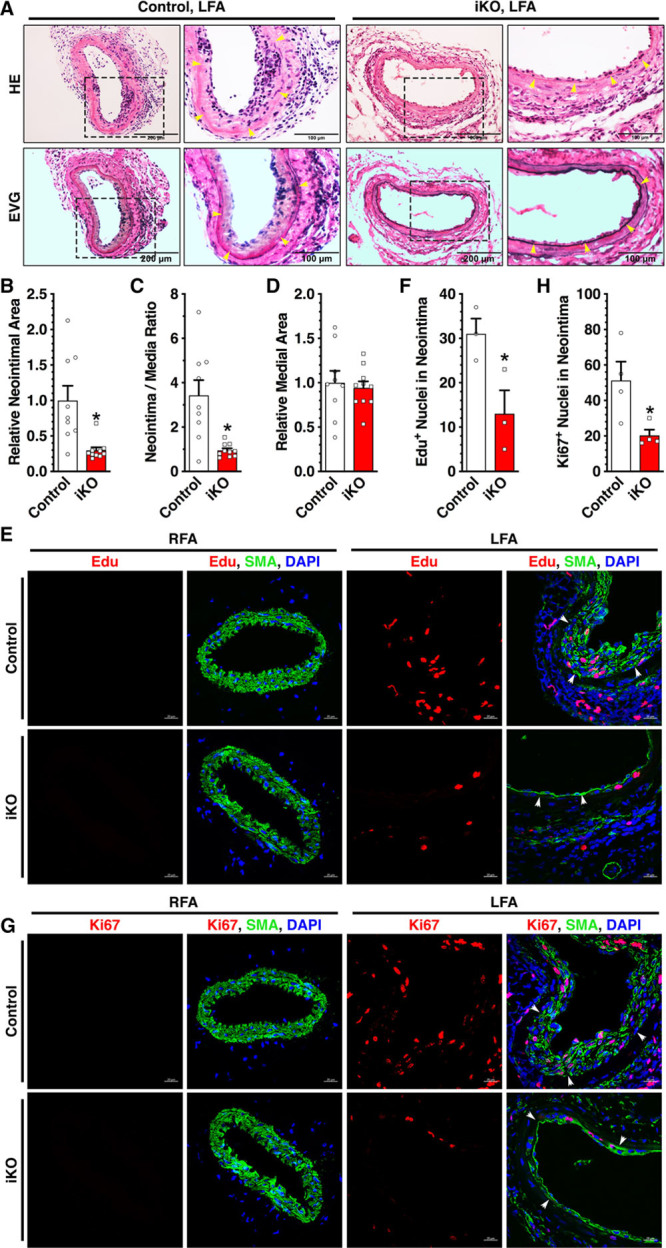
Smooth muscle (SM)–specific deletion of Tead (TEA domain transcription factor) 1 attenuates neointima formation by inhibiting vascular SM cell proliferation. A, Control or SM-specific Tead1 iKO (inducible knockout) mice were subjected to left femoral artery (LFA) wire injury. At 14 d post-injury, LFAs were harvested, and neointimal area was analyzed by hematoxylin and eosin (HE) or Elastic Van Gieson (EVG) staining. n=9 per group. The boxed area is magnified and shown to the right. Yellow arrowheads denote internal elastic lamina. B, Quantification of relative neointimal area, (C) neointima-to-media ratio, and (D) relative medial in LFA at 14 d post-injury from both control (set to 1) and iKO group mice. E, Control or iKO mice were subjected to LFA wire injury. At 14 d post-injury, mice were intraperitoneally injected with EdU (5-ethynyl-2′-deoxyuridine) 2 h before sacrifice. Isolated right femoral arteries (RFAs) or LFAs were subjected to EdU (red) and SMA (SM α-actin; green) costain. Nuclei were counterstained with DAPI (4’,6-diamidino-2-phenylindole; blue). F, Quantification of EdU-positive cells within neointimal area of LFA in both control and iKO group. G, Immunofluorescence staining was performed as described in E except for using antibodies for Ki67 (red) and SMA (green). White arrowheads in E and G denote the internal elastic lamina. H, Ki67-positive cells within neointimal area of LFA in both control and iKO mice were quantified and plotted. *P<0.05.
SM-Specific Deletion of Tead1 Inhibits mTORC1 Activation After Vascular Injury
Data described above demonstrated that TEAD1 expression correlates with enhanced mTORC1 signaling in VSMCs after arterial injury (Figure 1). We next tested the effects of SM-specific Tead1 iKO on vascular injury–induced mTORC1 activation. Western blot and immunofluorescence analysis demonstrated that compared with control mice, SM-specific Tead1 iKO significantly attenuated the induction of pS6 (phospho-S6; mTORC1 activation marker) and the proliferative marker pH3 in injured LFA (Figure 3A through 3C). However, SM-specific Tead1 iKO did not rescue the injury-induced downregulation of multiple contractile SM markers in LFA (Figure 3A and 3B; Online Figure IV). Together, these results suggest that specific deletion of Tead1 in VSMCs inhibits vascular injury–induced neointima formation by attenuating mTORC1 activation and VSMC proliferation.
Figure 3.

Smooth muscle (SM)–specific deletion of Tead (TEA domain transcription factor) 1 impairs mTORC1 (mechanistic target of rapamycin complex 1) signaling in the neointima. A, Control or SM-specific Tead1 iKO (inducible knockout) mice were subjected to left femoral artery (LFA) wire injury. At 7 d post-injury, right femoral arteries (RFAs) or LFAs were harvested and analyzed by Western blotting as indicated. B, Relative protein expression levels of the indicated proteins as shown in A were plotted. Expression of proteins in the RFA of the oil-treated control group was set as 1 (red dashed line). *P<0.05, vs control LFA injury group. C, Fourteen days post-injury, control RFA or injured LFA from control or iKO mice was isolated for immunofluorescence staining with antibodies for pS6 (phospho-ribosomal protein S6; red) or SMA (SM α-actin; green). Nuclei were counterstained with DAPI (4’,6-diamidino-2-phenylindole; blue). White arrowheads denote the internal elastic lamina. n=4 per group. MLCK indicates myosin light-chain kinase; pH3, phospho-histone 3; SM MHC, smooth muscle myosin heavy chain; and SM22α, smooth muscle protein 22-alpha.
Glutamine Transporter Slc1a5 Is a Direct Target Gene of TEAD1
To gain further insight on the mechanism underlying TEAD1-mediated mTORC1 activation and VSMC proliferation, we performed whole-transcriptome analysis by RNA-seq of intact aortic tissues isolated from SM-Tead1 iKO mice versus control mice. Data from the RNA-seq analysis demonstrated that SM-specific Tead1 iKO resulted in a significant downregulation of 145 genes and upregulation of 247 genes in aortic tissues (Figure 4A; Online Table I). Gene ontology analysis revealed that Tead1 iKO significantly affected the expression of genes involved in cell proliferation, differentiation, and migration (Figure 4B). Further analysis of the downregulated mRNAs revealed that SM-specific Tead1 iKO inhibited the expression of Slc1a5—a major glutamine transporter that was previously linked to mTORC1 activation in nonvascular cells (Figure 4C; Online Table I).29 Western blotting confirmed that SM-specific deletion of TEAD1 downregulated SLC1A5 expression at the protein level in intact aortic tissues (Figure 4D). Consistently, siRNA-mediated knockdown of endogenous TEAD1, but not other TEAD family genes, in HCASMCs specifically downregulated SLC1A5 mRNA expression levels by ≈50%, without affecting the expression of SLC7A5 and SLC38A1 (Figure 4E; Online Figure V). The TEAD1-dependent expression of SLC1A5 was confirmed at the protein level in HCASMCs (Figure 4F). Furthermore, we found that Slc1a5 expression was upregulated at the mRNA level in injured LFA 7 days post-injury, which was accompanied with upregulation of the proliferative marker Pcna and downregulation of SM contractile markers Acta2 (SMA) and Cnn1 (calponin; Figure 4G). Next, we attempted to test whether SLC1A5 is upregulated at the protein level in injured vessels. Because of the limited amount of protein that can be extracted from the relatively small-sized mouse femoral artery or lack of sensitivity of the SLC1A5 antibody for small amount of protein, we switched to utilize a rat carotid artery balloon injury model that is similar to mouse wire injury in resembling human postangioplasty restenosis,30 while providing a larger amount of protein. Using the protein lysate harvested from the balloon-injured rat carotid artery 7 days post-injury or uninjured contralateral controls, data from Western blotting assays demonstrated that TEAD1 was upregulated in injured arteries and correlated with induction of SLC1A5, pS6, PCNA, and pH3, with accompanying downregulation of contractile SM markers (Hic-5 [hydrogen peroxide–inducible clone 5], calponin, and SM22α; Online Figure VIA and VIB).
Figure 4.
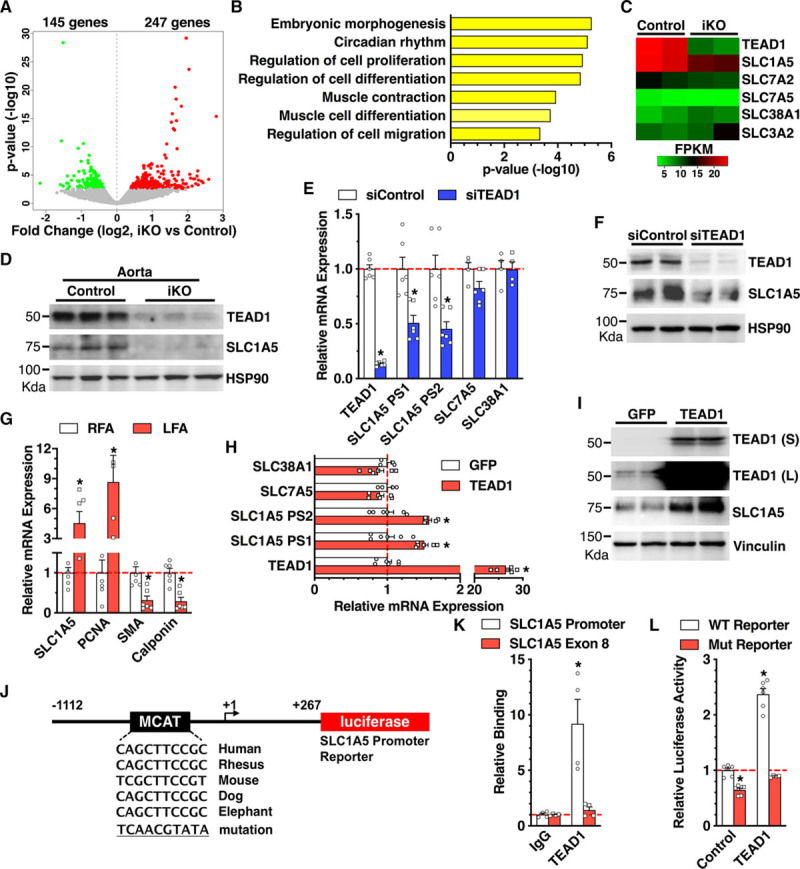
The glutamine transporter SLC1A5 (solute carrier family 1 member 5) is a direct target gene of TEAD (TEA domain transcription factor) 1. A, Volcano plot of the differentially expressed mRNAs between control and smooth muscle (SM)–specific Tead1 iKO (inducible knockout) mouse aortic tissues as revealed by RNA-sequencing. B, Gene ontology analysis was performed to show the biological functions related with the differentially expressed genes following ablation of Tead1 in adult vascular SM cells in mice. C, Heat map to show the expression of Tead1 and SLC (solute carrier family) family genes in control or SM-specific Tead1 iKO mouse aortic tissues. D, Aortic tissue lysates harvested from control or SM-specific Tead1 iKO were subjected to Western blot analysis. n=6 per group. E, Human coronary artery SM cells (HCASMCs) were transfected with scrambled control siRNA (siControl) or TEAD1 siRNA (siTEAD1) for 48 h. Total RNA was isolated for qRT-PCR (quantitative real-time polymerase chain reaction) analysis to determine mRNA expression as indicated. Two primer sets (PS) were used to assess SLC1A5 expression as shown. F, Similar to E, except that HCASMCs were harvested for Western blot analysis. G, Control right femoral arteries (RFAs) or wire-injured left femoral arteries (LFAs) from adult male C57BL/6 mice at 7 d post-injury were harvested, and total RNA was extracted for qRT-PCR analysis to determine mRNA expression using the gene-specific primers as indicated. The mRNA expression in control RFA was set to 1 (red dashed line). H, HCASMCs were transduced with GFP (green fluorescent protein) or TEAD1 adenovirus, and total RNA was isolated for qRT-PCR analysis to determine the relative mRNA expression. Cells transduced with GFP adenovirus served as control. I, Similar to H, except that HCASMCs were harvested for Western blot analysis. J, Schematic diagram to show generation of WT (wild type) or muscle CAT element (MCAT) mutation human SLC1A5 promoter–driven luciferase reporters. K, TEAD1 adenovirus was transduced into HCASMCs, and then chromatin was harvested for immunoprecipitation with TEAD1 antibody or IgG control. The precipitated DNA was amplified by real-time polymerase chain reaction using SLC1A5 promoter-specific primers that span the MCAT region depicted in J or primers localized in SLC1A5 exon 8 region. L, The luciferase reporter constructs harboring WT SLC1A5 promoter or mutation of predicted TEAD1-binding site in SLC1A5 promoter were cotransfected with TEAD1 expression plasmid into 10T1/2 cells. Forty-eight hours post-transfection, cells were harvested for dual luciferase assays. Reporter activity was normalized to a renilla luciferase internal control and expressed relative to the transfection with control empty plasmid (set to 1). FPKM indicates fragments per kilobase of transcript per million mapped reads; L, long exposure; and S, short exposure. *P<0.05.
Because arterial injury can induce both TEAD1 and SLC1A5 expression (Figures 1A and 4G; Online Figure VI), we next tested whether overexpression of TEAD1 in VSMCs can promote SLC1A5 expression. Indeed, adenovirus-mediated overexpression of TEAD1 in HCASMCs specifically upregulated SLC1A5 mRNA (Figure 4H) and protein (Figure 4I) expression, respectively. We next sought to delineate the mechanism by which TEAD1 regulates SLC1A5 gene expression in VSMCs. Bioinformatic analysis identified an evolutionarily conserved TEAD-binding element (MCAT) in the SLC1A5 gene promoter region (Figure 4J). Chromatin immunoprecipitation assays demonstrated an ≈9.2-fold enrichment of TEAD1 in the promoter region of SLC1A5 harboring the MCAT element but not in the SLC1A5 exon 8 region (Figure 4K). To test whether TEAD1 can directly activate SLC1A5 gene promoter, we generated luciferase reporters containing SLC1A5 promoter region harboring the WT or mutated MCAT site (Figure 4J). Data from luciferase reporter assays demonstrated that TEAD1 significantly enhanced SLC1A5 promoter activity by ≈2.4-fold. Mutation of the TEAD-binding MCAT site not only inhibited the basal activity of SLC1A5 gene reporter but also abolished TEAD1-dependent SLC1A5 promoter activation (Figure 4L). Together, these results demonstrate that SLC1A5 is a direct transcriptional target of TEAD1 and suggest that SLC1A5 may mediate the effects of TEAD1 on mTORC1 activation and VSMC proliferation.
TEAD1 Is Sufficient and Required to Promote VSMC Proliferation, Induce SLC1A5, and Activate mTORC1 Signaling
Data described above demonstrated that wire injury–induced and balloon injury–induced TEAD1 expression positively corelates with mTORC1 activation (Figure 1; Online Figure VI) and that SM-specific Tead1 iKO attenuates mTORC1 activation and neointimal cell proliferation in vivo (Figure 2). Therefore, we next sought to test the direct effects of TEAD1 on VSMC proliferation and mTORC1 activation in HCASMCs in vitro. Data from WST-1 (water soluble tetrazolium-1) colorimetric proliferation assay, EdU incorporation assay, and cell number counting revealed that overexpression of TEAD1 in HCASMCs is sufficient to promote VSMC proliferation (Figure 5A through 5D). Furthermore, TEAD1 overexpression specifically induced SLC1A5 expression and enhanced mTORC1 signaling as indicated by the hyperphosphorylation of mTORC1 substrates S6 and 4EBP1 (Figure 5E). Furthermore, overexpression of TEAD1 in HCASMCs neither affected the basal nor the activated Akt (protein kinase B) or ERK (extracellular signal-regulated kinase) signaling induced by acute treatment (30 minutes) with the potent mitogenic growth factor PDGF (platelet-derived growth factor)-BB,31 suggesting a selective function of TEAD1 on mTORC1 signaling activation (Figure 5E). Western blot data from our initial time course experiments revealed that PDGF-BB promoted a maximal expression of the proliferative marker pH3 by 2-day treatment, whereas it downregulated multiple SM contractile proteins after 5-day treatment (Online Figure VII). Therefore, we chose 2-day treatment of PDGF-BB to examine the effects of overexpression of TEAD1 on PDGF-BB–induced VSMC proliferation. We found that overexpression of TEAD1 had a slightly additive effect on the induced expression of the proliferative marker pH3 and downregulated expression of the cell cycle inhibitor p27 in the presence of PDGF-BB for 2 days (Figure 5F). Furthermore, to confirm the reproducibility of our findings above, the functional effects of overexpression of TEAD1 on SLC1A5 expression and mTORC1 activation were validated in an independent isolate of HCASMCs derived from a different human donor (Online Figure VIII). Together, these data demonstrate that TEAD1 is sufficient to promote VSMC proliferation, induce SLC1A5 expression, and activate mTORC1 signaling independent of PDGF-BB (Figure 5A through 5F). Consistent with the data from gain-of-function assays, knocking down endogenous TEAD1 in HCASMCs using specific siRNA against TEAD1 resulted in inhibition of VSMC proliferation as indicated by WST-1 proliferation assay and cell count analysis (Figure 5G and 5H). Furthermore, silencing TEAD1 led to downregulation of SLC1A5 protein expression, inhibition of 4EBP1 phosphorylation, and downregulation of the proliferative marker pH3. Meanwhile, silencing TEAD1 promoted the expression of the cell cycle inhibitor p27 and expression of the VSMC differentiation markers, Hic-5, SMA, and calponin (Figure 5I). Taken together, our TEAD1 gain-of-function and loss-of-function assays in HCASMCs demonstrate that TEAD1 is sufficient and required to induce SLC1A5 expression, activate mTORC1 signaling, and promote a differentiated-to-proliferative phenotypic switching of VSMCs in vitro.
Figure 5.

TEAD (TEA domain transcription factor) 1 is required and sufficient to promote vascular smooth muscle cell proliferation, upregulate SLC1A5 (solute carrier family 1 member 5), and activate mTORC1 (mechanistic target of rapamycin complex 1) signaling in vitro. A, Human coronary artery smooth muscle cells (HCASMCs) were transduced with GFP (green fluorescent protein) or TEAD1 adenovirus for WST-1 (water soluble tetrazolium-1) proliferation assay at day 5 post-transduction. B, HCASMCs were transduced with GFP or TEAD1 adenovirus for 48 h and then incubated with EdU (5-ethynyl-2′-deoxyuridine) overnight. Immunofluorescence staining was performed to visualize EdU incorporation (red). Cell nuclei were costained with DAPI (4′,6-diamidino-2-phenylindole; blue). C, The percentage of EdU+ cells vs total number of cells is plotted. D, After transduction with GFP control or TEAD1 adenovirus, HCASMCs were plated at equal number (day 0) and harvested for cell counting analysis at day 3 or day 5 post-transduction. n=3 per group. E, HCASMCs were transduced with GFP or TEAD1 adenovirus in the absence or presence of PDGF (platelet-derived frowth factor)-BB (50 ng/mL) for 30 min (m) and then harvested for Western blot analysis. n=3 per group. F, HCASMCs were transduced with GFP or TEAD1 adenovirus in the absence or presence of PDGF-BB (50 ng/mL) for 48 h and then harvested for Western blot analysis. G, HCASMCs were transfected with scrambled control siRNA (siControl) or TEAD1 siRNA (siTEAD1) for WST-1 proliferation assay. H, After transfection of silencing control or silencing TEAD1 RNA duplexes, HCASMCs were plated at equal number (day 0) and harvested for cell counting analysis at day 3 or day 5 post-transfection. n=3 per group. I, After transfection of silencing control or silencing TEAD1 RNA duplexes for 48 h, HCASMCs were harvested for Western blot analysis using the indicated primary antibodies. L indicates long exposure; and S, short exposure. *P<0.05.
SLC1A5 Mimics TEAD1 in Activating mTORC1 Signaling and Promoting VSMC Proliferation
Our data described above demonstrated that TEAD1 directly targets SLC1A5, activates mTORC1 signaling, and promotes VSMC proliferation (Figures 4 and 5). We next sought to determine whether SLC1A5 can mimic the function of TEAD1 in promoting mTORC1 activation and VSMC proliferation. Similar to overexpression of TEAD1, data from proliferation assays revealed that overexpression of SLC1A5 in HCASMCs promotes VSMC proliferation (Figure 6A through 6D). Furthermore, adenovirus-mediated overexpression of SLC1A5 was sufficient to enhance mTORC1 activity as indicated by enhanced phosphorylation of p70S6K and S6, without affecting the phosphorylation of Akt or ERK (Figure 6E). SLC1A5 overexpression also enhanced the expression of the proliferative marker pH3, while having no effect on the expression of VSMC differentiation markers or TEAD1 expression (Figure 6E). Conversely, depletion of endogenous SLC1A5 by siRNA resulted in inhibition of VSMC proliferation (Figure 6F and 6G), inhibition of 4EBP1 phosphorylation, and reduced the expression of the proliferative marker pH3 (Figure 6H). Together, these SLC1A5 gain-of-function and loss-of-function studies demonstrate that SLC1A5 mimics the effects of TEAD1 in promoting mTORC1 activation and VSMC proliferation.
Figure 6.

SLC1A5 (solute carrier family 1 member 5) activates mTORC1 (mechanistic target of rapamycin complex 1) signaling and promotes vascular smooth muscle cell proliferation. A, Human coronary artery smooth muscle cells (HCASMCs) were transduced with GFP (green fluorescent protein) or SLC1A5 adenovirus for WST-1 (water soluble tetrazolium-1) proliferation assay at day 3 post-viral transduction. B, Immunofluorescence for EdU (5-ethynyl-2′-deoxyuridine; red) was performed to assess HCASMC proliferation after GFP or SLC1A5 viral transduction for 3 d, and (C) the percentages of EdU+ cells to total cell numbers were quantified. Cell nuclei were counterstained with DAPI (4’,6-diamidino-2-phenylindole; blue). D, Numbers of HCASMCs were counted post 3 d of GFP or SLC1A5 viral transduction. n=5. E, Western blot was performed to examine the effects of myc-tagged SLC1A5 overexpression on mTORC1 signaling activation and smooth muscle contractile gene expression in HCASMCs. F, HCASMCs were transfected with scrambled control siRNA (siControl) or SLC1A5 siRNA (siSLC1A5) for WST-1 proliferation assay or (G) cell count analysis at day 3 post-transfection. n=3 per group. H, HCASMCs were transfected with control siRNA (siControl) or SLC1A5 siRNA (siSLC1A5) for 3 d and then cell lysates were harvested for Western blot analysis. L indicates long exposure; and S, short exposure. *P<0.05.
SLC1A5-Mediated Glutamine Uptake Is Required for TEAD1-Induced mTORC1 Activation and VSMC Proliferation
To further delineate the mechanism underlying SLC1A5-mediated VSMC proliferation, HCASMCs were cultured in medium with or without glutamine or treated with GPNA (γ-L-glutamyl-p-nitroanilide)32—an inhibitor that blocks glutamine uptake through SLC1A5 transporter—and then cells were harvested for Western blotting to assess mTORC1 signaling or for proliferation assays. Data from these experiments revealed that glutamine deprivation attenuates VSMC proliferation (Online Figure IXA) and that GPNA treatment impairs mTORC1 activation (Online Figure IXB) and VSMC proliferation (Online Figure IXC and IXD). These data suggest that glutamine uptake via SLC1A5 is critical for mTORC1 activation and VSMC proliferation. Next, we sought to test the role of SLC1A5-mediated glutamine uptake in TEAD1-dependent mTORC1 activation and VSMC proliferation. Pretreatment of HCASMCs with GPNA markedly attenuated TEAD1-mediated mTORC1 activation, as indicated by inhibition of TEAD1-mediated p70S6K and S6 phosphorylation (Figure 7A). GPNA treatment also significantly attenuated both basal and TEAD1-mediated VSMC proliferation (Figure 7B). Consistently, silencing of endogenous SLC1A5 with siRNA markedly attenuated TEAD1-mediated activation of mTORC1 signaling (Figure 7C) and blunted TEAD1-dependent VSMC proliferation (Figure 7D and 7E). Finally, we sought to test whether treatment of HCASMCs with rapamycin—a well-accepted mTORC1 inhibitor33—would abolish TEAD1-mediated VSMC proliferation. Similar to silencing SLC1A5 and blocking glutamine uptake by GPNA treatment, we found that rapamycin markedly attenuated both basal and TEAD1-mediated activation of mTORC1 signaling, without affecting basal TEAD1 expression (Figure 7F). Furthermore, rapamycin treatment led to inhibition of TEAD1-induced expression of the proliferative marker PCNA (Figure 7F). Collectively, these data demonstrate that TEAD1-induced VSMC proliferation occurs, at least in part, through TEAD1-dependent induction of SLC1A5-mediated glutamine uptake and subsequent activation of mTORC1 signaling.
Figure 7.
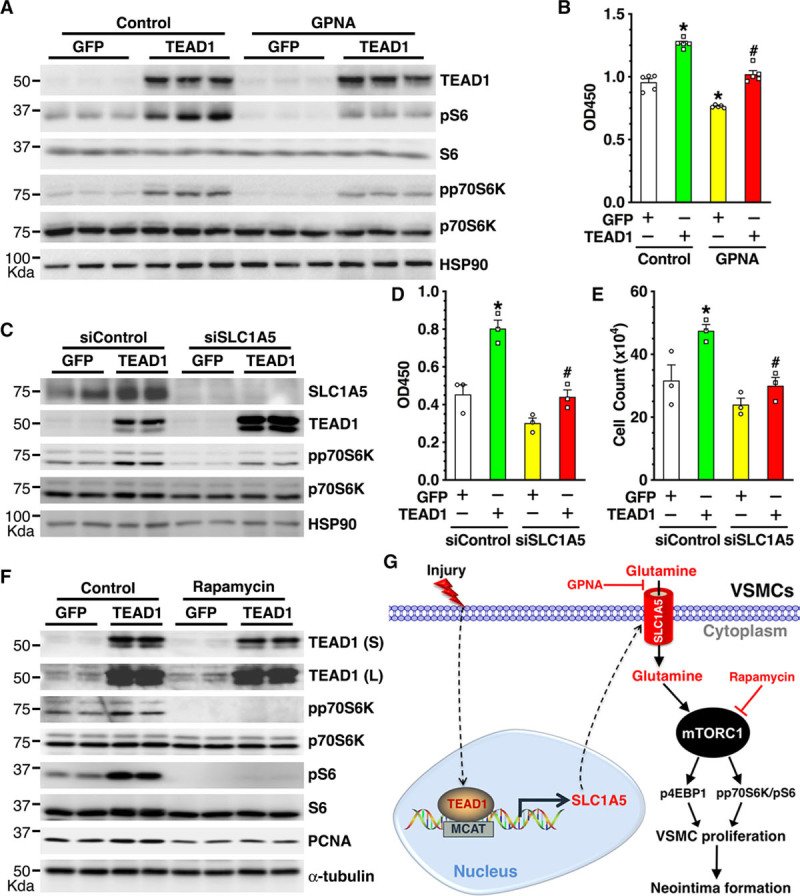
SLC1A5 (solute carrier family 1 member 5) and SLC1A5-mediated glutamine uptake are required for TEAD (TEA domain transcription factor) 1-dependent mTORC1 (mechanistic target of rapamycin complex 1) activation and vascular smooth muscle cell (VSMC) proliferation. A, Human coronary artery smooth muscle cells (HCASMCs) were transduced with GFP (green fluorescent protein) or TEAD1 adenovirus in the absence or presence of GPNA (γ-L-glutamyl-p-nitroanilide; 3 mmol/L)—a blocker for SLC1A5 transporter–mediated glutamine uptake, for 72 h and then cells were harvested for Western blot analysis or (B) WST-1 (water soluble tetrazolium-1) proliferation assay. *P<0.05, vs GFP transduction without GPNA treatment; #P<0.05, vs TEAD1 transduction without GPNA treatment. C, HCASMCs were transfected with scrambled control siRNA (siControl) or SLC1A5 siRNA (siSLC1A5) for 2 d and then transduced with control GFP or TEAD1 adenovirus for an additional 2 d, then VSMCs were harvested for Western blot. D, HCASMCs were transfected with siControl or siSLC1A5 for 2 d and then transduced with control GFP or TEAD1 adenovirus for an additional 3 d for WST-1 proliferation assay or (E) cell count analysis. *P<0.05, vs GFP transduction and siControl transfection; #P<0.05, vs TEAD1 transduction and siControl transfection. F, HCASMCs were transduced with GFP or TEAD1 adenovirus in the absence or presence of rapamycin (50 nM)—an inhibitor for mTORC1—for 72 h and then cells were harvested for Western blot analysis. G, Schematic diagram depicting the key findings of this study. We found that vascular injury induces TEAD1 expression, which in turn transcriptionally activates the glutamine transporter SLC1A5 expression in VSMCs. The overexpressed SLC1A5 enhances glutamine uptake, leading to mTORC1 activation, VSMC proliferation, and neointima formation in response to vascular injury. Blocking SLC1A5-mediated glutamine uptake by GPNA or inhibiting mTORC1 activation by rapamycin abolishes TEAD1-induced VSMC proliferation. L indicates long exposure; and S, short exposure.
Discussion
Our study uncovers a previously unrecognized role of TEAD1 in VSMC proliferation. Our data demonstrate that vascular injury induces TEAD1 expression. The injury-induced TEAD1 expression, in turn, transcriptionally promotes SLC1A5 expression, leading to enhanced glutamine uptake and activation of mTORC1 signaling, which results in enhanced VSMC proliferation that underlies the robust neointima formation in response to vascular injury (Figure 7G). Previous studies demonstrated that TEAD proteins are upregulated in many cancer types,7 which correlates with poor clinical outcome in patients with cancer.34 Consistent with these observations in cancer tissues, this study demonstrates that TEAD1 expression is induced in the neointimal area in response to arterial injury (Figure 1; Online Figure VI), suggesting that TEAD1 induction positively correlates with enhanced cell proliferation across different cell types. It will be intriguing to delineate the underlying mechanism of TEAD1 induction after arterial injury. It is likely that YAP acts as an upstream transactivator for TEAD1 expression since YAP expression was induced after arterial injury. Further investigation is needed to test this novel hypothesis. Mechanistically, multiple targets have been identified that mediate the growth promoting effects of TEADs/YAP in cancer cells or in VSMCs, including CTGF (connective tissue growth factor), cyr61 (cysteine-rich angiogenic inducer 61), and c-myc.35–40 In contrast to these earlier studies, data from RNA-seq analysis revealed that none of the genes listed above were altered in the SM-specific TEAD1 iKO aortic tissues (Online Table I), indicating that the underlying mechanism of TEAD1-mediated cell growth is cell context dependent.
To our knowledge, to date, there are no reports showing a direct association between TEAD1, SLC1A5 expression/mutation, or glutamine uptake with human vascular diseases. However, TEADs have been implicated to play roles in regulating cell cycle suppressor genes, CDKN2A (p16 and p14) and CDKN2B (p15), which were identified as human coronary artery disease risk alleles in 9p21.3.41 Furthermore, as revealed by ChIP-seq (chromatin immunoprecipitation sequencing) with the coronary artery disease–associated transcription factor TCF21 (transcription factor 21) in HCASMCs, TEADs co-occupy with TCF21 on many gene loci related to other independent coronary artery disease loci.42 Although our work provides a direct evidence that TEAD1 promotes VSMC proliferation, further investigations are needed to establish the causative role of TEAD1 in human vascular diseases.
A recent lineage-tracing study revealed that vascular injury–induced VSMC proliferation contributes far more than VSMC migration to the neointimal VSMC accumulation in vascular diseases.4 Our study demonstrates a critical role of TEAD1 in promoting VSMC proliferation and neointima formation. However, it appears that TEAD1 may also play a role in VSMC migration as demonstrated by the gene ontology analysis from our RNA-seq data (Figure 4B). Future work is needed to identify the potential target genes of TEAD1 and their roles in migration in VSMCs.
It has been reported that the transcriptional output of TEAD1 can be modulated by post-translational modifications such as autopalmitoylation43,44 and phosphorylation.45 Although we did not observe a significant change in the total protein of TEAD1 expression after PDGF-BB treatment (Figure 5F), It is possible that TEAD1 function in VSMCs can be regulated by post-translational modifications in addition to its expression level. Similarly, future investigations are needed to examine whether SLC1A5 can be post-translationally modified and determine whether such modifications have any effects on VSMC proliferation.
Several studies have shown that amino acid transporters are critical for cell proliferation, but the mechanisms regulating the expression of these transporters are poorly understood. A recent study demonstrated that silencing YAP—a major transcriptional cofactor of TEADs46—reduces the expression of SLC1A5 in cancer cells.47 However, the direct role of TEAD proteins in mediating YAP effects on SLC1A5 has not been explored. In this study, we demonstrate, for the first time, that TEAD1 directly binds and activates the SLC1A5 promoter in VSMCs, increasing SLC1A5 expression, thereby leading to the activation of mTORC1 signaling and enhanced VSMC proliferation (Figures 4 and 5). In addition to SLC1A5, Park et al48 recently demonstrated that YAP regulates the expression of 2 other amino acid transporters, namely SLC38A1 and SLC7A5, to activate mTORC1 signaling. Similarly, Hansen et al49 demonstrated that YAP induces the promoter activity and the expression of the high-affinity leucine transporter SLC7A5 to enhance mTORC1 signaling. However, in this study, we found that TEAD1 did not regulate the expression of SLC38A1 or SLC7A5 in HCASMCs (Figures 4E, 4H, and 5E). Together, these studies suggest that TEAD/YAP proteins may regulate different amino acid transporters in a cell context–dependent manner. Notably, while overexpression of TEAD1 per se is sufficient to activate SLC1A5/mTORC1 signaling pathway (Figure 5E; Online Figure VIII), TEAD1 modestly induces SLC1A5 mRNA expression (Figure 4H). We speculate there are 2 possibilities for this modest induction: (1) the endogenous TEAD1 and SLC1A5 are expressed at high levels in cultured HCASMCs to maintain the proliferative phenotype. Therefore, adenoviral-mediated overexpression of TEAD1 is unable to further dramatically potentiate its target SLC1A5 expression; or (2) it is likely that TEAD1 requires other cofactors such as YAP to cooperatively drive SLC1A5 expression in VSMCs. We previously reported that similar to TEAD1, YAP can promote VSMC proliferation in vitro and in vivo.50 Consistently, we found that YAP expression is also upregulated after wire injury (Figure 1A and 1B). These data suggest that TEAD1-dependent maximal activation of SLC1A5 likely requires binding to YAP or other unidentified cofactors. Future studies are required to test this possibility.
Previous studies have demonstrated a key role of SLC1A5 in enhancing mTORC1 activity in nonvascular cells that is mediated via enhanced glutamine uptake.29 Our current study extends these findings and reveals a previously unrecognized role of SLC1A5 and glutamine uptake in VSMC proliferation in vitro. Furthermore, our data suggest that SLC1A5 may mediate, at least in part, the effects of TEAD1 on mTORC1 activation and VSMC proliferation but does not mediate the effects of TEAD1 on VSMC dedifferentiation (Figure 6E). Future studies are required to test the specific role of SLC1A5 in vascular injury–induced neointima formation in vivo.
Although the role of TEADs in regulating VSMC proliferation seems clear, the role of TEADs in regulating VSMC differentiation is far more complex. During embryonic development, TEADs are required for the initial transcriptional activation of key SM differentiation genes, such as myocardin9 and Acta2,10 but are not required for the promoter activity of Acta2 in adult differentiated SM cells in vivo.10 Conversely, in adult SM, we previously demonstrated that TEAD1 promotes dedifferentiation of rat aortic VSMCs by disrupting myocardin/SRF interactions,12 suggesting that TEADs may enhance or downregulate SM differentiation genes in a temporal manner. In this study, we demonstrate that TEAD1 promotes dedifferentiation in HCASMCs in vitro (Figure 5F and 5I). On the contrary, we found that specific deletion of Tead1 in VSMCs of adult mice does not significantly affect the expression of SM differentiation markers in intact aortic tissues (Online Figure IIIC) or rescue their downregulated expression after arterial injury (Figure 3A and 3B). These discrepancies between our in vitro and in vivo observations may be attributed to a difference in the differentiation and proliferation states of VSMCs in culture versus intact vessels. In addition, after arterial injury, many factors other than TEAD1, such as PDGF-BB, are induced/released that likely contribute to dedifferentiation of VSMCs.51,52
Previous studies have demonstrated that rapamycin-eluting stents inhibit mTORC1 activation in both ECs and VSMCs, leading to disruption of re-endothelialization of injured vessels, and thus, are accompanied with an increased risk of in-stent thrombosis.53,54 Accordingly, in future studies, it will be important to determine whether TEAD1 has differential effects on VSMCs versus ECs in terms of mTORC1 signaling and proliferation. Because TEAD1 mRNA and protein levels are more abundant in human VSMCs than in human ECs (Online Figure I), the axis of TEAD1-SLC1A5-glutamine uptake could be a more favorable therapeutic target for treatment of restenosis without affecting recoverage of ECs. Furthermore, the critical role of glutamine in cancer cell growth and homeostasis suggests potential for developing new therapies that target glutamine metabolism.55 Our study suggests that antagonizing cellular glutamine uptake, which could potentially abrogate glutamine metabolism, may represent a novel efficacious approach for treating VSMC-driven vascular diseases.
In summary, our study unravels a novel mechanism of TEAD1-mediated VSMC proliferation and neointima formation via activation of the SLC1A5-glutamine uptake axis. Our study further suggests that targeting this axis is a promising therapeutic strategy to ameliorate vascular occlusive disease.
Acknowledgments
We thank Dr Paul Herring for a critical reading of the manuscript. We also thank Dr Stefan Offermanns for sharing SM MHC-CreERT2 mice.
Sources of Funding
The work at the laboratory of J. Zhou is supported by a grant from the National Heart, Lung, and Blood Institute, National Institutes of Health (HL132164). J. Zhou is a recipient of Established Investigator Award (17EIA33460468) from the American Heart Association. I. Osman and K. Dong are supported by postdoctoral fellowships (18POST34030400 and 19POST34450071, respectively) from the American Heart Association.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- 4EBP1
- eukaryotic initiation factor-4E–binding protein-1
- EC
- endothelial cell
- EdU
- 5-ethynyl-2′-deoxyuridine
- F
- flox
- GPNA
- γ-L-glutamyl-p-nitroanilide
- HCASMC
- human coronary artery smooth muscle cell
- HET
- heterozygous
- Hic-5
- hydrogen peroxide–inducible clone 5
- iKO
- inducible knockout
- KO
- knockout
- LFA
- left femoral artery
- MCAT
- muscle CAT element
- MHC
- myosin heavy chain
- MLCK
- myosin light-chain kinase
- mTORC
- mechanistic target of rapamycin complex 1
- p70S6K
- p70 ribosomal protein S6 kinase
- PCNA
- proliferating cell nuclear antigen
- pH3
- phospho-histone 3
- pS6
- phospho-ribosomal protein S6
- S6
- ribosomal protein S6
- SLC1A5
- solute carrier family 1 member 5
- SM
- smooth muscle
- SMA
- smooth muscle α-actin
- SRF
- serum response factor
- TEAD
- TEA domain transcription factor
- VSMC
- vascular smooth muscle cell
- WT
- wild type
- YAP
- yes-associated protein
In January 2019, the average time from submission to first decision for all original research papers submitted to Circulation Research was 15.24 days.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCRESAHA.118.314187.
Novelty and Significance
What Is Known?
Vascular smooth muscle (VSMC) proliferation is key to a number of occlusive vascular diseases that block the blood flow in arteries.
TEAD (TEA domain transcription factor) 1 is a transcription factor that has been shown to promote cancer cell proliferation.
mTORC1 (mechanistic target of rapamycin complex 1) is a master cell growth regulator, and its inhibitors (eg, rapamycin) are commonly used in the clinical setting to limit VSMC overgrowth-induced blood vessel blockage.
SLC1A5 (solute carrier family 1 member 5) is a key upstream activator for mTORC1 signaling and thereby for cancer cell proliferation.
What New Information Does This Article Contribute?
TEAD1 deficiency in VSMCs inhibits vascular injury–induced arterial occlusion due to impaired VSMC proliferation.
TEAD1 is upregulated after arterial injury and transcriptionally induces SLC1A5 expression, leading to mTORC1 signaling activation, thereby promoting VSMC proliferation and arterial occlusion.
In many occlusive vascular diseases, VSMCs undergo phenotypic switching to a hyperproliferative state. Identification of the mechanisms involved in VSMC proliferation will lead us toward better understanding the pathology of these diseases and ultimately designing new therapeutic agents for their treatment and prevention. Our study uncovers a previously unrecognized role of the transcription factor TEAD1 in VSMC proliferation. Our data demonstrate that vascular injury induces TEAD1 expression. The injury-induced TEAD1 expression, in turn, transcriptionally promotes the key glutamine transporter SLC1A5 expression, leading to enhanced glutamine uptake and activation of mTORC1 signaling, which results in enhanced VSMC proliferation that underlies the robust neointima formation in response to vascular injury. Our novel findings suggest that TEAD1-SLC1A5-glutamine uptake is a promising therapeutic axis to ameliorate occlusive vascular diseases in humans.
References
- 1.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 2.Herring BP, Hoggatt AM, Burlak C, Offermanns S. Previously differentiated medial vascular smooth muscle cells contribute to neointima formation following vascular injury. Vasc Cell. 2014;6:21. doi: 10.1186/2045-824X-6-21. doi: 10.1186/2045-824X-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bentzon JF, Majesky MW. Lineage tracking of origin and fate of smooth muscle cells in atherosclerosis. Cardiovasc Res. 2018;114:492–500. doi: 10.1093/cvr/cvx251. doi: 10.1093/cvr/cvx251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chappell J, Harman JL, Narasimhan VM, Yu H, Foote K, Simons BD, Bennett MR, Jørgensen HF. Extensive proliferation of a subset of differentiated, yet plastic, medial vascular smooth muscle cells contributes to neointimal formation in mouse injury and atherosclerosis models. Circ Res. 2016;119:1313–1323. doi: 10.1161/CIRCRESAHA.116.309799. doi: 10.1161/CIRCRESAHA.116.309799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin KC, Park HW, Guan KL. Regulation of the hippo pathway transcription factor TEAD. Trends Biochem Sci. 2017;42:862–872. doi: 10.1016/j.tibs.2017.09.003. doi: 10.1016/j.tibs.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao B, Ye X, Yu JD, Li L, Li WQ, Li SM, Yu JJ, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, Guan KL. Tead mediates yap-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou YH, Huang TT, Cheng ASL, Yu J, Kang W, To KF. The tead family and its oncogenic role in promoting tumorigenesis. Int J Mol Sci. 2016;17:15. doi: 10.3390/ijms17010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahoney WM, Jr, Hong JH, Yaffe MB, Farrance IK. The transcriptional co-activator TAZ interacts differentially with transcriptional enhancer factor-1 (TEF-1) family members. Biochem J. 2005;388:217–225. doi: 10.1042/BJ20041434. doi: 10.1042/BJ20041434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Creemers EE, Sutherland LB, McAnally J, Richardson JA, Olson EN. Myocardin is a direct transcriptional target of Mef2, Tead and Foxo proteins during cardiovascular development. Development. 2006;133:4245–4256. doi: 10.1242/dev.02610. doi: 10.1242/dev.02610. [DOI] [PubMed] [Google Scholar]
- 10.Gan Q, Yoshida T, Li J, Owens GK. Smooth muscle cells and myofibroblasts use distinct transcriptional mechanisms for smooth muscle alpha-actin expression. Circ Res. 2007;101:883–892. doi: 10.1161/CIRCRESAHA.107.154831. doi: 10.1161/CIRCRESAHA.107.154831. [DOI] [PubMed] [Google Scholar]
- 11.Azakie A, Lamont L, Fineman JR, He Y. Divergent transcriptional enhancer factor-1 regulates the cardiac troponin T promoter. Am J Physiol Cell Physiol. 2005;289:C1522–C1534. doi: 10.1152/ajpcell.00126.2005. doi: 10.1152/ajpcell.00126.2005. [DOI] [PubMed] [Google Scholar]
- 12.Liu F, Wang X, Hu G, Wang Y, Zhou J. The transcription factor TEAD1 represses smooth muscle-specific gene expression by abolishing myocardin function. J Biol Chem. 2014;289:3308–3316. doi: 10.1074/jbc.M113.515817. doi: 10.1074/jbc.M113.515817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallo R, Padurean A, Jayaraman T, Marx S, Roque M, Adelman S, Chesebro J, Fallon J, Fuster V, Marks A, Badimon JJ. Inhibition of intimal thickening after balloon angioplasty in porcine coronary arteries by targeting regulators of the cell cycle. Circulation. 1999;99:2164–2170. doi: 10.1161/01.cir.99.16.2164. [DOI] [PubMed] [Google Scholar]
- 14.Luo Y, Marx SO, Kiyokawa H, Koff A, Massagué J, Marks AR. Rapamycin resistance tied to defective regulation of p27Kip1. Mol Cell Biol. 1996;16:6744–6751. doi: 10.1128/mcb.16.12.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marx SO, Jayaraman T, Go LO, Marks AR. Rapamycin-FKBP inhibits cell cycle regulators of proliferation in vascular smooth muscle cells. Circ Res. 1995;76:412–417. doi: 10.1161/01.res.76.3.412. [DOI] [PubMed] [Google Scholar]
- 16.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE SIRIUS Investigators. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323. doi: 10.1056/NEJMoa035071. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 17.De Luca G, Dirksen MT, Spaulding C, et al. Drug-Eluting Stent in Primary Angioplasty (DESERT) Cooperation. Drug-eluting vs bare-metal stents in primary angioplasty: a pooled patient-level meta-analysis of randomized trials. Arch Intern Med. 2012;172:611–621; discussion 621. doi: 10.1001/archinternmed.2012.758. doi: 10.1001/archinternmed.2012.758. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu K, Kaira K, Tomizawa Y, Sunaga N, Kawashima O, Oriuchi N, Tominaga H, Nagamori S, Kanai Y, Yamada M, Oyama T, Takeyoshi I. ASC amino-acid transporter 2 (ASCT2) as a novel prognostic marker in non-small cell lung cancer. Br J Cancer. 2014;110:2030–2039. doi: 10.1038/bjc.2014.88. doi: 10.1038/bjc.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Yang L, An H, Chang Y, Zhang W, Zhu Y, Xu L, Xu J. High expression of Solute Carrier Family 1, member 5 (SLC1A5) is associated with poor prognosis in clear-cell renal cell carcinoma. Sci Rep. 2015;5:16954. doi: 10.1038/srep16954. doi: 10.1038/srep16954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Geldermalsen M, Wang Q, Nagarajah R, et al. ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene. 2016;35:3201–3208. doi: 10.1038/onc.2015.381. doi: 10.1038/onc.2015.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassanein M, Hoeksema MD, Shiota M, Qian J, Harris BK, Chen H, Clark JE, Alborn WE, Eisenberg R, Massion PP. SLC1A5 mediates glutamine transport required for lung cancer cell growth and survival. Clin Cancer Res. 2013;19:560–570. doi: 10.1158/1078-0432.CCR-12-2334. doi: 10.1158/1078-0432.CCR-12-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z, Friedrich GA, Soriano P. Transcriptional enhancer factor 1 disruption by a retroviral gene trap leads to heart defects and embryonic lethality in mice. Genes Dev. 1994;8:2293–2301. doi: 10.1101/gad.8.19.2293. [DOI] [PubMed] [Google Scholar]
- 23.Sawada A, Kiyonari H, Ukita K, Nishioka N, Imuta Y, Sasaki H. Redundant roles of Tead1 and Tead2 in notochord development and the regulation of cell proliferation and survival. Mol Cell Biol. 2008;28:3177–3189. doi: 10.1128/MCB.01759-07. doi: 10.1128/MCB.01759-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen T, Yin Q, Yu L, Hu G, Liu J, Zhang W, Huang L, Su H, Wang M, Zhou J. Characterization of mice carrying a conditional tead1 allele. Genesis. 2017;55:e23085. doi: 10.1002/dvg.23085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wirth A, Benyó Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horváth B, Maser-Gluth C, Greiner E, Lemmer B, Schütz G, Gutkind JS, Offermanns S. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–68. doi: 10.1038/nm1666. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 26.Osman I, Fairaq A, Segar L. Pioglitazone attenuates injury-induced neointima formation in mouse femoral artery partially through the activation of AMP-activated protein kinase. Pharmacology. 2017;100:64–73. doi: 10.1159/000471769. doi: 10.1159/000471769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sata M, Maejima Y, Adachi F, Fukino K, Saiura A, Sugiura S, Aoyagi T, Imai Y, Kurihara H, Kimura K, Omata M, Makuuchi M, Hirata Y, Nagai R. A mouse model of vascular injury that induces rapid onset of medial cell apoptosis followed by reproducible neointimal hyperplasia. J Mol Cell Cardiol. 2000;32:2097–2104. doi: 10.1006/jmcc.2000.1238. doi: 10.1006/jmcc.2000.1238. [DOI] [PubMed] [Google Scholar]
- 28.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 29.Nicklin P, Bergman P, Zhang B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clowes AW, Reidy MA, Clowes MM. Mechanisms of stenosis after arterial injury. Lab Invest. 1983;49:208–215. [PubMed] [Google Scholar]
- 31.Ferns GA, Raines EW, Sprugel KH, Motani AS, Reidy MA, Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991;253:1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- 32.Esslinger CS, Cybulski KA, Rhoderick JF. Ngamma-aryl glutamine analogues as probes of the ASCT2 neutral amino acid transporter binding site. Bioorg Med Chem. 2005;13:1111–1118. doi: 10.1016/j.bmc.2004.11.028. doi: 10.1016/j.bmc.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 33.Habib A, Finn AV. Antiproliferative drugs for restenosis prevention. Interv Cardiol Clin. 2016;5:321–329. doi: 10.1016/j.iccl.2016.02.002. doi: 10.1016/j.iccl.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Knight JF, Shepherd CJ, Rizzo S, Brewer D, Jhavar S, Dodson AR, Cooper CS, Eeles R, Falconer A, Kovacs G, Garrett MD, Norman AR, Shipley J, Hudson DL. TEAD1 and c-Cbl are novel prostate basal cell markers that correlate with poor clinical outcome in prostate cancer. Br J Cancer. 2008;99:1849–1858. doi: 10.1038/sj.bjc.6604774. doi: 10.1038/sj.bjc.6604774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, Liu CY, Zha ZY, Zhao B, Yao J, Zhao S, Xiong Y, Lei QY, Guan KL. TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition. J Biol Chem. 2009;284:13355–13362. doi: 10.1074/jbc.M900843200. doi: 10.1074/jbc.M900843200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai D, Ho KC, Hao Y, Yang X. Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res. 2011;71:2728–2738. doi: 10.1158/0008-5472.CAN-10-2711. doi: 10.1158/0008-5472.CAN-10-2711. [DOI] [PubMed] [Google Scholar]
- 37.Mamada H, Sato T, Ota M, Sasaki H. Cell competition in mouse NIH3T3 embryonic fibroblasts is controlled by the activity of Tead family proteins and Myc. J Cell Sci. 2015;128:790–803. doi: 10.1242/jcs.163675. doi: 10.1242/jcs.163675. [DOI] [PubMed] [Google Scholar]
- 38.Feng X, Liu P, Zhou X, Li MT, Li FL, Wang Z, Meng Z, Sun YP, Yu Y, Xiong Y, Yuan HX, Guan KL. Thromboxane A2 activates YAP/TAZ protein to induce vascular smooth muscle cell proliferation and migration. J Biol Chem. 2016;291:18947–18958. doi: 10.1074/jbc.M116.739722. doi: 10.1074/jbc.M116.739722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimura TE, Duggirala A, Smith MC, White S, Sala-Newby GB, Newby AC, Bond M. The Hippo pathway mediates inhibition of vascular smooth muscle cell proliferation by cAMP. J Mol Cell Cardiol. 2016;90:1–10. doi: 10.1016/j.yjmcc.2015.11.024. doi: 10.1016/j.yjmcc.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen B, Liu G. WWC3 inhibits intimal proliferation following vascular injury via the Hippo signaling pathway. Mol Med Rep. 2018;17:5175–5183. doi: 10.3892/mmr.2018.8484. doi: 10.3892/mmr.2018.8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Almontashiri NA, Antoine D, Zhou X, Vilmundarson RO, Zhang SX, Hao KN, Chen HH, Stewart AF. 9p21.3 coronary artery disease risk variants disrupt TEAD transcription factor-dependent transforming growth factor β regulation of p16 expression in human aortic smooth muscle cells. Circulation. 2015;132:1969–1978. doi: 10.1161/CIRCULATIONAHA.114.015023. doi: 10.1161/CIRCULATIONAHA.114.015023. [DOI] [PubMed] [Google Scholar]
- 42.Sazonova O, Zhao Y, Nürnberg S, Miller C, Pjanic M, Castano VG, Kim JB, Salfati EL, Kundaje AB, Bejerano G, Assimes T, Yang X, Quertermous T. Characterization of TCF21 downstream target regions identifies a transcriptional network linking multiple independent coronary artery disease loci. PLoS Genet. 2015;11:e1005202. doi: 10.1371/journal.pgen.1005202. doi: 10.1371/journal.pgen.1005202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan P, Han X, Zheng B, DeRan M, Yu J, Jarugumilli GK, Deng H, Pan D, Luo X, Wu X. Autopalmitoylation of TEAD proteins regulates transcriptional output of the Hippo pathway. Nat Chem Biol. 2016;12:282–289. doi: 10.1038/nchembio.2036. doi: 10.1038/nchembio.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noland CL, Gierke S, Schnier PD, Murray J, Sandoval WN, Sagolla M, Dey A, Hannoush RN, Fairbrother WJ, Cunningham CN. Palmitoylation of TEAD transcription factors is required for their stability and function in hippo pathway signaling. Structure. 2016;24:179–186. doi: 10.1016/j.str.2015.11.005. doi: 10.1016/j.str.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 45.Lin KC, Moroishi T, Meng Z, Jeong HS, Plouffe SW, Sekido Y, Han J, Park HW, Guan KL. Regulation of Hippo pathway transcription factor TEAD by p38 MAPK-induced cytoplasmic translocation. Nat Cell Biol. 2017;19:996–1002. doi: 10.1038/ncb3581. doi: 10.1038/ncb3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 2001;15:1229–1241. doi: 10.1101/gad.888601. doi: 10.1101/gad.888601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edwards DN, Ngwa VM, Wang S, Shiuan E, Brantley-Sieders DM, Kim LC, Reynolds AB, Chen J. The receptor tyrosine kinase epha2 promotes glutamine metabolism in tumors by activating the transcriptional coactivators yap and taz. Sci Signal. 2017;10:eaan4667. doi: 10.1126/scisignal.aan4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park YY, Sohn BH, Johnson RL, et al. Yes-associated protein 1 and transcriptional coactivator with PDZ-binding motif activate the mammalian target of rapamycin complex 1 pathway by regulating amino acid transporters in hepatocellular carcinoma. Hepatology. 2016;63:159–172. doi: 10.1002/hep.28223. doi: 10.1002/hep.28223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hansen CG, Ng YL, Lam WL, Plouffe SW, Guan KL. The Hippo pathway effectors YAP and TAZ promote cell growth by modulating amino acid signaling to mTORC1. Cell Res. 2015;25:1299–1313. doi: 10.1038/cr.2015.140. doi: 10.1038/cr.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X, Hu G, Gao X, Wang Y, Zhang W, Harmon EY, Zhi X, Xu Z, Lennartz MR, Barroso M, Trebak M, Chen C, Zhou J. The induction of yes-associated protein expression after arterial injury is crucial for smooth muscle phenotypic modulation and neointima formation. Arterioscler Thromb Vasc Biol. 2012;32:2662–2669. doi: 10.1161/ATVBAHA.112.254730. doi: 10.1161/ATVBAHA.112.254730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marijianowski MMH, Giese NA, Chronos NAF, Cui JH, Hanson SR. Anti-pdgf beta-receptor antibody inhibits neointima formation in primates. Circulation. 1997;96:195. [Google Scholar]
- 52.Banai S, Wolf Y, Golomb G, Pearle A, Waltenberger J, Fishbein I, Schneider A, Gazit A, Perez L, Huber R, Lazarovichi G, Rabinovich L, Levitzki A, Gertz SD. PDGF-receptor tyrosine kinase blocker AG1295 selectively attenuates smooth muscle cell growth in vitro and reduces neointimal formation after balloon angioplasty in swine. Circulation. 1998;97:1960–1969. doi: 10.1161/01.cir.97.19.1960. [DOI] [PubMed] [Google Scholar]
- 53.Viñals F, Chambard JC, Pouysségur J. p70 S6 kinase-mediated protein synthesis is a critical step for vascular endothelial cell proliferation. J Biol Chem. 1999;274:26776–26782. doi: 10.1074/jbc.274.38.26776. [DOI] [PubMed] [Google Scholar]
- 54.Walpoth BH, Hess OM. Late coronary thrombosis secondary to a sirolimus-eluting stent. Circulation. 2004;110:e309; author reply e309. doi: 10.1161/01.CIR.0000141422.05934.DC. doi: 10.1161/01.CIR.0000141422.05934.DC. [DOI] [PubMed] [Google Scholar]
- 55.Still ER, Yuneva MO. Hopefully devoted to Q: targeting glutamine addiction in cancer. Br J Cancer. 2017;116:1375–1381. doi: 10.1038/bjc.2017.113. doi: 10.1038/bjc.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]


